A kind of preparation method of celecoxib
A technology of celecoxib and reaction temperature, applied in the direction of organic chemistry, can solve the problems of obvious amplification effect, low product yield, low productivity, etc., achieve easy post-treatment operation, mild reaction conditions, and avoid regioisomers The effect of generation of impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
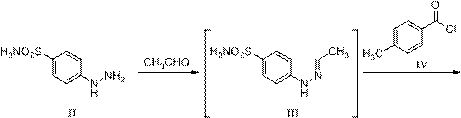
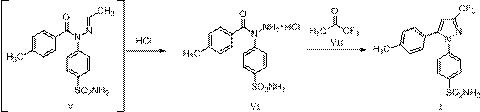
[0039] (1) Preparation of 4-[1-(4-methylbenzoyl)hydrazino]benzenesulfonamide hydrochloride (VI)
[0040] Add 200 g (1.07 mol) of 4-hydrazinobenzenesulfonamide, 2 L of toluene, and dropwise 47.1 g (1.07 mol) of acetaldehyde into a 5 L reaction flask, and react at 20 °C for 4 h. Cool down to 0°C, add 165 g (1.07 mol) p-toluoyl chloride dropwise, and react at 20°C for 3 h. Cool down to 0°C, add 390 g (3.21 mol) of 30% hydrochloric acid ethanol, react at 0°C for 2 h, after the reaction is complete, concentrate under reduced pressure at 55°C to remove the solvent. Slurry with 1.6 L of methyl tert-butyl ether for 2 h, filter and dry to obtain 350 g of 4-[1-(4-methylbenzoyl)hydrazino]benzenesulfonamide hydrochloride, yield 95.9%, HPLC purity 98.44%.
[0041] (2) Preparation of Celecoxib (I)
[0042] Add 100 g (0.89 mol) of 1,1,1-trifluoroacetone, 1.3 L of 80% aqueous ethanol to a 5 L reaction flask, add 320 g (0.94 mol) of 4-[1-(4-methylbenzoyl ) hydrazino]benzenesulfonamide hydr...
Embodiment 2
[0044] (1) Preparation of 4-[1-(4-methylbenzoyl)hydrazino]benzenesulfonamide hydrochloride (VI)
[0045] Add 200 g (1.07 mol) of 4-hydrazinobenzenesulfonamide, 2 L of toluene, and 49.3 g (1.12 mol) of acetaldehyde into a 5 L reaction flask, and react at 70 °C for 2 h. Cool down to 0°C, add 173 g (1.12 mol) p-toluoyl chloride dropwise, and react at 30°C for 2.5 h. Cool down to 0°C, add 520 g (4.28 mol) of 30% hydrochloric acid ethanol, react at 0°C for 3 hours, after the reaction is complete, concentrate under reduced pressure at 55°C to remove the solvent. Slurry with 1.6 L of methyl tert-butyl ether for 2 h, filter and dry to obtain 351 g of 4-[1-(4-methylbenzoyl)hydrazino]benzenesulfonamide hydrochloride, yield 96.1%, HPLC The purity is 99.08%.
[0046] (2) Preparation of Celecoxib (I)
[0047] Add 105 g (0.94 mol) 1,1,1-trifluoroacetone, 1.3 L isopropanol to a 5 L reaction flask, add 320 g (0.94 mol) 4-[1-(4-methylbenzoyl) Hydrazino]benzenesulfonamide hydrochloride, rea...
Embodiment 3
[0049](1) Preparation of 4-[1-(4-methylbenzoyl)hydrazino]benzenesulfonamide hydrochloride (VI)
[0050] Add 200 g (1.07 mol) of 4-hydrazinobenzenesulfonamide and 2 L of 2-methyltetrahydrofuran into a 5 L reaction flask, add 56.4 g (1.28 mol) of acetaldehyde dropwise, and react at 50°C for 2.5 h. Cool down to 0°C, add 331 g (2.14 mol) p-toluoyl chloride dropwise, and react at 40°C for 2 h. Cool down to 20°C, add 650 g (5.35 mol) of 30% hydrochloric acid ethanol, react at 30°C for 1.5 h, after the reaction is complete, concentrate under reduced pressure at 55°C to remove the solvent. Slurry with 1.6 L of methyl tert-butyl ether for 2 h, filter and dry to obtain 342 g of 4-[1-(4-methylbenzoyl)hydrazino]benzenesulfonamide hydrochloride, yield 93.7%, HPLC The purity is 97.09%.
[0051] (2) Preparation of Celecoxib (I)
[0052] Add 114 g (1.02 mol) 1,1,1-trifluoroacetone, 1.2 L methanol to a 5 L reaction flask, add 290 g (0.85 mol) 4-[1-(4-methylbenzoyl)hydrazino] Benzenesulfona...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com