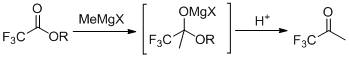
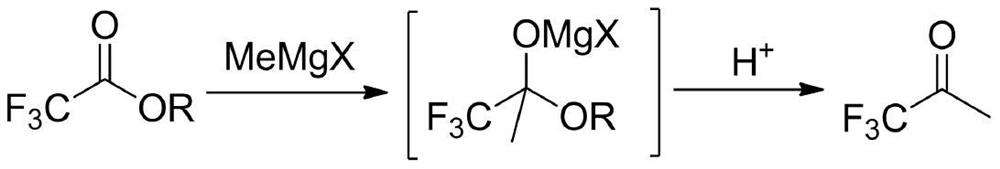
The preparation method of 1,1,1-trifluoroacetone
A technology of trifluoroacetate and molar ratio, applied in the preparation of organic compounds, preparation of carbon-based compounds, chemical instruments and methods, etc., can solve the problems of harsh reaction conditions, large pollution, low yield, etc., and achieve low cost , Simple operation, convenient purification effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] In a dry four-neck flask (equipped with a rectification column), under the protection of nitrogen, add 900mL (1.8mol) of 2mol / L tetrahydrofuran solution of methylmagnesium chloride, stir, add 11.4g (0.075mol) of cesium fluoride, and cool down to - A mixed solution of 213.1 g (1.5 mol) of ethyl trifluoroacetate and 375 mL of tetrahydrofuran was added dropwise at 5°C. After dropping, slowly rise to 30°C for 1 hour reaction. Below 10°C, adjust the pH to 1 with 20% hydrochloric acid. Slowly raise the temperature, distill under normal pressure directly, and collect the fraction at 22°C~23°C in a cold trap at -40°C to obtain 153.7g of colorless liquid, namely TFK, with a GC purity of 99.5% (area normalization method) and a yield of 91.5%.
Embodiment 2
[0023] With the technological operation step of embodiment 1, different conditions are:
[0024] Add 1800mL (1.8mol) of tetrahydrofuran solution of 1mol / L methylmagnesium chloride, 4.4g (0.075mol) of potassium fluoride, drop it up to 20°C for 6 hours, and obtain 140.7g of colorless liquid, namely TFK, with a GC purity of 99.2%. Yield 83.7%.
Embodiment 3
[0026] With the technological operation step of embodiment 1, different conditions are:
[0027] Add 600 mL (1.8 mol) of 3 mol / L tetrahydrofuran solution of methylmagnesium chloride, 3.2 g (0.075 mol) of sodium fluoride, drop it up to 30 ° C for 4 hours, and obtain 142.9 g of a colorless liquid, namely TFK, with a GC purity of 99.1%. Yield 85.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com