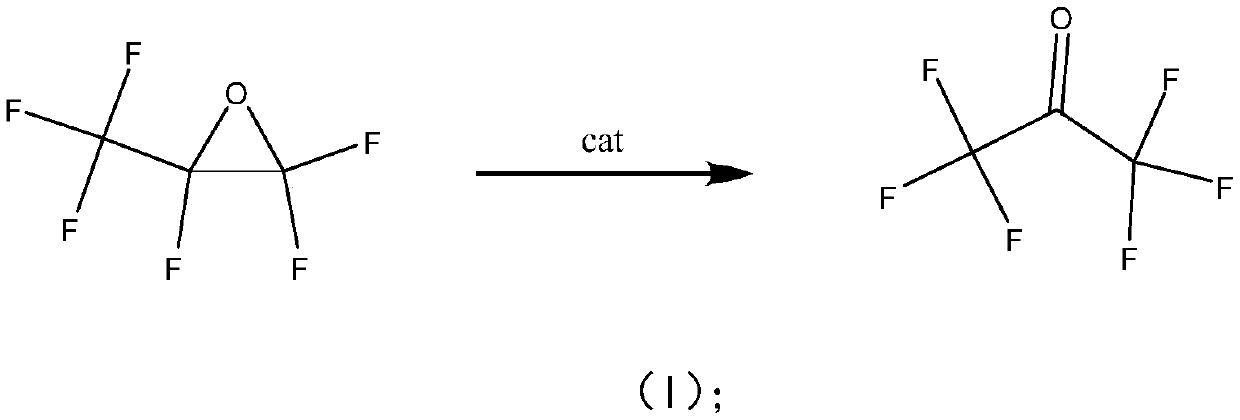
Method for preparing hexafluoroacetone by isomerizing hexafluoropropylene oxide
A technology of hexafluoropropylene oxide and hexafluoroacetone, which is applied in the preparation of heterocyclic compounds and organic chemistry, can solve the problems of complex preparation, many catalyst components, and high reaction temperature, and achieve simple preparation, low requirements for reaction conditions, and highly selective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
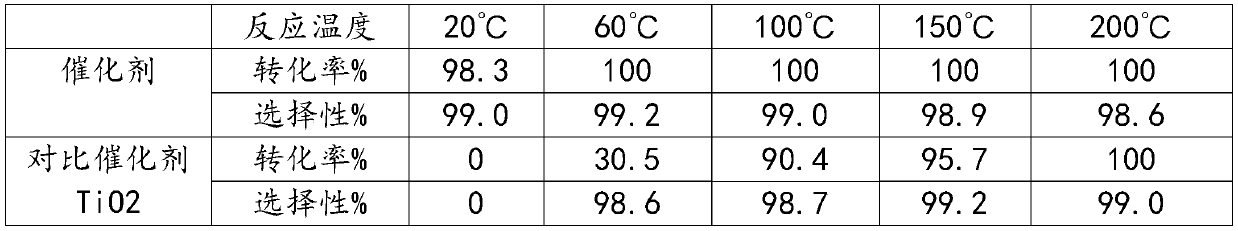
[0016] Embodiment: Preparation of catalyst: Dry ZSM-5 molecular sieve (silicon-aluminum ratio is 50) in an oven at 120° C. for 4 hours, take 10 g and put it in a tube furnace, feed N2 to heat up to 350° C., and change to R22 gas, Flow rate is 30ml / min, fluoride for 4 hours, change to N2 and cool down to room temperature. Tablets take 20-40 mesh as the catalyst.
[0017] Hexafluoropropylene oxide catalytic isomerization prepares hexafluoroacetone: the reaction test is carried out in a fixed bed reactor, a stainless steel reaction tube (inner diameter 10mm, length 300mm), catalyzer 5.0ml (20-40 mesh) is loaded into, and the reaction temperature is 20°C, 60°C, 100°C, 150°C, 200°C, the operating pressure is normal pressure, and the reaction space velocity is 300h-1. The reaction product is analyzed and determined by a gas chromatography analysis method, and then passed into deionized water for absorption to generate hexafluoroacetone hydrate. The reaction results are shown in Ta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com