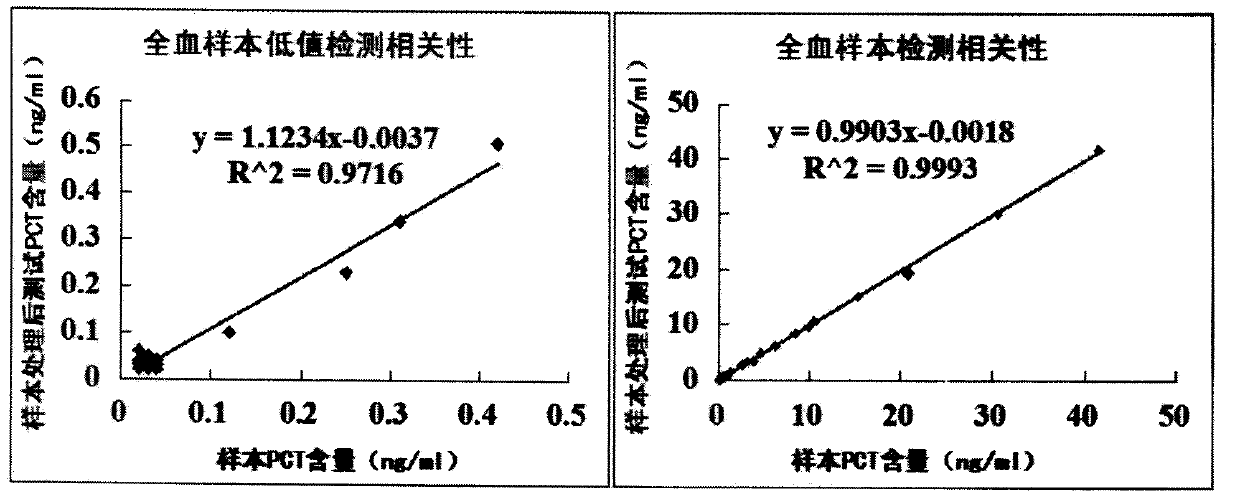
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
49 results about "Conglutination reaction" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
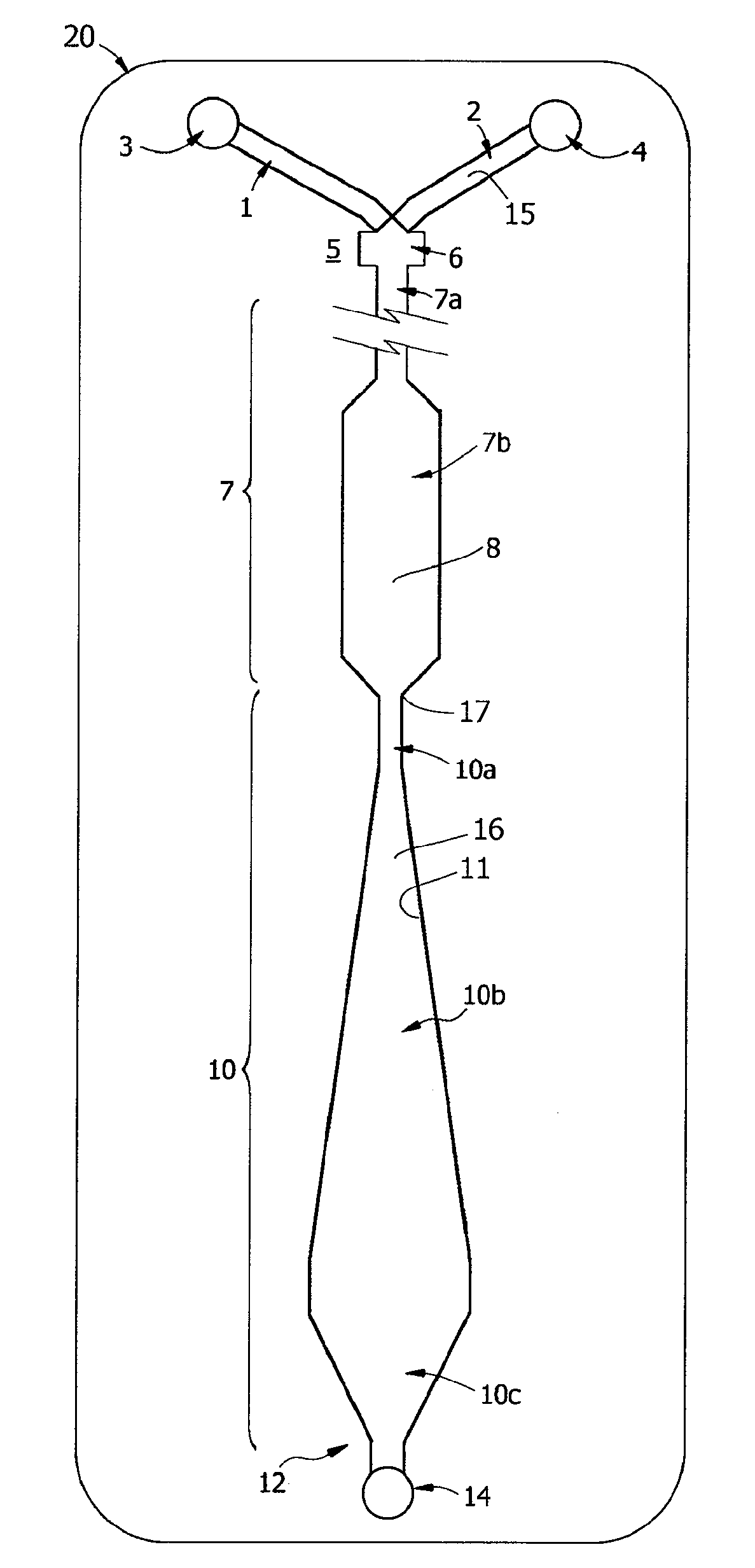
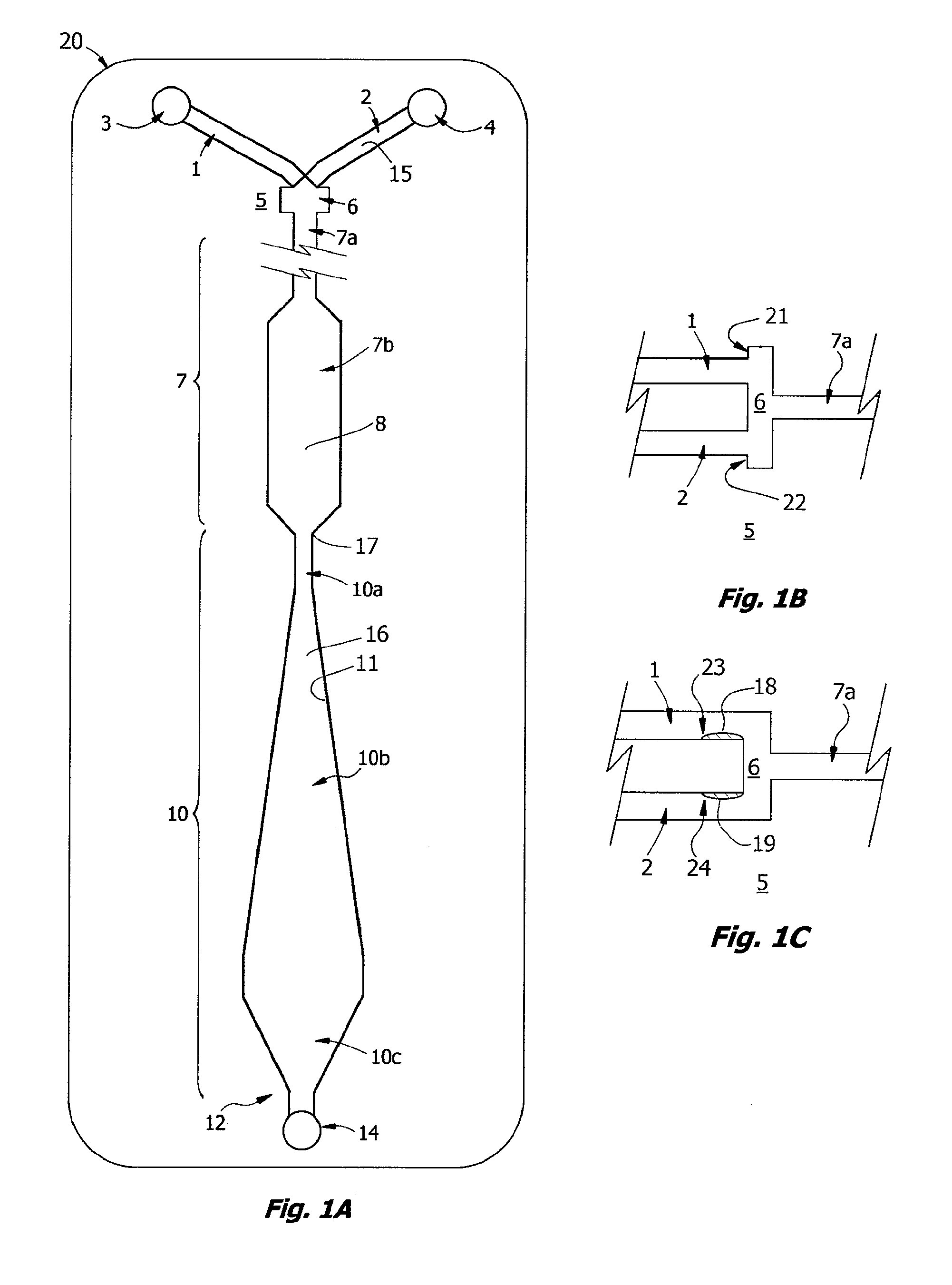
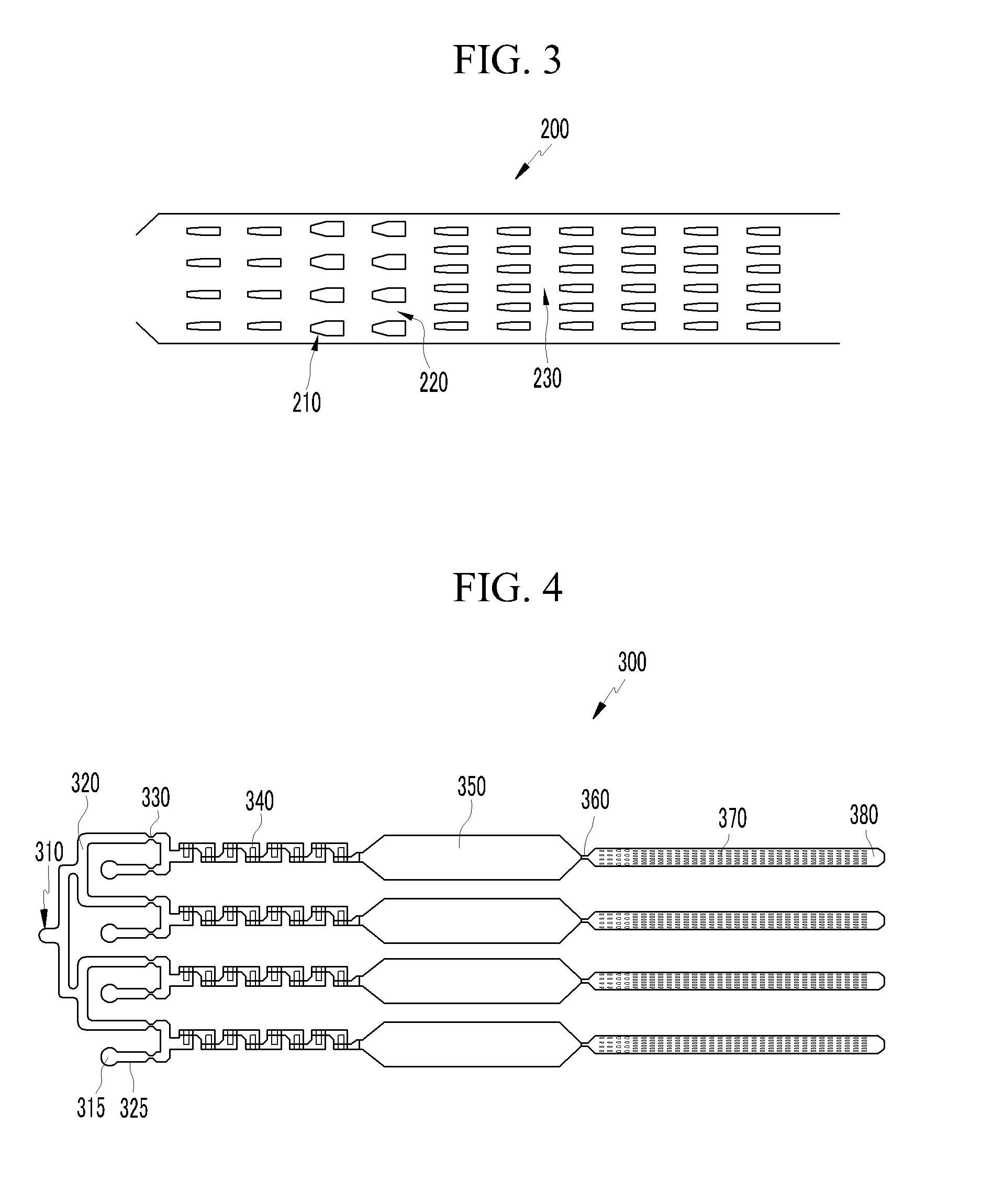
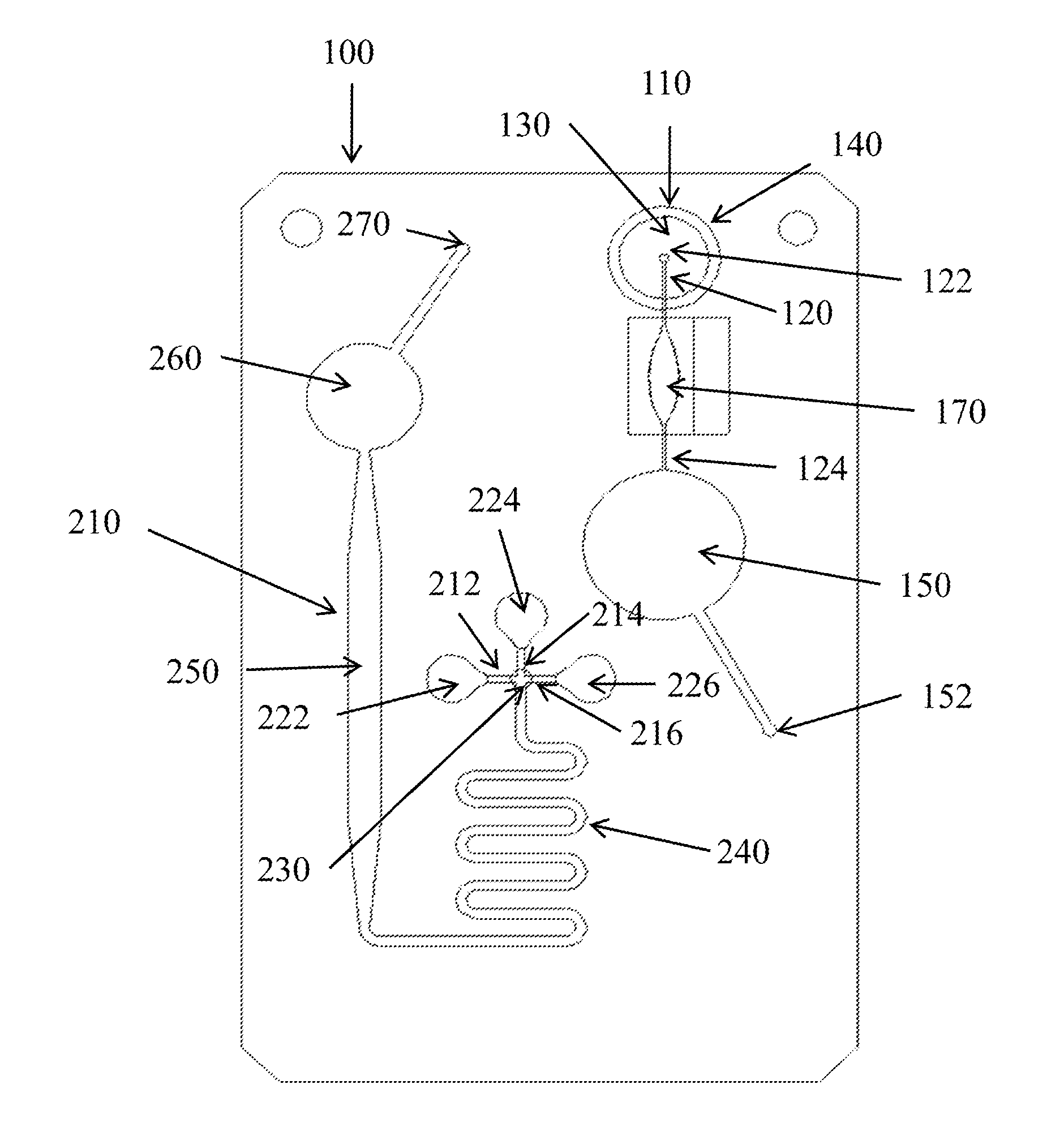
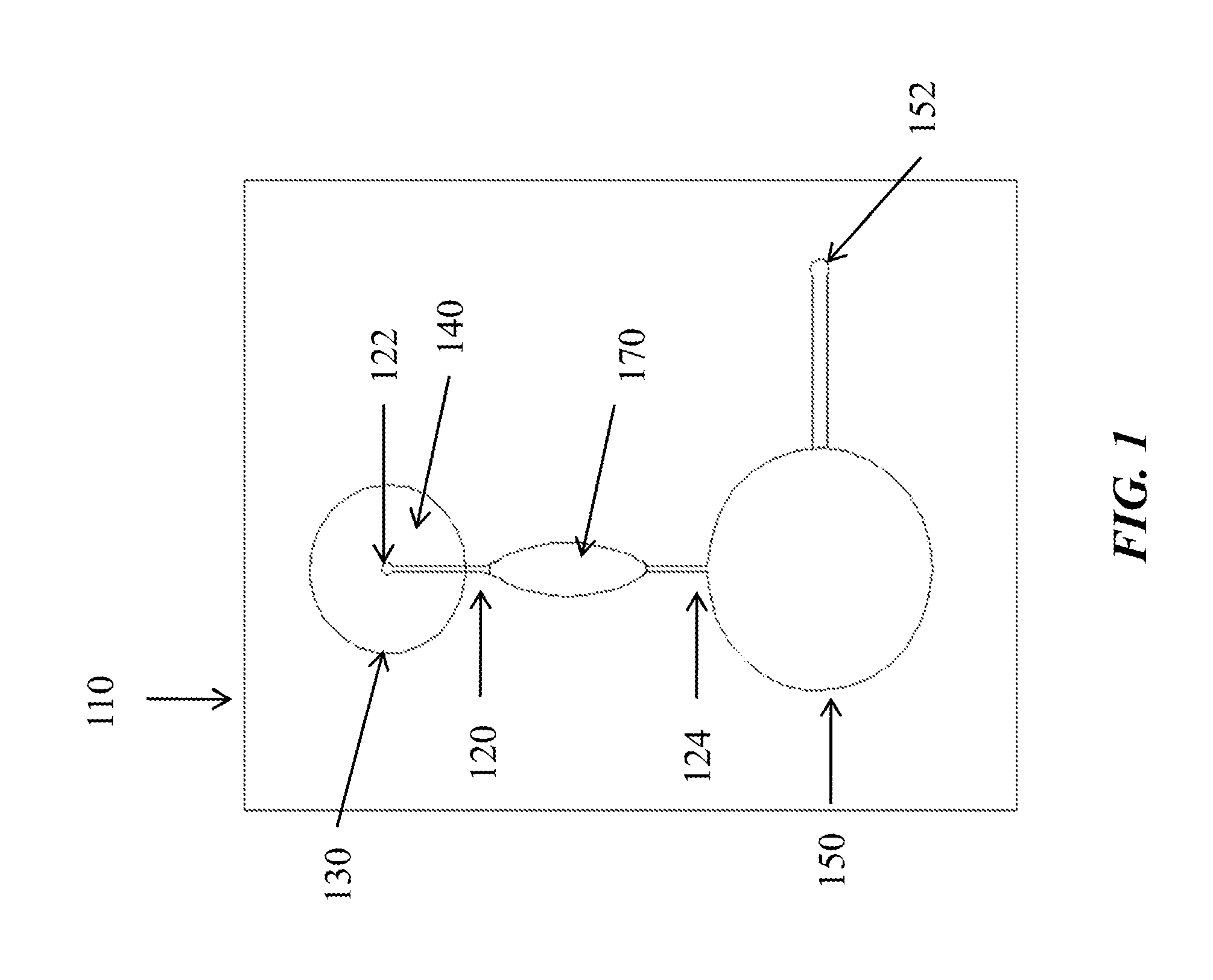
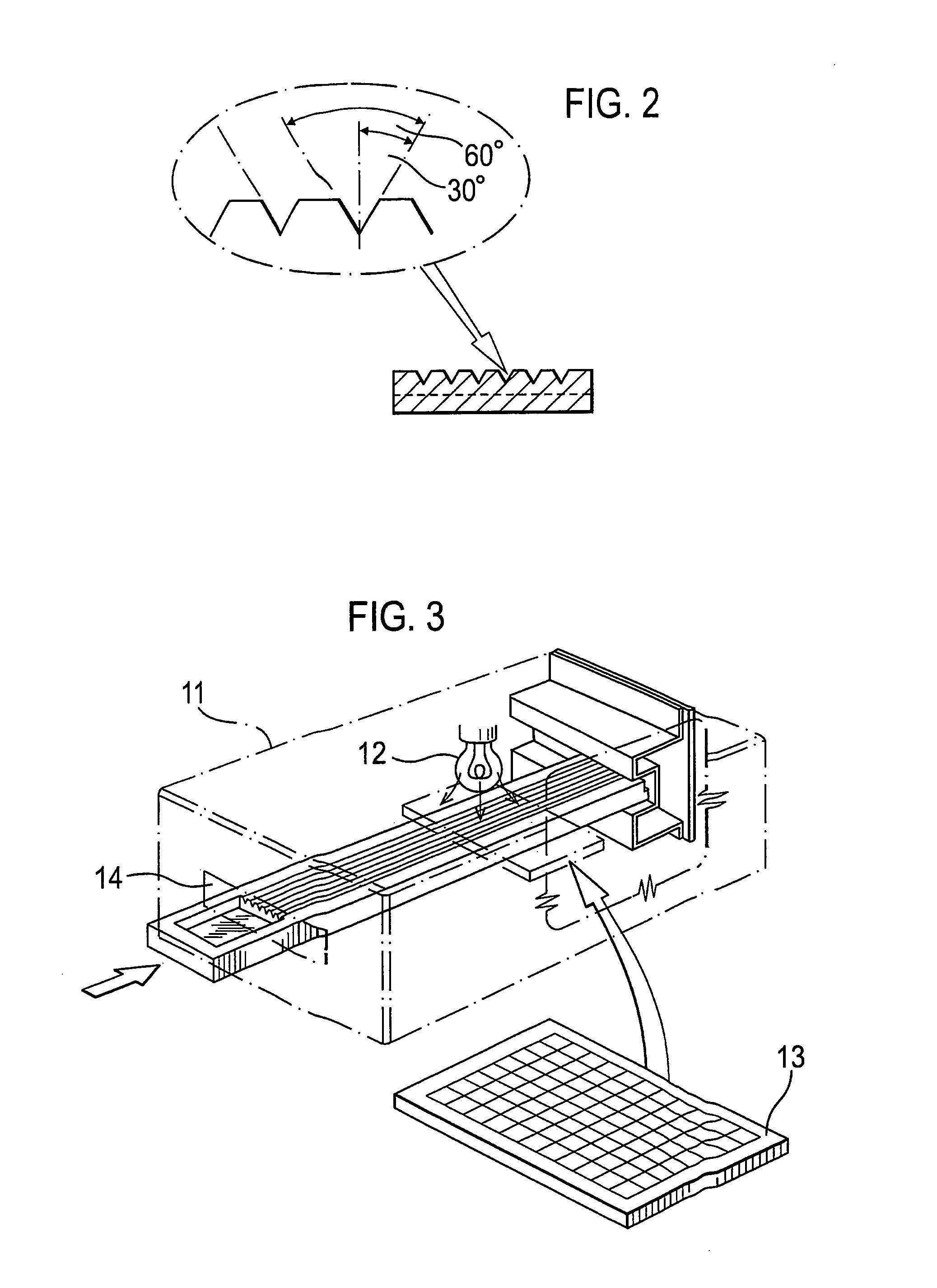
Microfluidic apparatus and methods for performing blood typing and crossmatching
ActiveUS20100112723A1Small dimensionIncrease ratingsBiocideBioreactor/fermenter combinationsAntigenGroup A - blood
Microfluidic cartridges for agglutination reactions are provided. The cartridges include a microfluidic reaction channel with at least two intake channels, one for an antigen-containing fluid and the other for an antibody-containing fluid, conjoined to a reaction channel modified by incorporation of a downstream flow control channel. At low Reynolds Number, the two input streams layer one on top of the other in the reaction channel and form a flowing, unmixed horizontally-stratified laminar fluid diffusion (HLFD) interface for an extended duration of reaction. Surprisingly, the design, surface properties, and flow regime of microfluidic circuits of the present invention potentiate detection of antibody mediated agglutination at the stratified interface. Antigen:antibody reactions involving agglutination potentiated by these devices are useful in blood typing, in crossmatching for blood transfusion, and in immunodiagnostic agglutination assays, for example.
Owner:PERKINELMER HEALTH SCIENCES INC
Serum amyloid protein A detection method and reagent
InactiveCN106568978AMeet clinical needsQuick checkBiological material analysisBiological testingAntigenCross-link
The invention discloses a serum amyloid protein A detection method. The serum amyloid protein A detection method comprises following steps: serum SAA antigen of a tested body is mixed with a latex cross-linked substance coated with SAA antibody, in vitro agglutination reaction is carried out, and quantitative determination of the content of SAA in serum is carried out. The invention also discloses a serum amyloid protein A detection reagent. The serum amyloid protein A detection method and the reagent possesses following advantages: linearity range is wide, detection is rapid, detection sensitivity is high, accuracy is high, cost is low, and the reagent is stable.
Owner:PUREBIO LAB NINGBO
Immunoassay and immunoassay apparatus
InactiveUS20030082662A1Highly accurate immunoassaySimple correctionBioreactor/fermenter combinationsBiological substance pretreatmentsValue setAgglutination
An immunoassay comprises the steps of: (a) mixing a whole blood sample with sensitized insoluble carrier particles smaller than erythrocytes to cause an immune agglutination reaction; (b) introducing the resulting immune agglutination reaction mixture including agglutinated particles and unagglutinated particles to a flow cell, irradiating the particles passing through the flow cell with laser light, and detecting scattered lights generated thereby; (c) setting a threshold value for distinguishing unagglutinated particles from agglutinated particles and a threshold value for distinguishing the agglutinated particles from blood cells with regard to intensity of the scattered light; and (d) distinguishing and counting the unagglutinated particles, the agglutinated particles and the blood cells from the scattered lights detected in the step (b), in reference to the threshold values set in the step (c).
Owner:SYSMEX CORP
Microfluidic biochip for blood typing based on agglutination reaction
InactiveUS7718420B2Efficient mixingSmall amountBioreactor/fermenter combinationsBiological substance pretreatmentsGroup A - bloodPoint of care
The present invention is directed to a microfluidic biochip based on an agglutination reaction that is frequently used in qualitative typing in the diagnostic medicine field by realizing a specimen inlet, a reagent inlet, a split microchannel, transfer microchannels, a chaos micromixer, a reaction microchamber, a microfilter, a passive microvalve, and an outlet on a plastic microchip. Particularly, the biochip of the present invention is characterized in that portability thereof is superior and a small amount (about 1 μl) of each of a specimen and a reagent is used. In addition, the biochip of the present invention can be cheaply made through conventional photolithography, electroplating, injection molding, and bonding. Therefore, by utilizing the microfluidic biochip for blood typing according to the present invention, a point-of-care diagnosis for performing blood typing based on an agglutination reaction at any place becomes possible.
Owner:DONG SUNG KIM
Microfluidic devices and methods for performing serum separation and blood cross-matching
ActiveUS20160109467A1Bioreactor/fermenter combinationsBiological substance pretreatmentsSerum samplesBlood capillary
Microfluidic cartridges or devices for serum separation and blood cross-match analysis are provided. The devices may include a serum separation subcircuit alone or in combination with a solute mixing subcircuit. The serum separation subcircuit promotes on-cartridge clotting of a blood sample and manipulates the flow of the separated serum sample for subsequent cross-match analysis with a second blood sample, for example. The solute mixing subcircuit includes at least two intake channels, one for a whole blood sample from, for example, a blood donor and the other for the separated serum sample from, for example, a transfusion recipient. The solute mixing subcircuit further includes a serpentine mixing channel conjoined to a downstream channel. Under vacuum generated by a conjoined finger pump, the two input streams fill the serpentine mixing and downstream channels due to capillary action, enabling visualization of an agglutination reaction.
Owner:PERKINELMER HEALTH SCIENCES INC
Immunoassay, reagent for immunoassay, and production method of the same
InactiveUS7399644B2Effectively fixedEfficient processingPeptide/protein ingredientsMicrobiological testing/measurementAntigenAgglutination
An assay method for determining presence of a target antibody or antigen in a specimen qualitatively or quantitatively by mixing the specimen with an antigen or antibody immunologically reactive with the target antibody or antigen, and assaying the level of the immunological agglutination reaction, wherein the reactive antigen or antibody is effectively immobilized on the carrier via an amino acid sequence capable of binding to the carrier.
Owner:CANON KK
Immunological latex turbidimetry method and reagent therefor
InactiveUS20020182752A1Easy and rapid and high-sensitivity analysisImprovement of such an avoidanceBiological material analysisBiological testingAntigenProteinase activity
An immunological latex turbidimetry method for analyzing an antigen or antibody in a sample, comprising steps of: (1) bringing a sample which may contain the antigen or antibody to be analyzed into contact with a protease-treated albumin; and (2) bringing a mixture obtained in the above step (1) into contact with latex particles carrying an antibody or antigen specifically reacting with the antigen or antibody to be assayed, and analyzing a turbidity caused by a latex agglutination reaction, is disclosed. Further, an immunological latex turbidimetry reagent comprising (1) a first component containing a protease-treated albumin, and (2) a second component containing latex particles carrying an antibody or antigen specifically reacting with an antigen or antibody to be assayed is also disclosed.
Owner:MITSUBISHI CHEM MEDIENCE
Enterovirus 71 type latex agglutination detection kit, preparation and application
The invention relates to an enterovirus 71 type latex agglutination detection kit, preparation and application. The kit comprises the following substances: (1) a carboxylated latex reagent sensitizing a prokaryotic expression enterovirus 71 type VP1 specific antigen; (2) a carboxylated latex reagent sensitizing three strains of monoclonal mixed antibodies aiming at enterovirus 71 type VP1; (3) enterovirus 71 type positive-negative standard serum, an inactivated virus solution and PBS; and (4) a toothpick or a plastic cement rod which contains a glass slide platform for using the sentization latex to perform aggregation reaction and is used to uniformly mix latex and to-be reacted serum. The kit can detect EV71 antigen in samples of different sources, and overcomes the disadvantages that the sensitized antigen is less in amount and unstable, sensitized protein is easy to fall off, and the like when protein sensitizes common latex. The detection method is applicable to enterovirus 71 type serum epidemiological investigation, clinic assistant diagnosis, regulation and control on EV71 virus replication, anti-virus therapy medicine screening and other scientific research fields.
Owner:绍兴市疾病预防控制中心
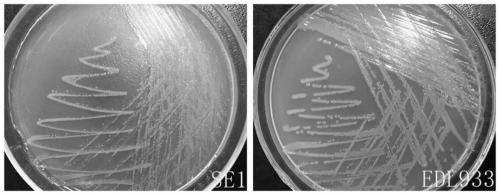
Inert carrier Escherichia coli and its potential application
ActiveCN110257276AImprove and refine specificity bottlenecksBacteriaBiological material analysisEscherichia coliAgglutination assay
The present invention relates to an inert carrier Escherichia coli and its potential application, the Escherichia coli isolated strain has the inert carrier property, and is expected to be developed into a carrier strain for an indirect agglutination test. The inert carrier Escherichia coli is preserved in the China General Microorganisms Collection and Management Center (CGMCC), and a preservation address is Beijing, China, a preservation number is CGMCC No. 17339, a preservation date is March 18, 2019, a classification name is Escherichia coli, and a strain code is SE1. The Escherichia coli isolated strain is from a healthy flock, the bacteria number at a higher working concentration does not cause any macroscopic agglutination reaction with various chicken serums of different genetic backgrounds, and does not generate non-specific agglutination reaction with chicken source serum, so that the Escherichia coli is called as an inert carrier Escherichia coli. The application provides an inert carrier strain and its potential application for developing a simple and rapid indirect agglutination assay.
Owner:YANGZHOU UNIV
Inert carrier indirect agglutination test detection system and application thereof
ActiveCN111537712ANo self-condensationSelf-condensation does not occurBiological material analysisDepsipeptidesSerum/Whole bloodAntigen
The invention discloses an inert carrier indirect agglutination test detection system and application thereof. The system comprises inert carrier bacteria S9 and a complex S9-P which is shown and expressed on the surface of the inert carrier bacteria S9 and carries a P-antibody factor. The system only carries the P-antibody factor, is single in component and specific in targeting, generates macroscopic positive particle agglutination reaction with whole blood or serum of chicken infected by pullorum disease and salmonella typhimurium under a certain concentration condition, and does not generate non-specific cross agglutination reaction with chicken-derived serum and whole blood of different backgrounds infected by non-pullorum disease and salmonella typhimurium. The S9-P is based on a glass plate agglutination reaction operation platform. The S9-P is simple and convenient to operate, sensitive and rapid in reaction, macroscopic in agglutination reaction particles and clear and easy inresult judgment, tests and result judgment are completed within two minutes, and the S9-P is suitable for targeted specific detection of pullorum disease and salmonella gallinarum infection in chicken flocks and has a good application prospect in on-site monitoring and diagnosis of the chicken flocks.
Owner:YANGZHOU UNIV
Hybridoma cell strain capable of secreting anti-human AB blood type IgM type monoclonal antibodies, monoclonal antibodies, and application
ActiveCN110903396AImmunoglobulins against blood group antigensTissue cultureBALB/cAntiendomysial antibodies
The invention provides a hybridoma cell strain capable of secreting anti-human AB blood type IgM type monoclonal antibodies. The preservation number of the hybridoma cell strain is CGMCC NO. 18535. The invention also provides the monoclonal antibody secreted by the hybridoma cell strain capable of secreting anti-human AB blood type IgM type monoclonal antibodies, and application of the monoclonalantibody. A preparation method comprises following steps: taking a human AB type erythrocyte suspension as an antigen to immunize BALB / c mice; carrying out immunization, cell fusion, screening and cloning to obtain a hybridoma cell strain 2D12 capable of stably subculturing and secreting anti-human AB blood type IgM type monoclonal antibodies; the secreted 2D12 antibody can agglutinate with humanA-type red blood cells, B-type red blood cells and AB-type red blood cells, no agglutination reaction with human O-type red blood cells is caused, the secreted 2D12 antibody is an anti-AB-type blood type monoclonal antibody, and has antibody titer of 512.
Owner:苏州国科思倍达生物技术有限公司
Fast erythrocyte removal method applicable to whole blood detection
InactiveCN104111333ASolve the problem that the whole blood test cannot be realizedDisease diagnosisMouse monoclonal antibodyAgglutination
Owner:叶森
Fast check reagent of Salmonella causing food poisoning
InactiveCN101565737AFull of nutritionEasy to prepareMicrobiological testing/measurementAgainst vector-borne diseasesSource typeFood poisoning
The invention relates to a fast check reagent of Salmonella causing food poisoning, in particular to a fast check reagent of food source type Salmonella aertrycke, Salmonella suipestifer, Salmonella enteritidis, TAB vaccine and salmonella B and a preparation method thereof. The fast check reagent has the characteristics of high speed, high accuracy, high sensitivity, and the like. Compared with a traditional method, the fast check reagent has an application value and has an important meaning for guiding clinical diagnosis and treatment and avoiding abusing antibacterial medicines. Specific Salmonella polyvalent and monovalent serum are added in enriched liquid, the check diagnosis process is shortened to 2-6 hours, the serum agglutination reaction of specific serum can be utilized at the time of enriching, and the identification accuracy is larger than 97 percent. The fast check reagent has little time consumption, high sensitivity and strong accuracy when checking pathogenic bacteria.
Owner:于维森
Polypeptide inducing apoptosis
InactiveCN1422333AGood anti-tumor effect in vivoProlong survival timePeptide/protein ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsApoptosisMonoclonal antibody
A reconstituted polypeptide characterized by inducing apoptosis in nuclear blood cells having integrin associated protein (IAP) without causing the agglutination of erythrocytes. This reconstituted polypeptide contains at least two H chain V domains and at least two L chain V domains of a monoclonal antibody which induces apoptosis in nuclear blood cells having IAP. This reconstituted polypeptide is useful as a remedy for blood diseases such as leukemia.
Owner:CHUGAI PHARMA CO LTD
Rabbit Klebsiella pneumoniae agglutinogen and application of rabbit Klebsiella pneumoniae agglutinogen
The invention relates to the technical field of organisms, in particular to a rabbit Klebsiella pneumoniae agglutination reaction experimental technique. A rabbit Klebsiella pneumoniae agglutinogen is prepared from rabbit Klebsiella pneumoniae liquid dyed by fuchsin dye liquid. An application method concretely belongs to an application method in agglutination reaction experiments: the rabbit Klebsiella pneumoniae agglutination is combined with agglutinin, and the agglutination result is judged. The rabbit Klebsiella pneumoniae agglutinogen has the advantages that expensive instruments and reagents are not needed, the cost is low, the speed is high, the operation is simple and convenient, the technical requirements on operators are low, the result is intuitional, the rabbit Klebsiella pneumoniae agglutinogen is applied to basic level site detection application, the detection antigen of a rabbit Klebsiella pneumoniae microagglutination reagent kit is dyed, agglutination particles or agglutination blocks are red, the observation is easier, and the detecting method can be used for quantificationally detecting the rabbit serum antibody valence, can also be used for qualitatively detecting the rabbit Klebsiella pneumoniae antigen and is particularly suitable for on-site rabbit Klebsiella pneumoniae diagnosis for basic level veterinary stations and warrens.
Owner:CHONGQING ACAD OF ANIMAL SCI
Generic inert carrier salmonella and potential application thereof
ActiveCN111500504AImprove and refine featuresImprove and perfect the technical bottlenecks where the sensitivity needs to be improvedBacteriaMicroorganism based processesBiotechnologyZoology
The invention discloses generic inert carrier salmonella S9H and a potential application thereof. The generic inert carrier salmonella S9H is obtained by continuously culturing inert carrier bacteriaS9 in vitro by using an LB solid and liquid culture medium for passage to the fortieth generation, under the condition of the quantity of bacteria with working concentration, the non-specific agglutination reaction with serum and whole blood of human sources, mouse sources, cattle sources, pig sources and poultry sources (including chickens, ducks, gooses, turkeys, pigeons and quails) can be avoided, the characteristics of carrying and surface expression displaying human sources, mouse sources, cattle sources, pig sources and poultry sources (including chickens, ducks, gooses, turkeys, pigeonsand quails) for different resistance factors are realized, the generic inert carrier salmonella S9H can be applied to development of an indirect agglutination test detection method for simply, conveniently and quickly detecting human and various animal antigens or infected antibodies, improves and perfects the technical bottleneck of poor specificity and sensitivity of an existing agglutination test for agglutination antigen and antibody detection, and has a wide application value and market prospect.
Owner:YANGZHOU UNIV
Sargassum thunbergii lectin and preparation method thereof
InactiveCN102690339AHigh activityPeptide preparation methodsAlgae/lichens peptidesIon exchangeGlycoprotein
The invention discloses a sargassum thunbergii lectin and a preparation method thereof, wherein the preparation method of the sargassum thunbergii lectin is characterized by selecting sargassum thunbergii as a raw material, carrying out lyophilization, pulverization, immersion, centrifugation, ammonium sulfate fractionation, DEAE-52 ion-exchange chromatography and SephadexG-200 gel filtration chromatography, thus producing the sargassum thunbergii lectin with a molecular weight of 17 kD. The sargassum thunbergii lectin of the invention can agglutinate a wide range of cells, has agglutinative functions for erythrocytes of rabbits, dogs, sheep, crucians, chickens and humans (A, B, AB), and the like, wherein minimum concentrations of agglutination reactions with rabbit and chicken erythrocytes are 4.4mg / ml and 0.55 mg / ml respectively, and shows hemagglutinin activity for rabbit erythrocytes even after heating treatment at 100 DEG C for 30 min (the activity decreases by 75%). A sugar inhibition experiment shows that the sargassum thunbergii lectin is only inhibited by glycoproteins as bovine thyroglobulin and gamma-globulin, and the minimum inhibition concentrations are 1.25 mg.mL<-1> and 2.5 mg.mL<-1> respectively.
Owner:DALIAN OCEAN UNIV +1
Multimer type discrimination and detection method for multimer-forming polypeptide
InactiveUS20140370620A1Convenient and prompt detecting procedureEasy accessBiological material analysisMaterial analysis by optical meansImaging analysisBiochemistry
The present invention relates to a method for selectively detecting a multimer type multimer-forming polypeptide in a biological sample, the method comprising: (a) bringing the biological sample into contact with an agglutination reaction inducing agent to induce the formation of an aggregate in an analysis target, the agglutination reaction inducing agent being a particle in which a specific antibody is surface-bonded with the multimer-forming polypeptide; (b) obtaining an image with respect to the aggregate of step (a); and (c) analyzing a size or a shape of the aggregate by using the image. Step (a), step (b), or steps (a) and (b) are performed on a microchip having a microchannel. The image analysis is performed using a coefficient according to the size of the aggregate in a predetermined volume provided by the microchannel. In the case where the multimer type of multimer-forming polypeptide is present in the biological sample, the size of the aggregate is larger than the size of an aggregate of a monomer type control group. According to the present invention, unlike in a detection method using chemiluminescence immunoanalysis of the related art, an image with respect to an agglutination reaction target is obtained and then a size or a shape of an aggregate is analyzed so as to determine whether or not an analysis target is present in a biological sample and to determine the quantity of the analysis target. Also, it is possible to detect a multimer type multimer-forming polypeptide by just analyzing an image acquired from the sample so that the detection process is made more convenient and quick.
Owner:NANOENTEK
Application of reaction of microbial bacterial liquid and serum vesicles in detection of microbial infection
PendingCN110938670AEasy to detectConvenient guidanceMicrobiological testing/measurementAgainst vector-borne diseasesBiotechnologyMicroorganism
The invention discloses an application of a reaction of a microbial bacterial liquid and serum vesicles in detection of microbial infection. The inventors unexpectedly discover in the study that the patient serum extract and the bacterial liquid can be subjected to an agglutination reaction, by detecting the number and type of agglutination blocks, the type of the microbial infection can be determined conveniently, quickly and accurately. Through some embodiments of the invention, the microbial infection can be conveniently and quickly detected, the detection results are reliable, and clinicalmedication can be better guided.
Owner:NANFANG HOSPITAL OF SOUTHERN MEDICAL UNIV
Microfluidic chip, method, kit and system for detecting protein antigen
The invention relates to a micro-fluidic chip, a method, a kit and a system for detecting a protein antigen. The method for detecting the protein antigen comprises the following steps: mixing a to-be-detected sample with a reaction reagent, preparing the mixture of the to-be-detected sample and the reaction reagent into a plurality of liquid drops by adopting a microfluidic technology, wherein the reaction reagent comprises a marker labelled antibody and an electrolyte providing agent, the marker labelled antibody can be specifically combined with the protein antigen in a to-be-detected sample to form an antigen-antibody compound, a plurality of antigen-antibody compounds are wrapped in the liquid drop, and the plurality of antigen-antibody compounds can be subjected to agglutination reaction under the action of the electrolyte providing agent; and detecting the intensity of a signal corresponding to the agglutination reaction in each liquid drop formed by the to-be-detected sample and the reaction reagent after the agglutination reaction, and analyzing to determine the amount of the protein antigen in the to-be-detected sample. The detection method is simple and convenient, does not need multi-step sample adding and cleaning, and is small in reagent dosage and good in reproducibility.
Owner:SHENZHEN YHLO BIOTECH
Detection method for agglutination test of rabies virus neutralizing antibody cells
InactiveCN102539757AAccurate Protection CapabilityAvoid false positivesMaterial analysisAntigenViral glycoprotein
The invention provides a detection method for an agglutination test of rabies virus neutralizing antibody cells. In the detection method, cells for expressing rabies virus glycoprotein are taken as antigens and carrier particles; and through agglutination reaction with human, dog and cat serum antibodies, whether the rabies virus neutralizing antibodies in the serum reach an immune protection level can be judged. By the detection method, the problems that the conventional detection method for the rabies virus neutralizing antibodies is long in detection time, complicated in operation, high in detection cost and the like can be solved.
Owner:MILITARY VETERINARY RES INST PLA MILITARY MEDICAL ACAD OF SCI
Agglutination reaction system
InactiveUS8685715B2Complex and expensive to manufactureProvide costBioreactor/fermenter combinationsBiological substance pretreatmentsAnalyteAgglutination
The present invention provides an agglutination based assay system for determining the presence and / or amount of analyte in a sample comprising a test device having one or more capillary pathways comprising detection regions adapted for non-visual detection of a sample which is releasably engageable with a reader which comprises detection means for detecting the sample at the detection regions in each of said capillary pathways and electronic means for indicating the presence and / or amount of analyte.
Owner:PLATFORM DIAGNOSTICS LTD
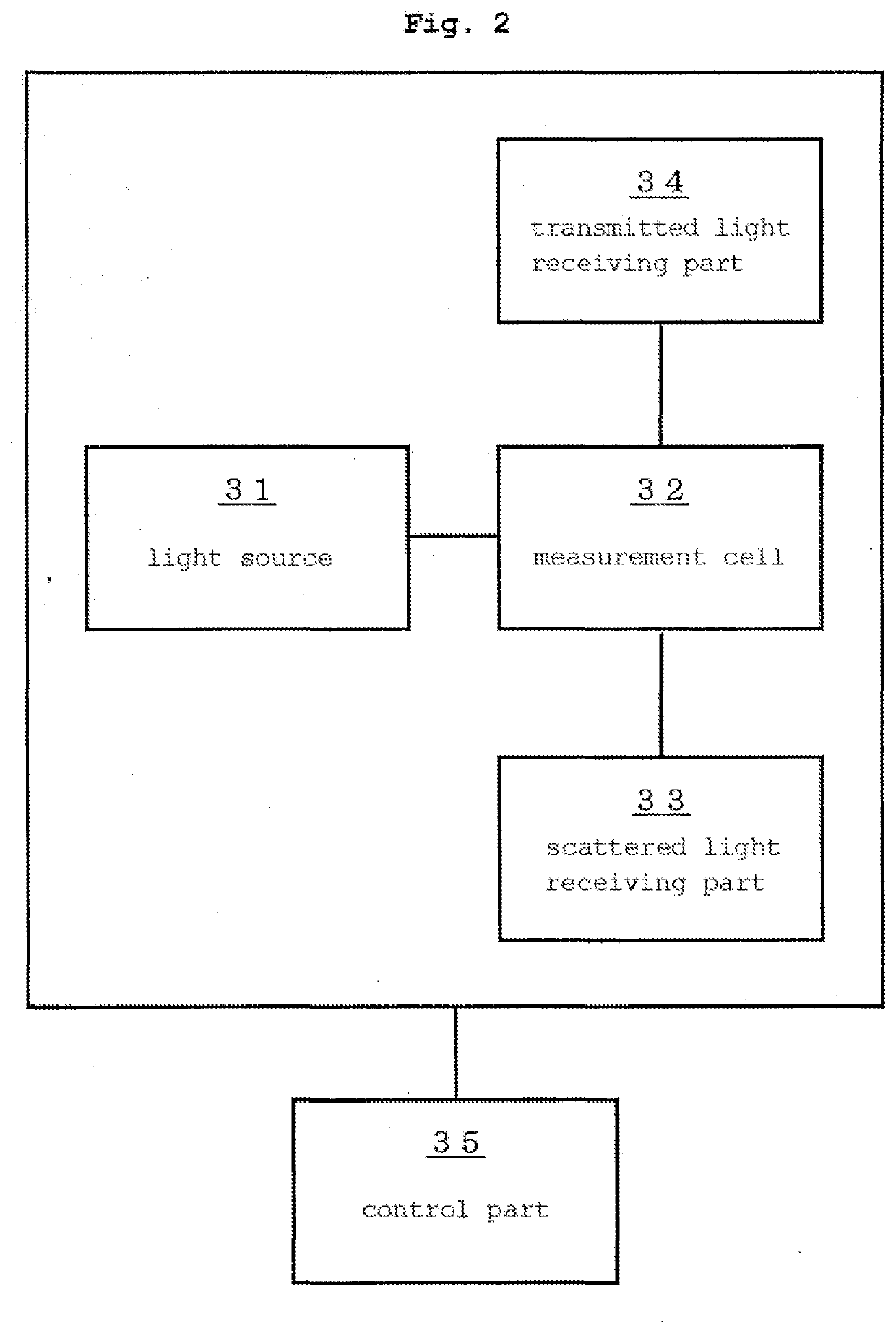
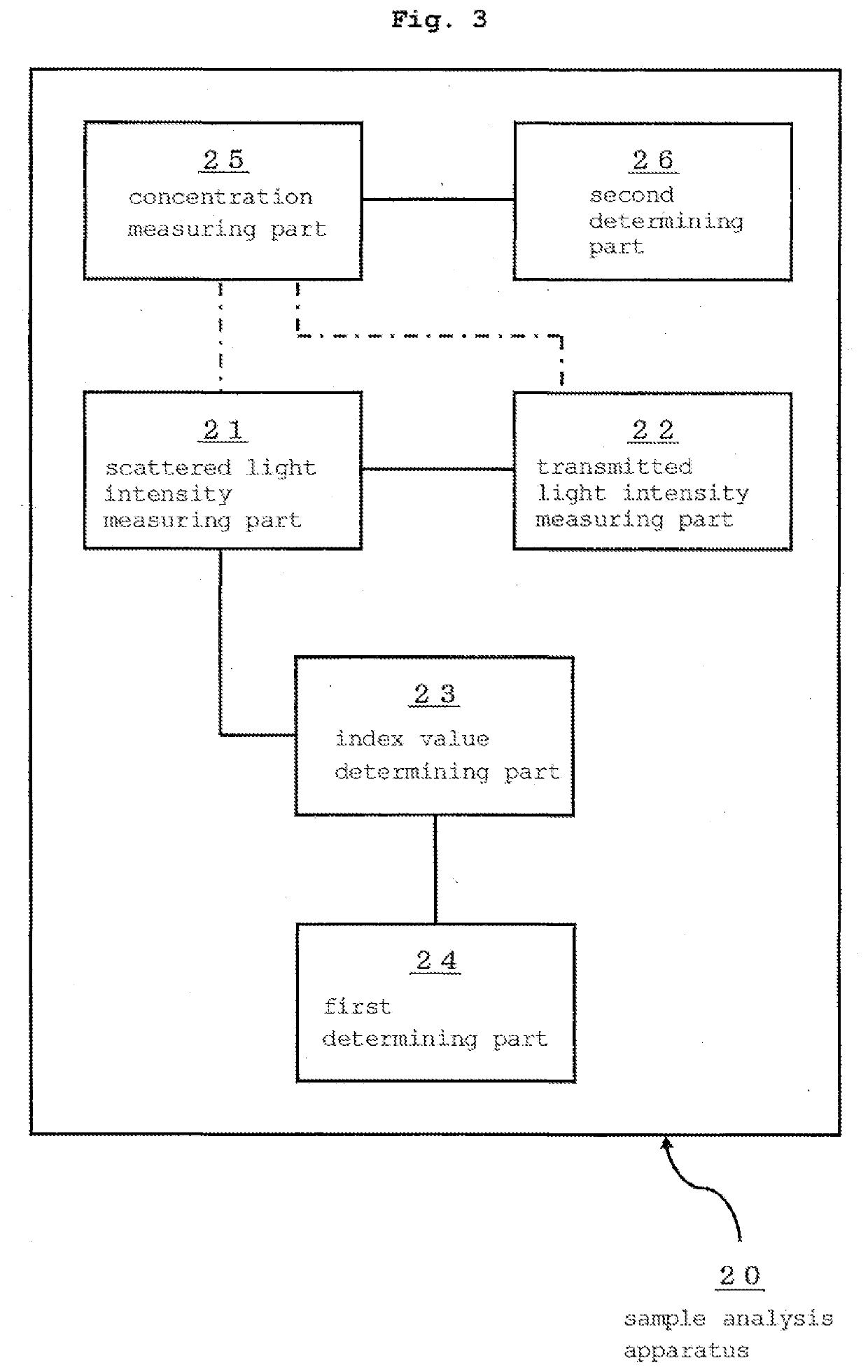
Method for assessing appropriateness of test substance concentration in concentration measurement using immunoagglutination reaction, and sample analysis device having processing unit for same
InactiveUS20200080935A1Material analysis by observing effect on chemical indicatorScattering properties measurementsAnalytical chemistryConglutination reaction
A method and a sample analysis apparatus having a processing part for practicing the method, the method including a step of, with respect to an immunoagglutination reaction that occurred in the reaction solution between the test substance and a binding partner thereof, determining an index value relating to a difference between a changing speed of a scattered light intensity in a first period of the reaction and a changing speed of a scattered light intensity in a second period of the reaction, based on a scattered light intensity of the reaction solution in the first period and a scattered light intensity of the reaction solution in the second period, and a step of determining whether the concentration of the test substance is within a proper range by comparing the index value and a predetermined threshold value.
Owner:HORIBA LTD
Generic inert carrier escherichia coli and potential application thereof
ActiveCN111560341AImprove and refine featuresImprove and perfect the technical bottlenecks where the sensitivity needs to be improvedBacteriaBiological material analysisBiotechnologyEscherichia coli
The invention discloses generic inert carrier salmonella S9H and a potential application thereof. The generic inert carrier salmonella S9H is obtained by continuously culturing inert carrier bacteriaS9 in vitro by using an LB solid and liquid culture medium for passage to the fortieth generation, under the condition of the quantity of bacteria with working concentration, the non-specific agglutination reaction with serum and whole blood of human sources, mouse sources, cattle sources, pig sources and poultry sources (including chickens, ducks, gooses, turkeys, pigeons and quails) can be avoided; due to the characteristics of carrying and surface expression displaying human sources, mouse sources, cattle sources, pig sources and poultry sources (including chickens, ducks, gooses, turkeys,pigeons and quails) for different resistance factors are realized, the generic inert carrier salmonella S9H can be applied to development of an indirect agglutination test detection method for simply,conveniently and quickly detecting human and various animal antigens or infected antibodies, improves and perfects the technical bottleneck of poor specificity and sensitivity of an existing agglutination test for agglutination antigen and antibody detection. The product has wide application value and market prospect.
Owner:YANGZHOU UNIV
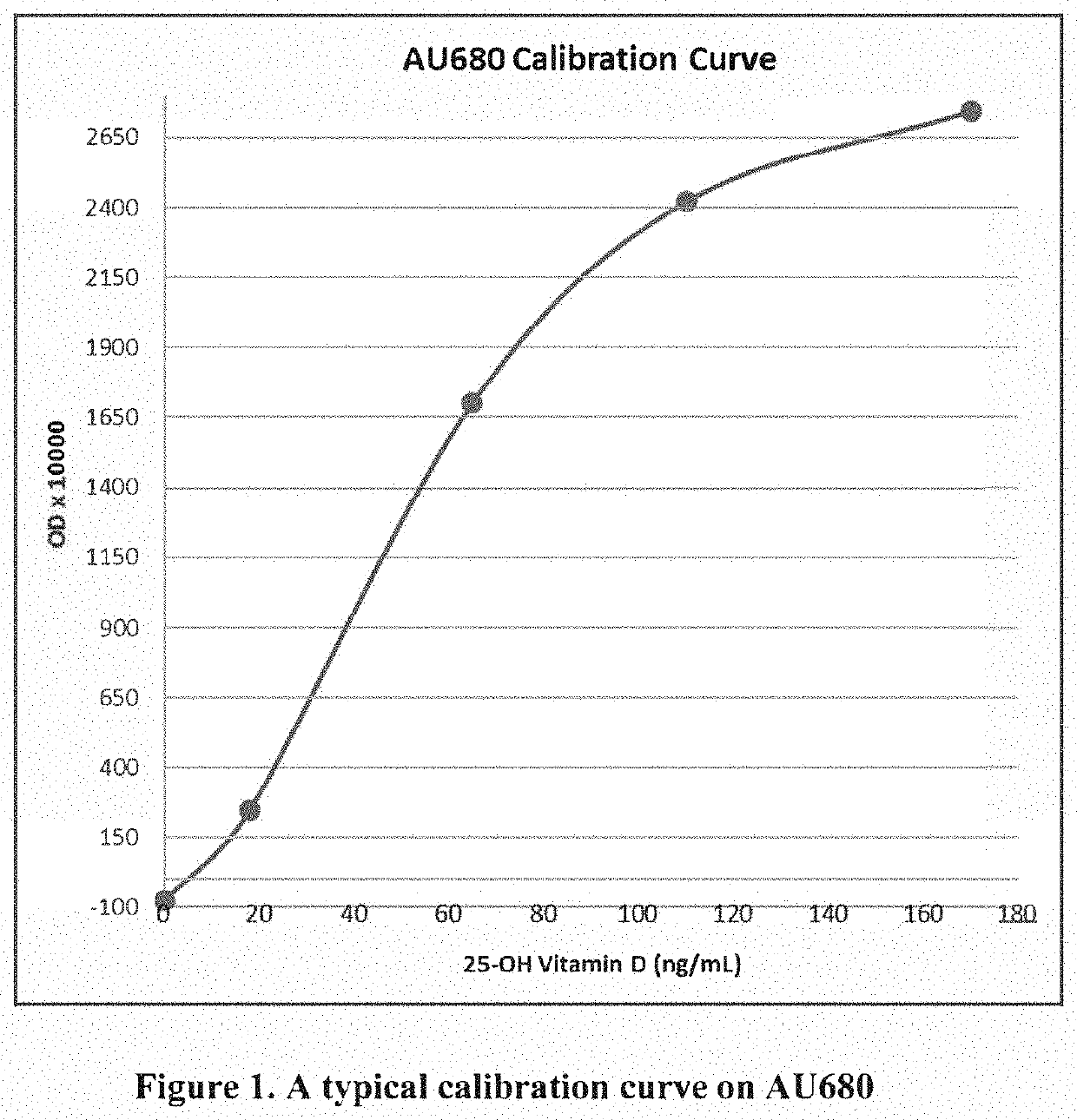
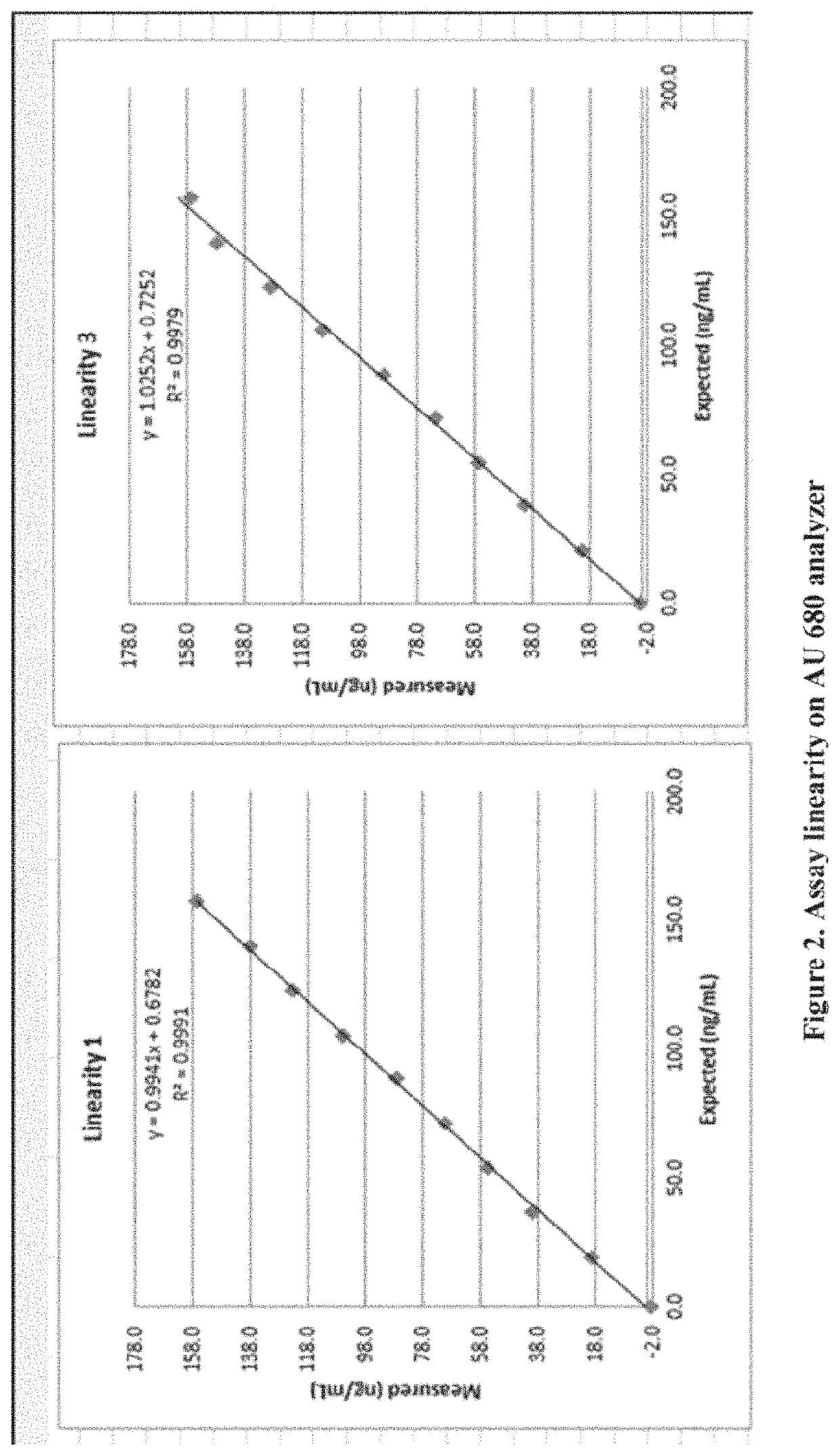
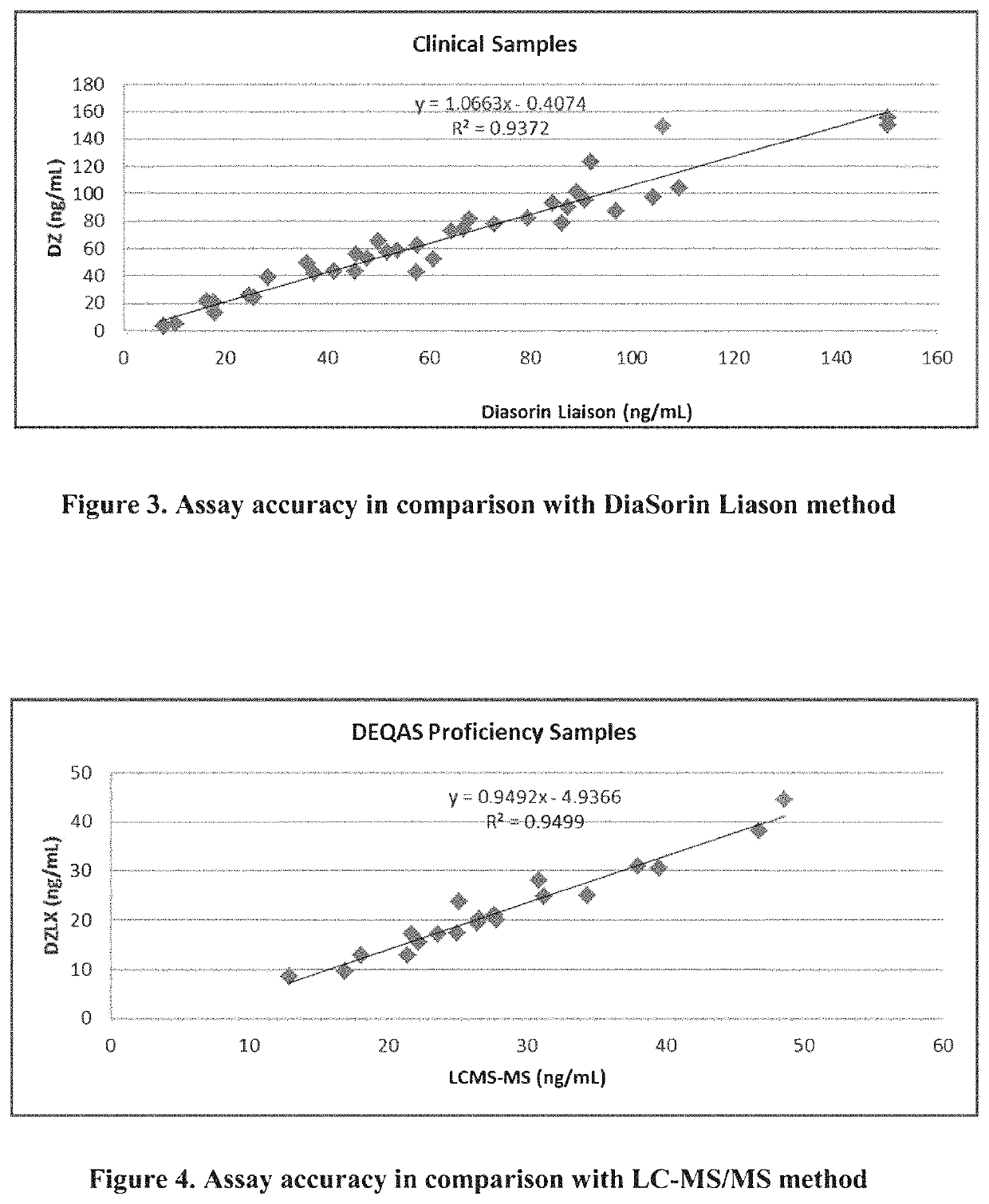
Methods and kits for assaying a vitamin D moiety
ActiveUS11435367B2Organic active ingredientsBiological testingAntiendomysial antibodiesOrganic chemistry
The present invention provides methods and compositions, e.g., kits, for assaying a vitamin D moiety in a sample, comprising or using, inter alia, a buffer that is capable of dissociating a vitamin D moiety from its binding protein and / or a buffer of acidic pH, and at least two antibodies, e.g., at least two monoclonal antibodies, that are separately conjugated to particles, e.g., latex particles, wherein at least one of said antibodies (or the first antibody) has a specific binding affinity towards the vitamin D moiety, and at least another said antibody (or the second antibody) has a specific binding affinity towards the complex formed between the first antibody and the vitamin D moiety, if present in said sample. In some embodiments, the optical change due to the agglutination reaction between the antibodies and the vitamin D moiety is measured for determination of the amount of vitamin D content in the samples. Kits and reaction mixtures for assaying a vitamin D moiety in a sample are also provided.
Owner:DIAZYME LAB INC
Fast check reagent of colon bacillus 0157 causing food poisoning
InactiveCN101566623AFull of nutritionEasy to prepareMicrobiological testing/measurementBiological testingBiotechnologyFormulary
The invention relates to a fast check reagent of colon bacillus 0157 causing food poisoning, in particular to a formula of a fast check reagent of colon bacillus 0157 causing enterorrhagia caused by food poisoning and a preparation method thereof. The invention breaks through a traditional sectional check means and adds specific colon bacillus 0157 polyvalent and monovalent serum in culture enriched liquid, the check diagnosis process is shortened to 2-6 hours, the serum agglutination reaction of specific serum can be utilized at the time of enriching, and the identification accuracy is larger than 97 percent. The fast check reagent has the characteristics of high speed, high accuracy, high sensitivity, and the like. Compared with a traditional method, the fast check reagent has an application value, has an important meaning for guiding clinical diagnosis and treatment and avoiding abusing antibacterial medicines and has an obvious advantage in the application of rapidly identifying and diagnosing colon bacillus 0157 which is a food poisoning pathogenic bacterium.
Owner:于维森
Preparation method of pork blood tofu
PendingCN110637989AEnsure the safety of blood sourcesStable tasteMeat/fish preservation by heatingBiotechnologyEngineering
The invention provides a preparation method of pork blood tofu, and belongs to the technical field of food processing. In order to ensure the safety of blood sources, animal quarantine is performed onmixed pork blood, and continuous processing can be performed after the inspection is passed. After a blood vessel ruptures, a platelet aggregation reaction occurs, and therefore, blood is coagulated.In order to prevent the agglutination reaction, sodium citrate is used in an early stage to prevent blood coagulation, and then a coagulant is added in a later stage to completely coagulate a product, so that a stable product taste is ensured. High-pressure all water sterilization is performed after collected filtered liquid and coagulant are mixed so that the pork blood tofu can be stored at a room temperature, and product safety is guaranteed.
Owner:正大食品(襄阳)有限公司
A kind of pool-checking red blood cell blood group irregular antibody detection kit based on solid-phase agglutination technology and preparation method
ActiveCN110174519BImprove coating efficiencyReduce testing costsBiological testingAgainst vector-borne diseasesLow ionic strengthFreeze-drying
The present invention relates to a detection kit based on solid-phase agglutination technology and a preparation method for the detection kit of erythrocyte irregular blood group antibodies, belonging to the technical field of kit preparation. (2) Low ionic strength solution; (3) Indicator system; (4) Negative control serum; (5) Positive control serum; the preparation method includes: (1) Preparation of pool-detection solid phase agglutination reaction microplate; (2) ) Preparation of low ionic strength solution; (3) Preparation of indicator system; (4) Preparation of negative control serum; (5) Preparation of positive control serum; The freeze-drying preservation technology and the high-sensitivity two-step indicator system can effectively solve the problems of low sensitivity, difficult to preserve red blood cells, and easy to miss detection in the current clinical irregular antibody detection methods, and provide a cost-effective detection for clinical first-line detection work. Reagent test kit.
Owner:广州血液中心
A method and device for judging the detection result of Treponema pallidum antibody
ActiveCN107064503BAccurate judgmentImplement automatic detectionMaterial analysisImaging processingBlood plasma
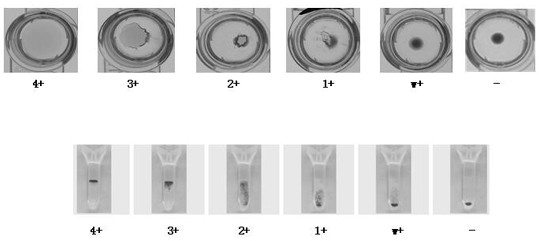
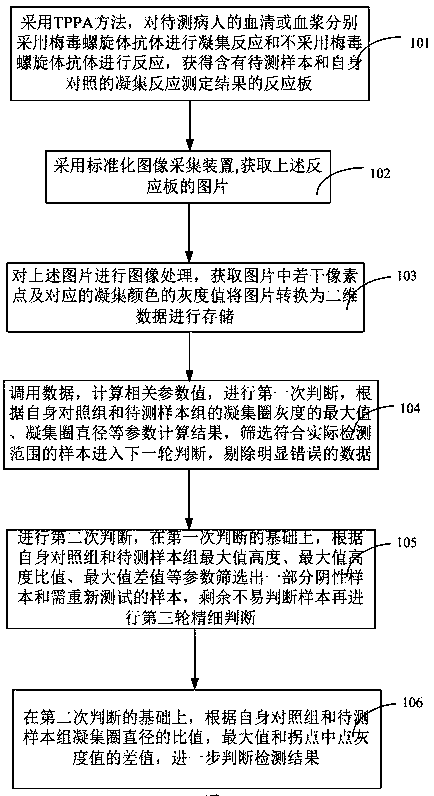
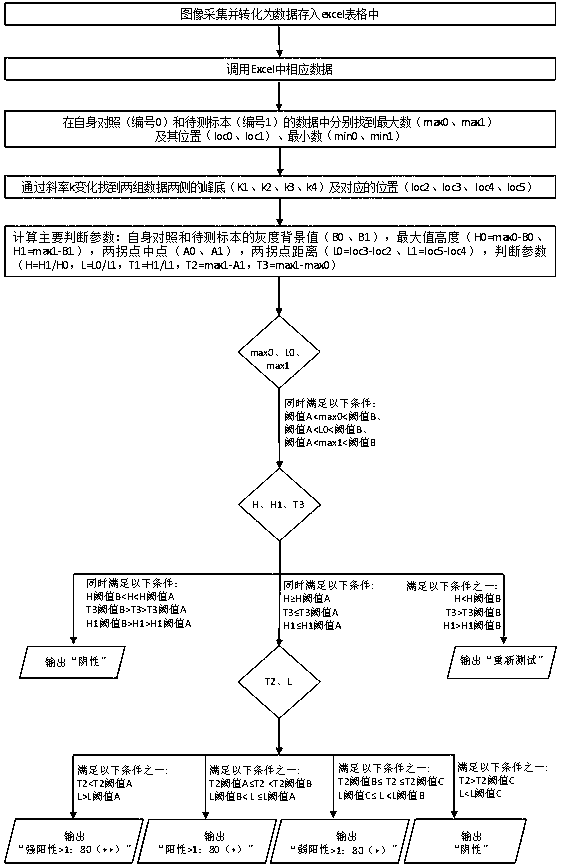
The invention discloses a method and a device for judging the detection result of a treponema pallidum antibody. The method comprises the following steps: performing agglutination reaction on serum or plasma of a to-be-detected patient by the treponema pallidum antibody and performing reaction on the serum or plasma of the to-be-detected patient without the treponema pallidum antibody separately by a treponema pallidum gelatin agglutination test method to acquire respective sample reaction plates; obtaining pictures of a to-be-detected sample and the control sample reaction plates; performing imaging processing on the pictures to acquire a plurality of pixel points and corresponding agglutination color pixel values from the pictures, and converting the pictures into two-dimensional data for storage; comparing parameters, such as shape, peak value, ratio of peak height to peak width, peak valley, difference value between peak height and peak valley and agglutination ring size, of agglutination color gray value curves of a to-be-detected group and a control group with a standard threshold value to judge the detection result. According to the method and the device for judging the detection result of the treponema pallidum antibody, the detection result of the treponema pallidum antibody can be judged more accurately and the detection efficiency is improved.
Owner:上海兰卫医学检验所股份有限公司
Sargassum thunbergii lectin and preparation method thereof
InactiveCN102690339BHigh activityPeptide preparation methodsAlgae/lichens peptidesAmmonium sulfate fractionationCentrifugation
The invention discloses a sargassum thunbergii lectin and a preparation method thereof, wherein the preparation method of the sargassum thunbergii lectin is characterized by selecting sargassum thunbergii as a raw material, carrying out lyophilization, pulverization, immersion, centrifugation, ammonium sulfate fractionation, DEAE-52 ion-exchange chromatography and SephadexG-200 gel filtration chromatography, thus producing the sargassum thunbergii lectin with a molecular weight of 17 kD. The sargassum thunbergii lectin of the invention can agglutinate a wide range of cells, has agglutinative functions for erythrocytes of rabbits, dogs, sheep, crucians, chickens and humans (A, B, AB), and the like, wherein minimum concentrations of agglutination reactions with rabbit and chicken erythrocytes are 4.4mg / ml and 0.55 mg / ml respectively, and shows hemagglutinin activity for rabbit erythrocytes even after heating treatment at 100 DEG C for 30 min (the activity decreases by 75%). A sugar inhibition experiment shows that the sargassum thunbergii lectin is only inhibited by glycoproteins as bovine thyroglobulin and gamma-globulin, and the minimum inhibition concentrations are 1.25 mg.mL<-1> and 2.5 mg.mL<-1> respectively.
Owner:DALIAN OCEAN UNIV +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com