Generic inert carrier salmonella and potential application thereof
An inert carrier, the technology of Salmonella, applied in the field of biomedical technology detection, can solve the problems of affecting and affecting the effect of epidemic disease purification, epidemic disease purification, interference detection and diagnosis results, etc., to achieve perfect specificity, improve specificity, huge The effect of application value and market prospect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1 The acquisition and verification of the pan-type inert carrier bacteria S9H
[0042] The inert carrier Salmonella S9 (preservation number is CGMCCNo.17340) was inoculated in the LB liquid medium, shaken at 37°C for 12 hours, then sucked 30 μL of the bacterial liquid into the LB solid medium for streak culture, and cultured at 37°C for 16-18 hours to obtain the first For the second-generation colony, pick the second-generation single colony and inoculate it in LB liquid medium. According to the same conditions as above, use LB liquid and solid medium as a cycle to cultivate alternately. Starting from the 40th generation, the single colony obtained is pantropic. Type inert carrier bacteria S9H, in fact, continues to be passed down forty-first to sixty generations, and any generation of them also has the above-mentioned characteristics of pan-type inert carrier bacteria S9H.
[0043] Use the primers of the fimW gene of Salmonella species reported by the applicant...
Embodiment 2
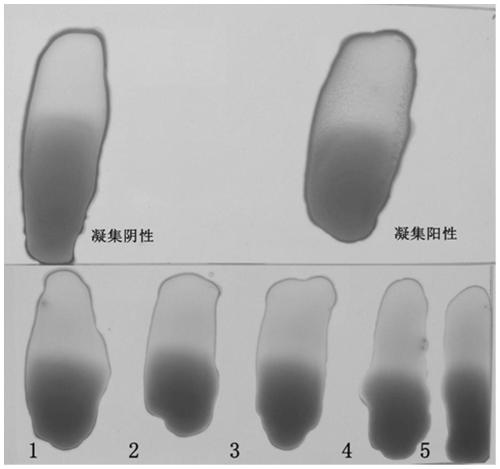
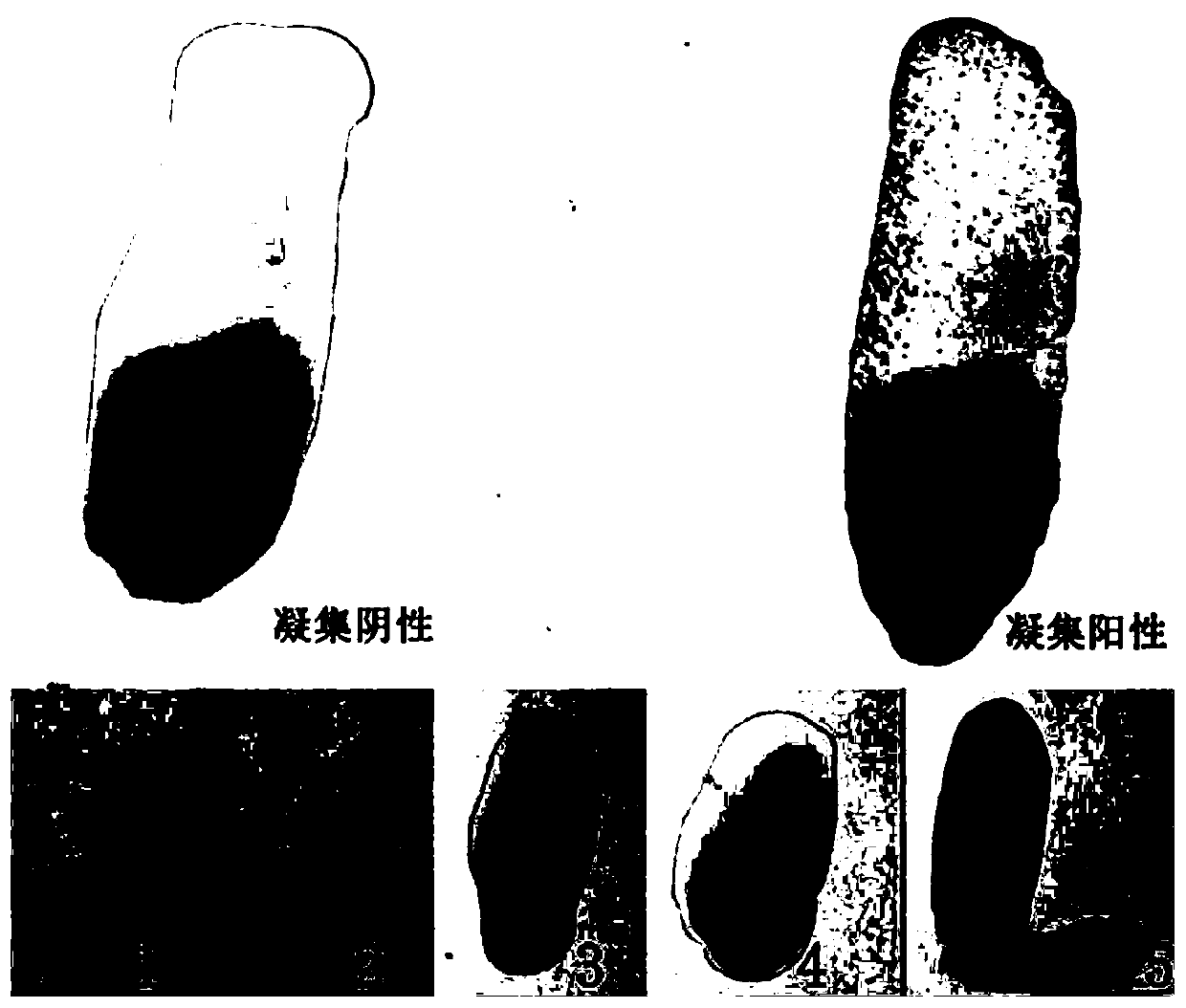
[0052]Example 2 Carrier bacterium Salmonella S9H and human and animal source serum and whole blood of different backgrounds without non-specific agglutination
[0053] According to the method of Example 1, the carrier bacterium S9 was alternately subcultured with LB agar and LB liquid medium for 40 generations to obtain the pan-type carrier bacterium Salmonella S9H, and the bacterial liquid was centrifuged at 4°C 4000rpm for 10min, and the supernatant was discarded. Resuspended in saline, centrifuged and washed three times, and then resuspended to a concentration gradient of different concentrations of bacteria. Before the test, the bacterial liquid was mixed with a vortex instrument, and the agglutination test was performed with sterile saline and SPF chicken serum to ensure that the test bacterial liquid had no self-agglutination and no non-specific agglutination. In the ultra-clean bench (20 ° C ~ 25 ° C), take several pieces of ordinary glass plates with clean surfaces, an...
Embodiment 3
[0060] Example 3 Test and verification of the surface expression of the carrier bacterium Salmonella S9H and the ability to carry the avian-derived Salmonella antigenic factor P
[0061] (1) Amplification of gene p encoding antigenic factor P expressed by avian Salmonella
[0062] According to the whole genome sequence of Salmonella pullorum ATCC 9120 strain in NCBI GenBank (NCBI accession number: CP012347.1), the whole genome sequence of Salmonella pullorum S44987_1 strain (NCBI accession number: LK931482.1), the whole genome sequence of Salmonella pullorum S06004 strain ( NCBI accession number: CP006575.1), whole genome sequence of Salmonella pullorum QJ-2D-Sal strain (NCBI accession number: CP022963.1), whole genome sequence of Salmonella gallinarum typhi 287 / 91 strain (NCBI accession number: AM933173.1) , Salmonella gallinarum typhi 9184 strain genome sequence (NCBI accession number: CP019035.1) published full-length genome sequence, search and compare the full-length frag...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com