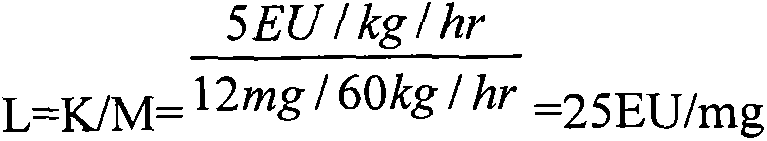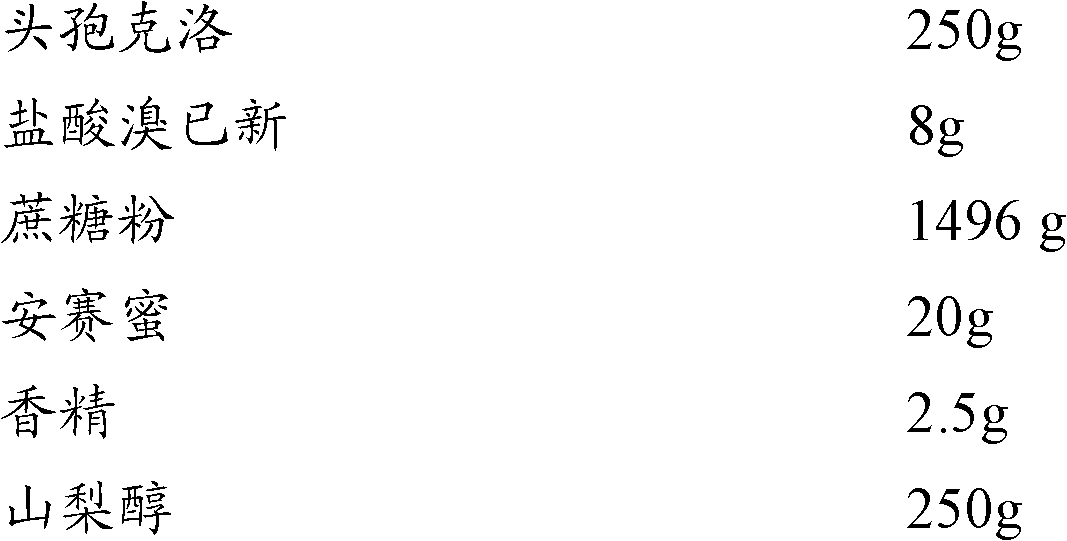Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
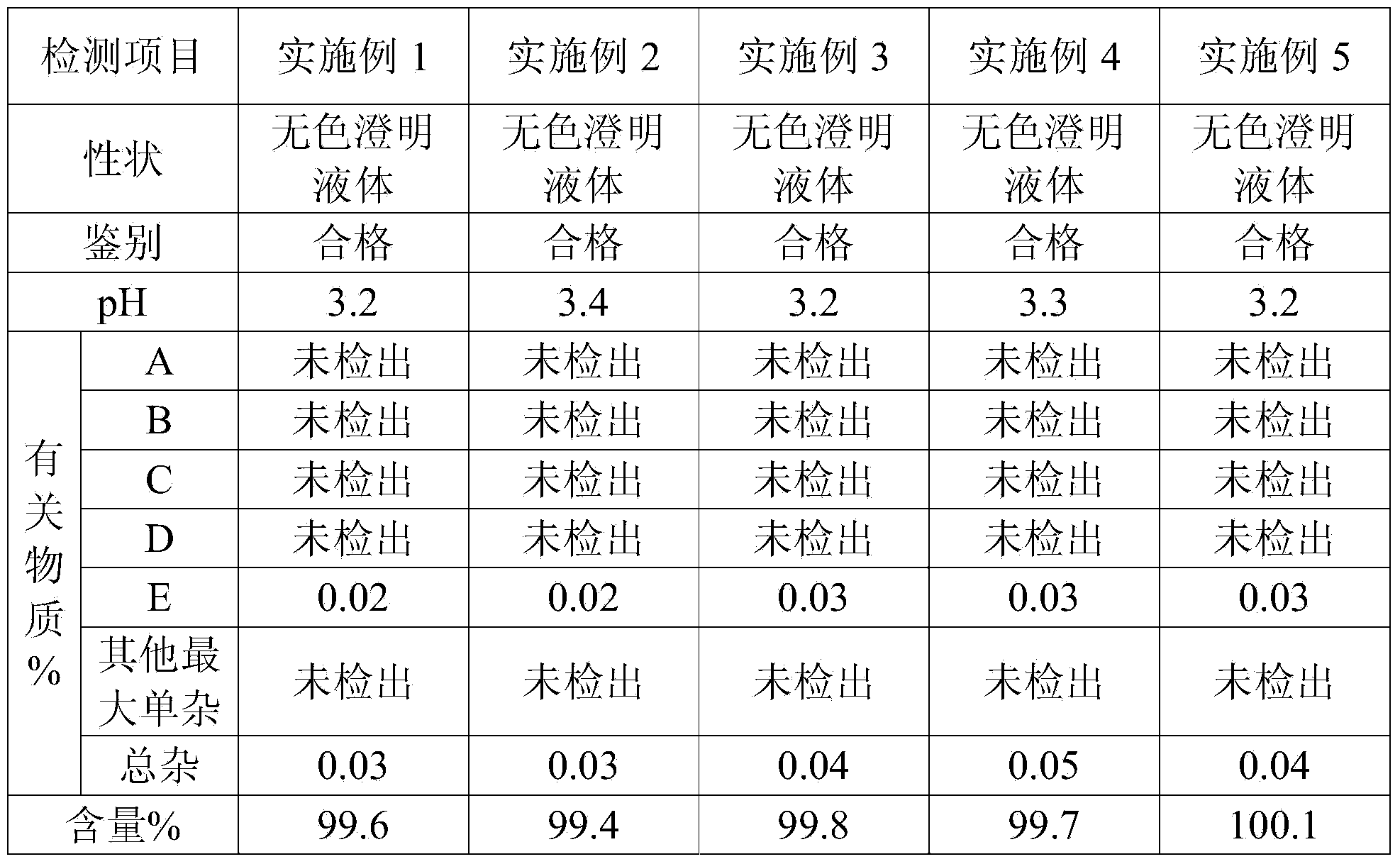
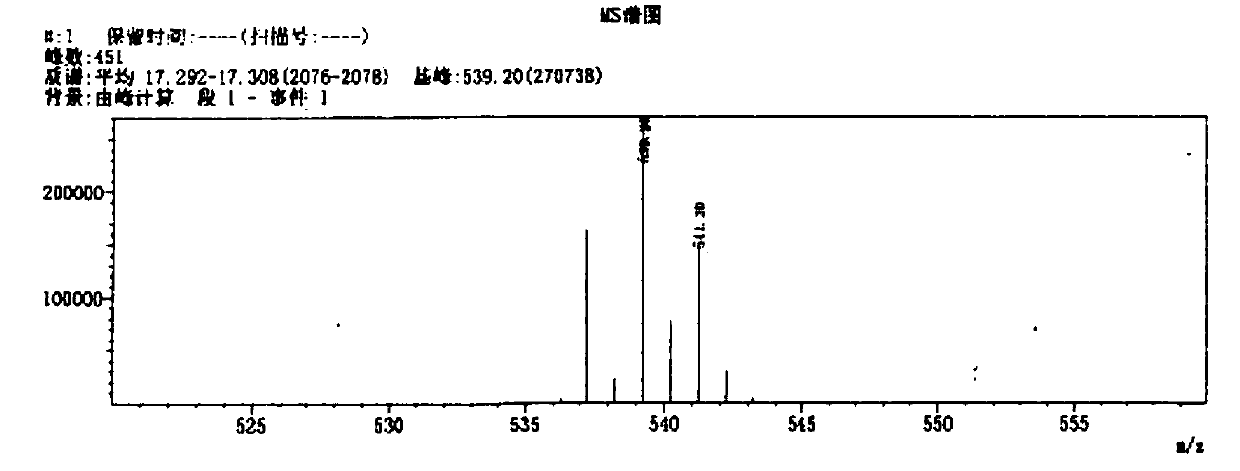
118 results about "Bromhexine hydrochloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
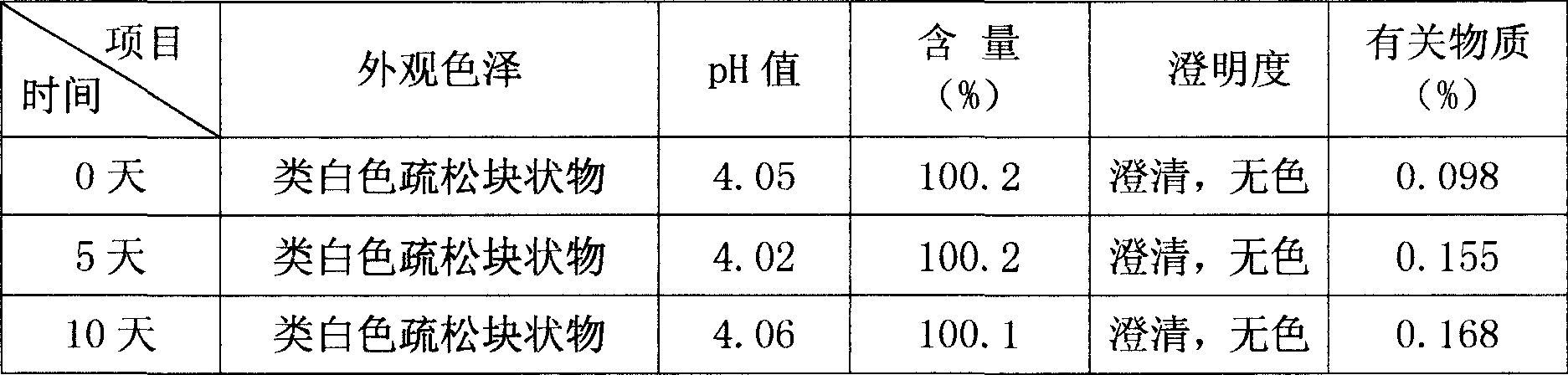
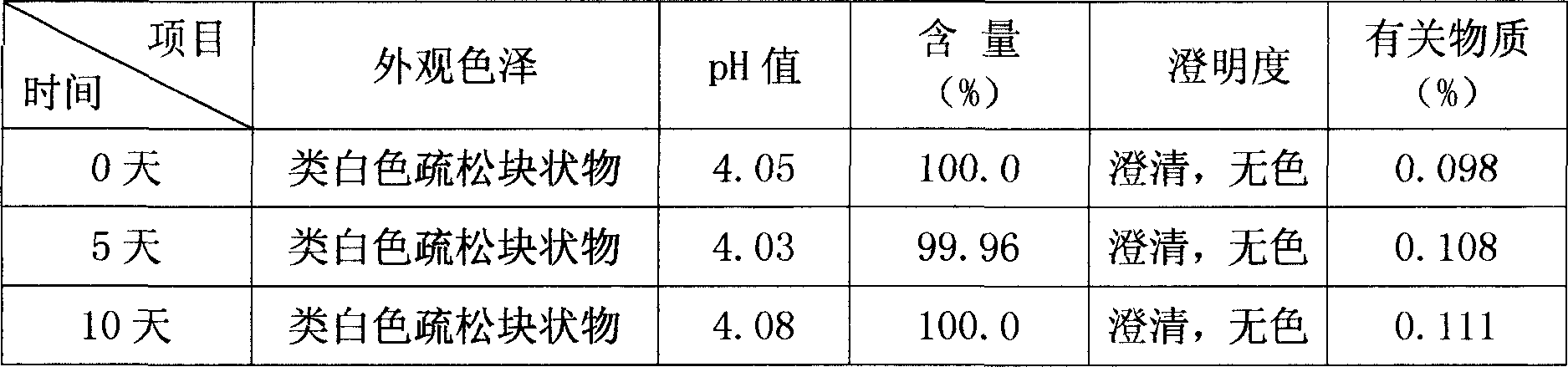
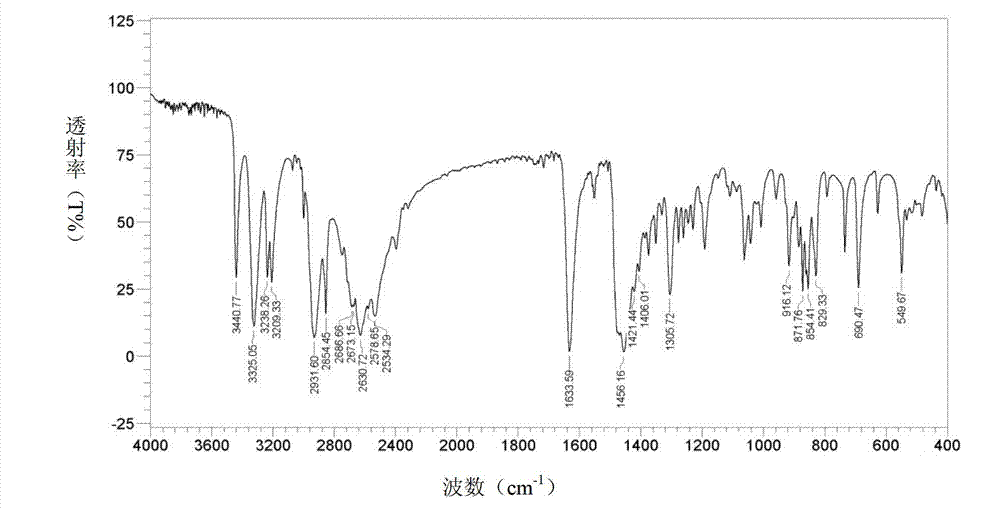
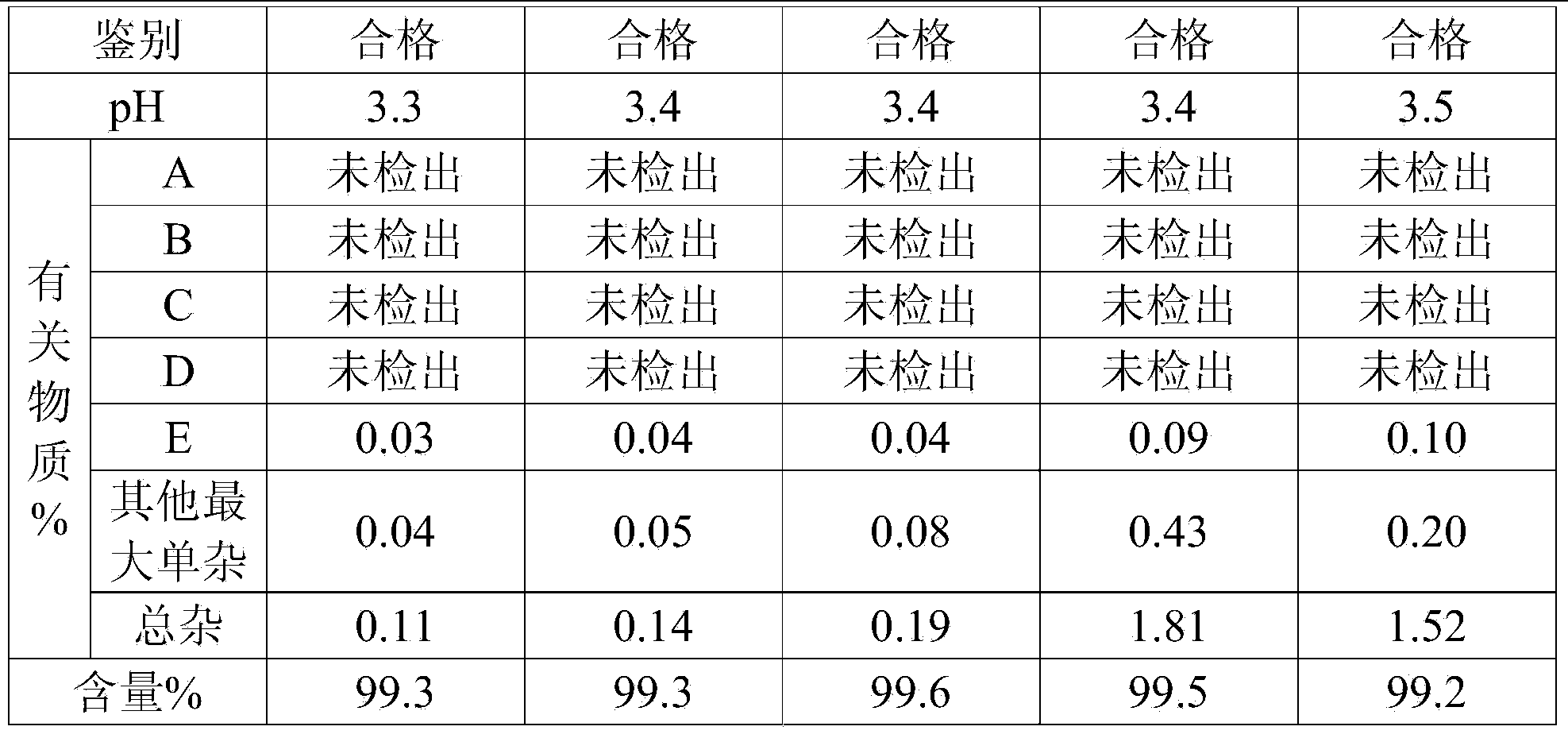
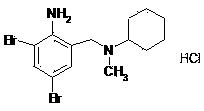
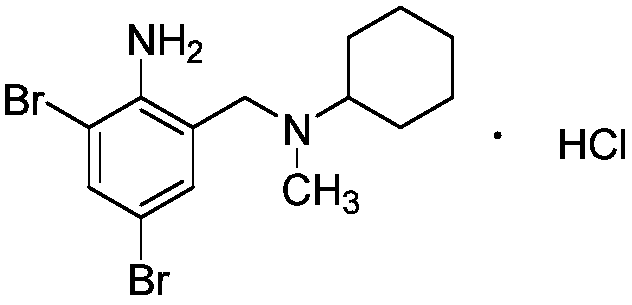
Bromhexine hydrochloride is a hydrochloride resulting from the reaction of equimolar amounts of bromhexine and hydrogen chloride. It is used as a mucolytic for the treatment of respiratory disorders associated with productive cough (i.e. a cough characterised by the production of sputum).
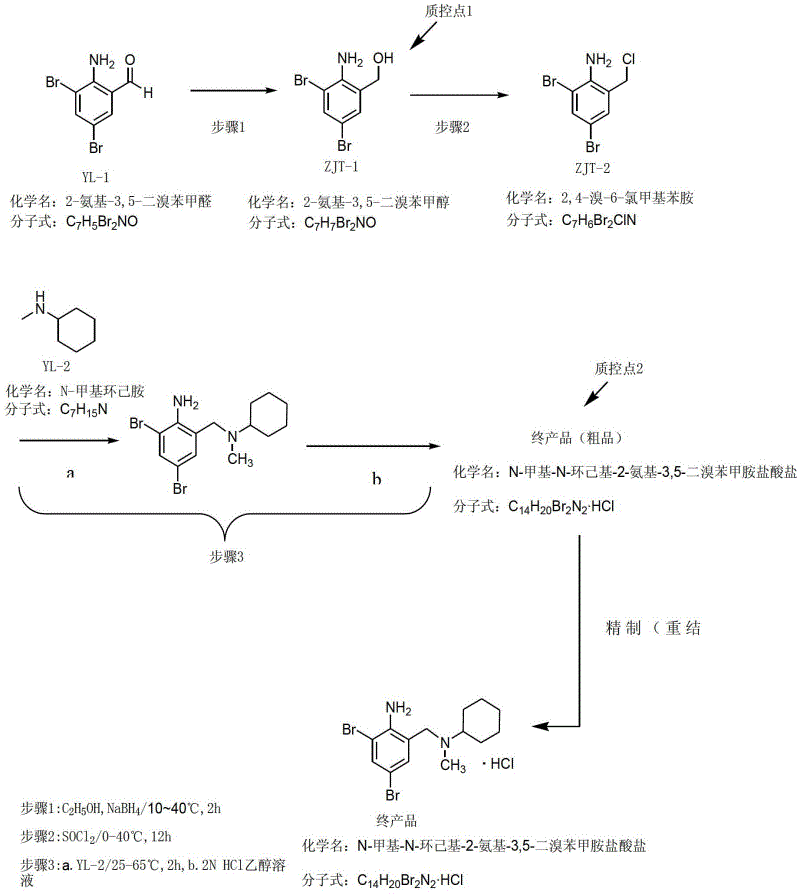
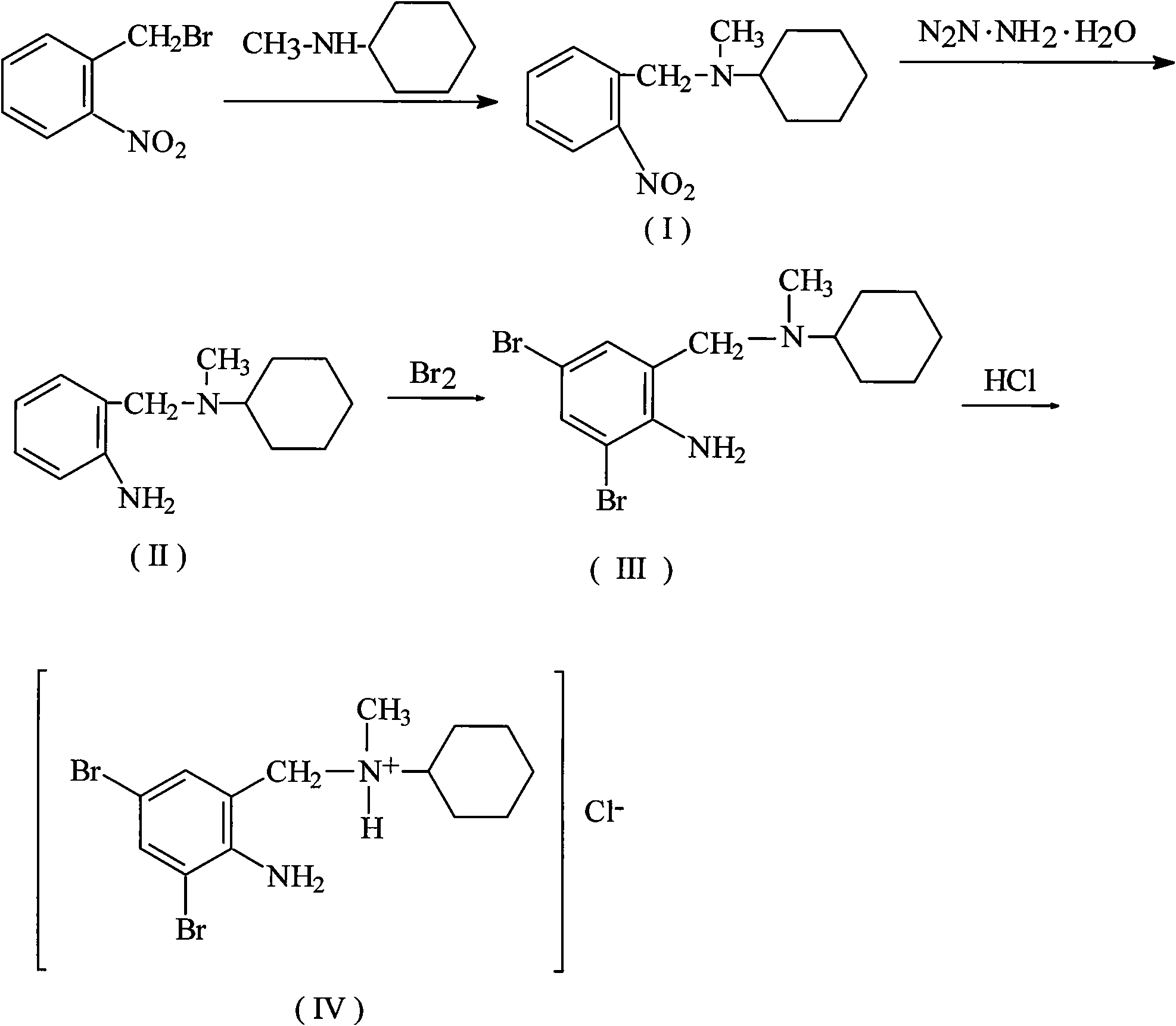
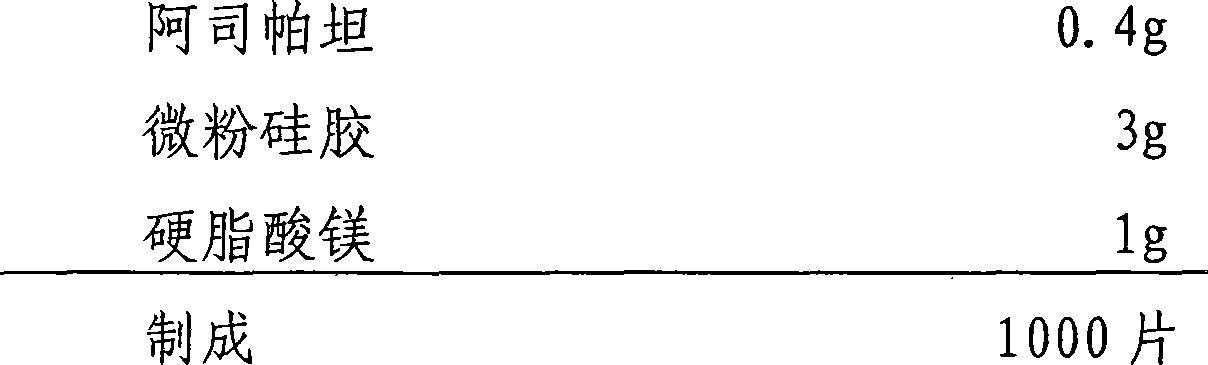
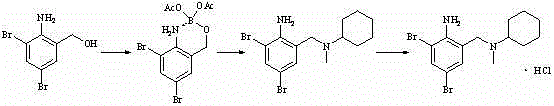
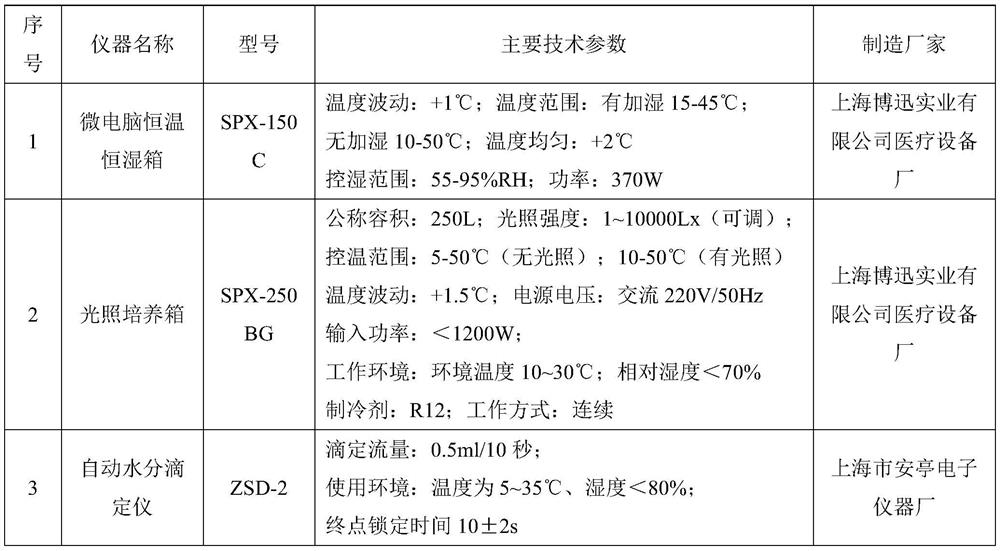
Method for preparing bromhexine hydrochloride
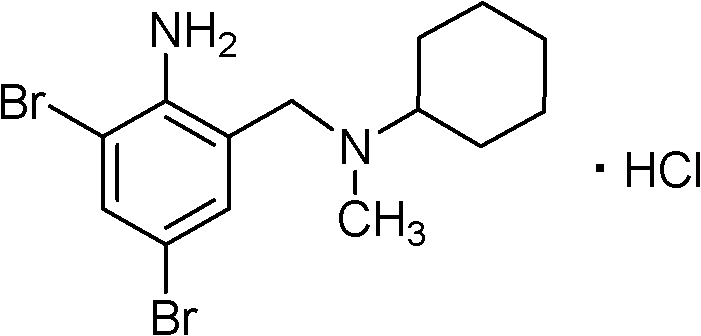
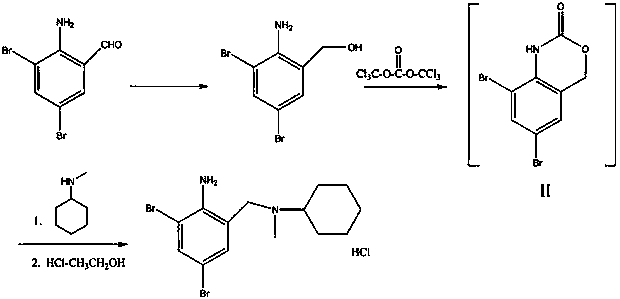
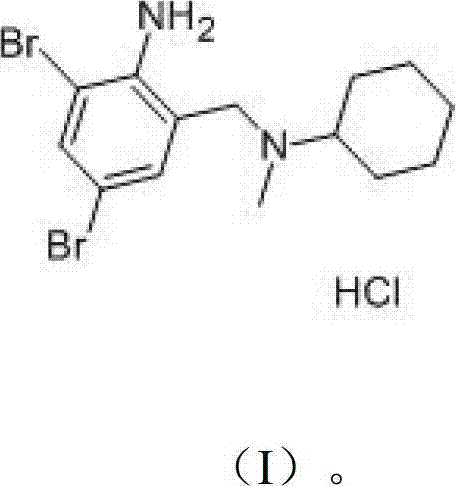
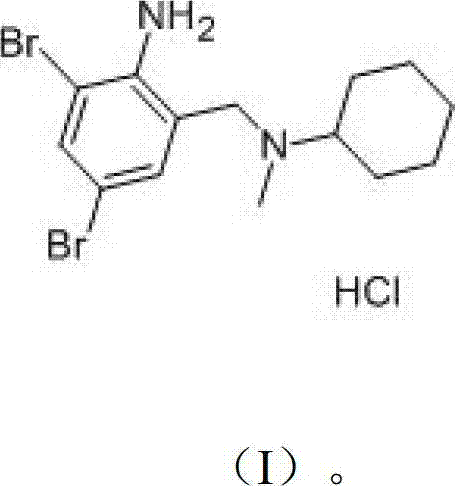
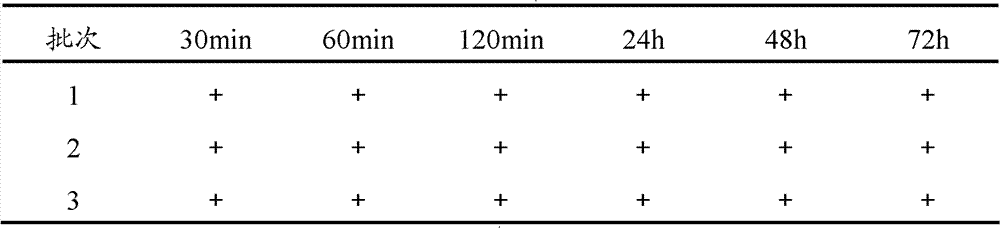
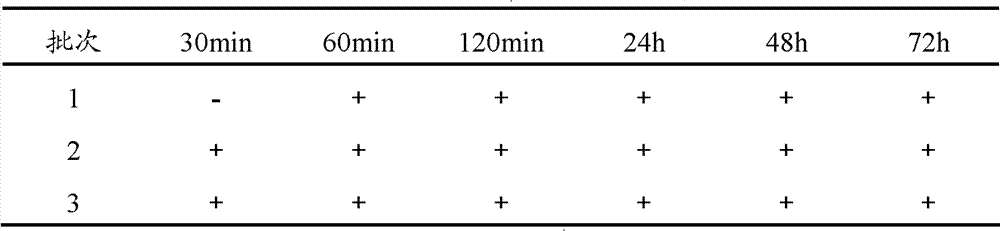
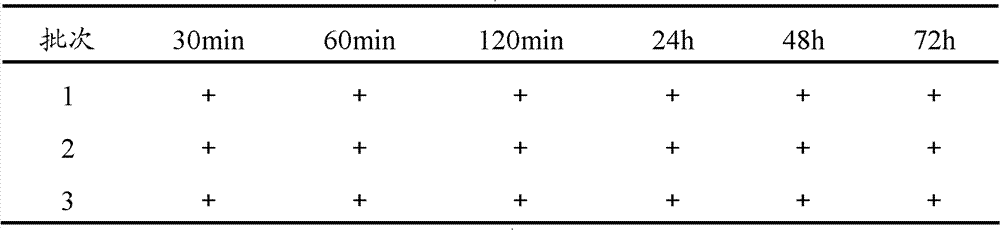
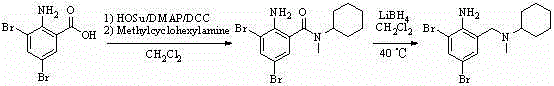
The invention provides a method for preparing bromhexine hydrochloride, which comprises the steps as follows: (1) 2-amino-3,5-dibromo benzaldehyde and reducing agents are in a reduction reaction to generate 2-amino-3,5-dibromo benzyl alcohol; (2) 2-amino-3, 5-dibromo benzyl alcohol that is obtained in the step (1) reacts with chlorinating agents to generate 2, 4-bromine-6-chloride methylaniline; and (3) 2,4-bromine-6-chloride methylaniline obtained in the step (2) and N-methylcyclohexylamine are in an amination reaction, and then 2, 4-bromine-6-chloride methylaniline and HCl salification agents are in a salification reaction, so that bromhexine hydrochloride is obtained. The preparation method adopts multiple advanced technologies, is easy to get starting materials, and has the advantages of stable property of intermediates, extremely low environment pollution, high yield coefficient of products and high purity.
Owner:SHIJIAZHUANG DONGFANG PHARMA
Bromhexine Hydrochloride aseptic freeze-drying formulation for injection and its preparation method
InactiveCN1557285AImprove bioavailabilityAvoid degradationPowder deliveryOrganic active ingredientsActive componentFreeze-drying
The present invention discloses freeze dried bromhexine hydrochloride preparation for injection and its preparation process. The preparation consists of bromhexine hydrochloride as active component, excipient, acidity regulator and injection water. The preparation process includes preparing clear water solution of water insoluble bromhexine hydrochloride with the acidity regulator, adding excipient and freeze drying. The freeze dried bromhexine hydrochloride preparation has raised sability, high bioavailability, less oxidating degradation, no upset reaction and clinical use convenience.
Owner:马小歧
Production method of bromhexine hydrochloride
InactiveCN101817754AFewer synthetic stepsMild reaction conditionsAmino preparation from aminesMixersHydrazine compoundAir tightness
The invention discloses a production method of bromhexine hydrochloride, which uses a multi-function reaction kettle with a special kettle bottom valve as the reaction vessel, uses a special filter as the purifier, uses 2-nitrobenzyl bromide and N-methyl cyclohexylamine as the starting materials, enables the product generated by the reaction under the condition of normal temperature and pressure to the react with hydrazine hydrate under the catalysis of Raney's nickel, and produces the bromhexine hydrochloride by brominating substitution reaction, chlorine hydride salification reaction, purification and drying. Compared with the disclosed production process of bromhexine hydrochloride and the bromhexine hydrochloride produced by the current synthesis device, the production method of the invention has the advantages of less reaction steps, moderate reaction condition, convenient purification and simple operation; the used special device has the advantages of good heat insulation, oxidation resistance, air tightness and environmental protection effects and thorough reaction; and the reaction device has the advantages of simple structure, convenient operation, easy disassembly / assembly and cleaning, high filter efficiency, reaction quality and yield, and stable product quality.
Owner:HONGYI SCI & TECH CO LTD NANCHANG
Bromhexine hydrochloride injection and preparation method and application thereof
ActiveCN104306329AImprove stabilitySimple preparation processOrganic active ingredientsPharmaceutical delivery mechanismActive componentBromhexine hydrochloride
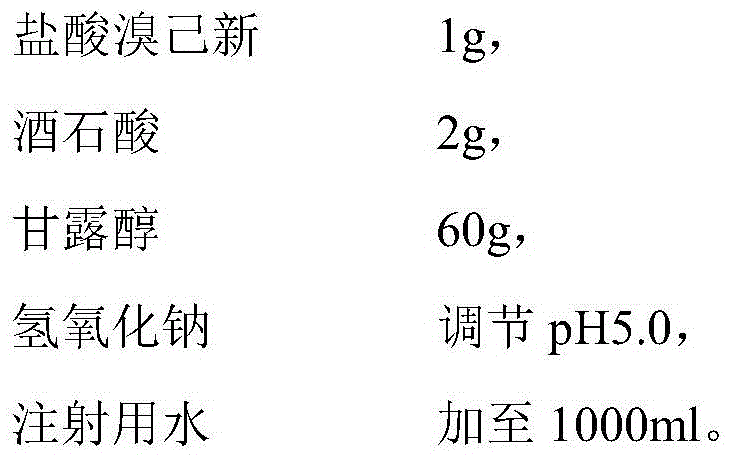
The invention provides bromhexine hydrochloride injection and a preparation method thereof. The bromhexine hydrochloride injection comprises an active component bromhexine hydrochloride, and tartaric acid, a stabilizing agent, sodium hydroxide and water for injection. The bromhexine hydrochloride injection has the advantages of low contents of relevant materials, high stability and the like.
Owner:HEBEI RENHE YIKANG PHARMA
A kind of bromhexine hydrochloride injection and its preparation method and application
ActiveCN102293741AImprove stabilityReduce usageOrganic active ingredientsPharmaceutical delivery mechanismMedicineInjection solution
The invention provides a bromhexine hydrochlorie injection, which contains bromhexine hydrochlorie and glucose and does not contain ethanol. Without ethanol, the injection can be used together with antibiotic drugs and has good stability. In accord with national regulations for pharmaceutical preparations, the injection has stable and reliable quality, and therefore can be used safely. The invention also provides a preparation method of the bromhexine hydrochlorie injection, comprising the steps of: preparing an isotonic water solution of glucose, then predissolving the bromhexine hydrochlorie in the isotonic water solution, and conducting an ultrasonic treatment. According to the method of the invention, the use of ethanol in the process of preparing a liquid preparation of bromhexine hydrochlorie can be avoided successfully, and possible adverse reactions during clinical application are avoided. With a process simple for operation, the method provided in the invention improves the stability of the injection.
Owner:HANKANG BIOCHEMICAL & PHARMA CO LTD
Bromhexine hydrochloride injection and its preparation method
The present invention discloses a bromhexine hydrochloride injection and its preparation method. It is prepared by using bromhexine hydrochloride and medicinal carrier, and can be directly used for intravenous injection.
Owner:张嵩
Bromhexine hydrochloride compound and pharmaceutical composition thereof
ActiveCN103145564AThe prescription process is simpleImprove stabilityOrganic active ingredientsAmino compound purification/separationPharmaceutical SubstancesMedicinal chemistry
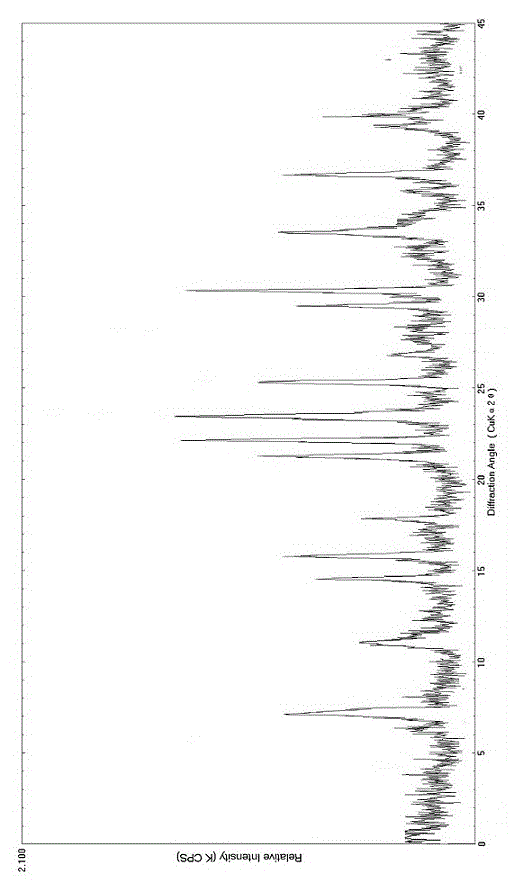
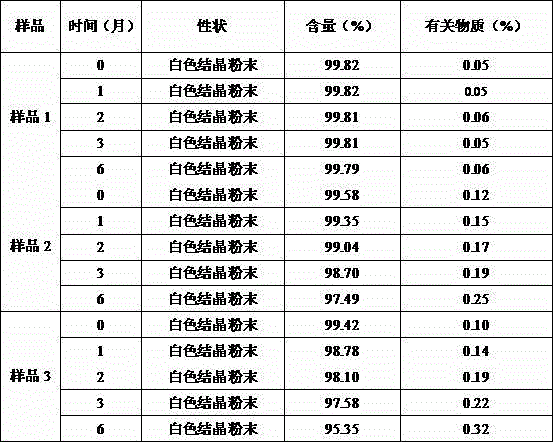
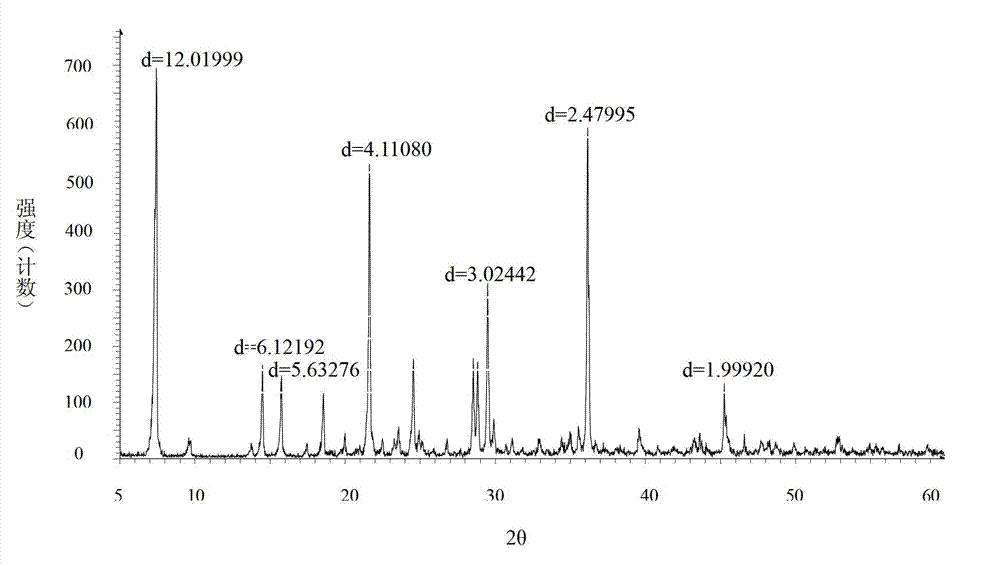
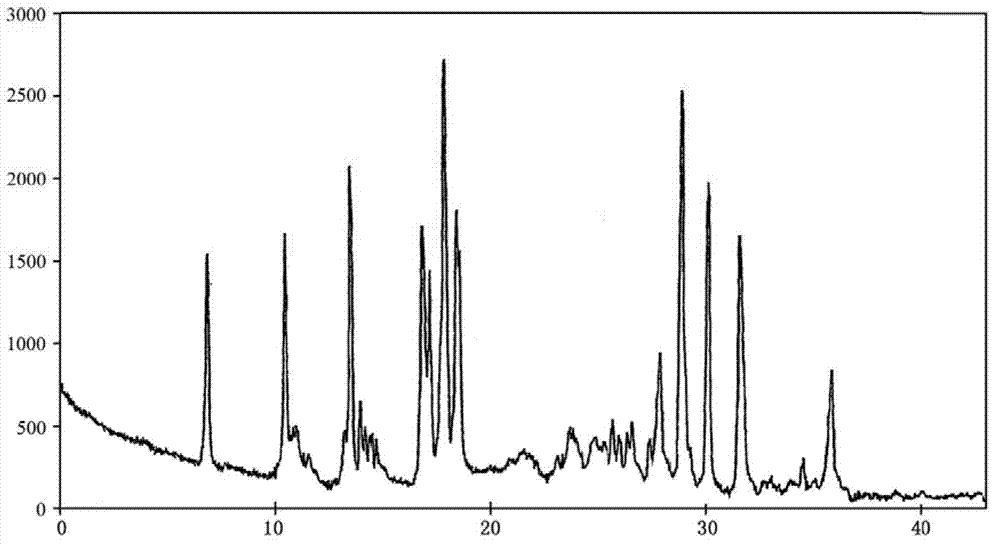
The invention relates to a bromhexine hydrochloride compound. The bromhexine hydrochloride compound is a crystal body and determined by using an X-ray powder diffraction method, and the characteristic peaks of the bromhexine hydrochloride compound are shown in a map in which 2theta plus or minus 0.2 degree is equal to 7.0 degrees, 10.9 degrees, 14.6 degrees, 15.8 degrees, 17.9 degrees, 21.1 degrees, 22.2 degrees, 23.4 degrees, 25.2 degrees, 29.3 degrees, 30.3 degrees, 33.4 degrees, 36.7 degrees and 39.6 degrees. The invention further provides a preparation and a pharmaceutical composition preparation which comprise the bromhexine hydrochloride compound, wherein the preparations can be prepared to form powder injection, freeze-dried powder injection, water injection and tablets. The formulation technology of the powder injection, freeze-dried powder injection, water injection and tablets of the bromhexine hydrochloride compound provided by the invention is simple in process, the stability of the bromhexine hydrochloride compound is obviously improved, and the safety and effectiveness of medication are improved.
Owner:湖北美林药业有限公司
Compound cefaclor suspension and preparation method thereof
InactiveCN101912368AGood dissolution effectPromote dissolutionAntibacterial agentsOrganic active ingredientsBiotechnologyCellulose
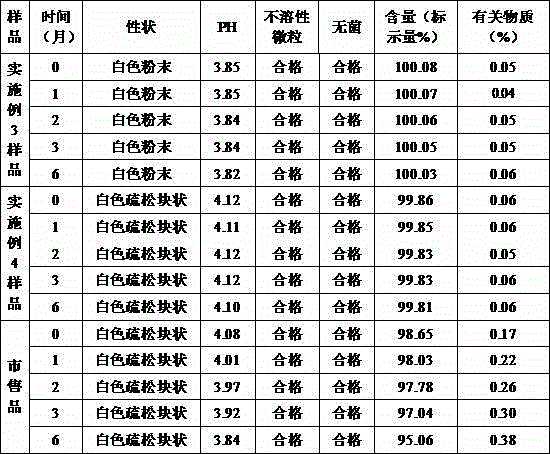
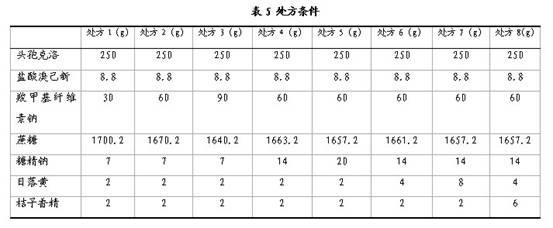
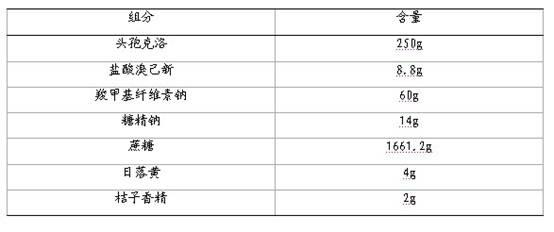
The invention discloses a compound cefaclor suspension and a preparation method thereof, belonging to the field of pharmaceutical preparations. The compound cefaclor suspension comprises the following components in percentage by weight: 4-50 percent of cefaclor, 0.14-1.75 percent of bromhexine hydrochloride, 0.5-6 percent of macromolecule suspending agent, 0.1-1.8 percent of sweetener, 38.45-95.256 percent of cane sugar, 0.003-1 percent of sunset yellow and 0.001-1 percent of flavoring orange essence. The preparation method comprises the following steps of: after uniformly mixing the macromolecule suspending agent sodium carboxymethylcellulose with the cane sugar, adding an ethanol-water solution containing the sunset yellow and the sweetener saccharin sodium; and after preparing a soft material, granulating and drying, uniformly mixing with the cefaclor, the bromhexine hydrochloride and the flavoring orange essence to obtain the compound cefaclor suspension. The compound cefaclor suspension can be used for treating respiratory tract mild-to-severe infection caused by sensitive bacteria, tonsillitis, chronic bronchitis acute exacerbation, pneumonia, nasosinusitis, and the like.
Owner:UNIV OF SHANGHAI FOR SCI & TECH +1
Compound cefaclor dispersible tablet
InactiveCN1666743AEasy to take and carryHigh degree of industrialization of productionAntibacterial agentsOrganic active ingredientsDiluentDissolution
The invention relates to a new drug from of cefaclor belonging to heavy oral cephalosporin antibacterial drugs, comprising following compositions in weight fractions: cefaclor is 250 deals in non water cefaclor; bromhexine hydrochloride is 8.77 deals in bromhexinum; filling agent is 0-140 deals selected from lactose, white dextrine, starch or / and compressible starch; diluent is 30-140 deals selected from microcrystalline cellulose; disintegrating adminicle is 20-150 deals selected from L-HPC or / and crospovidone; lubricating agent is 7-15 deals selected from micropowder silica gel or / and magnesium. The compound cefaclor dispersible tablet provided can disintegrate quickly in the body, improve its dissolution, promote absorption, and have high bioavailability.
Owner:黄本东
Stable bromhexine hydrochloride sodium chloride injection composition
ActiveCN104434786AGuaranteed sterilityOrganic active ingredientsPharmaceutical delivery mechanismAcetic acidSodium Chloride Injection
The invention provides a high-capacity bromhexine hydrochloride sodium chloride injection composition. The bromhexine hydrochloride sodium chloride injection provided by the invention is prepared from bromhexine hydrochloride, sodium chloride, tartaric acid, glacial acetic acid and injection water. The bromhexine hydrochloride sodium chloride injection provided by the invention has the advantages of low content of related substances, high stability and the like.
Owner:HEBEI RENHE YIKANG PHARMA
Bromhexine hydrochloride crystal as well as preparation method and application of crystal
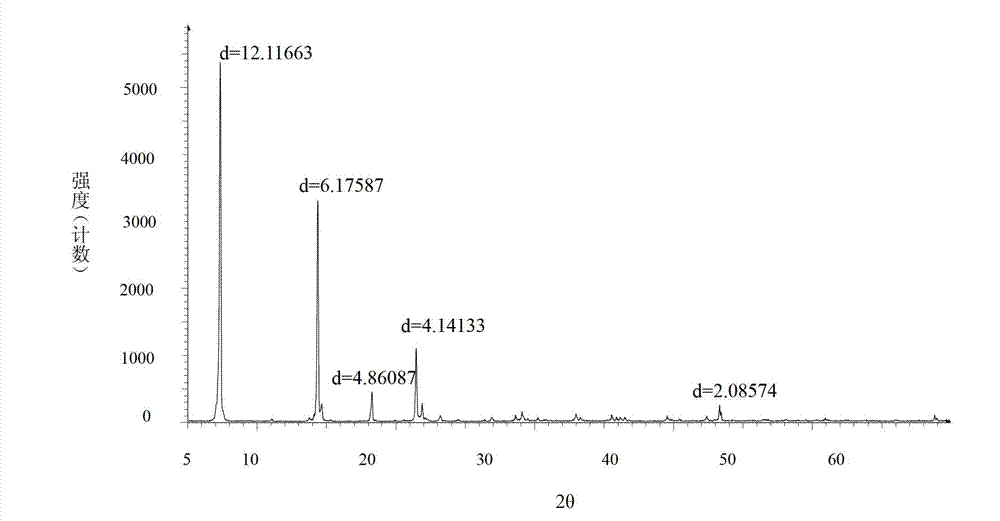
ActiveCN102924295AHigh purityImprove quality and safetyOrganic active ingredientsAmino compound purification/separationPhysical chemistryAqueous solubility
The invention provides a bromhexine hydrochloride crystal as well as a preparation method and application of the bromhexine hydrochloride crystal. According to the crystal, an X-ray powder diffraction picture under the radiation of Cu KAlpha1 has diffraction peaks in a part with reflection angles 2 Theta of 7.29, 14.33 and 24.439. The crystal can obviously improve the water solubility of bromhexine hydrochloride, and is beneficial for the preparation of an injection; and relative materials can be further reduced, so that the safety of the preparation can be improved.
Owner:SHIJIAZHUANG DONGFANG PHARMA
Oral liquid composition of bromhexine hydrochloride
ActiveCN103893116AImprove stabilitySimple preparation processOrganic active ingredientsPharmaceutical delivery mechanismAlcoholMaltitol
The invention provides an oral liquid composition of bromhexine hydrochloride and a preparation method of the oral liquid composition. The oral liquid composition of the bromhexine hydrochloride contains bromhexine hydrochloride, cool alcohol, maltitol and newtol. The oral liquid composition of the bromhexine hydrochloride has the advantages of low related substance content and good stability.
Owner:HEBEI RENHE YIKANG PHARMA
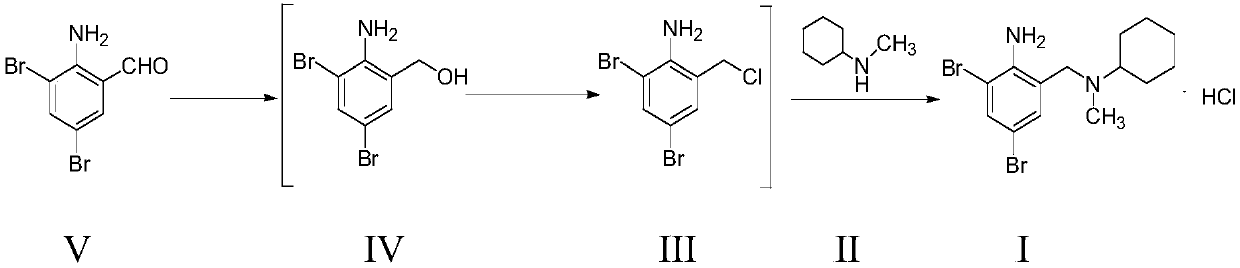
Preparation method of bromhexine hydrochloride
ActiveCN104003887AReduce usageHigh yieldAmino preparation by functional substitutionBenzaldehydeCombinatorial chemistry
The invention provides a preparation method of bromhexine hydrochloride. 2-amino-3,5-dibromo benzaldehyde is taken as a raw material, and reduction, amination and salifying are carried out, so that the bromhexine hydrochloride is obtained, wherein amination and salifying are completed in one step, and an intermediate product is not needed to be separated. The preparation method of the bromhexine hydrochloride has the advantages that reaction steps are reduced, and yield and purity of the bromhexine hydrochloride product are improved.
Owner:JIANGXI DONGFU PHARMA CO LTD
Bromhexine hydrochloric acid orally disintegrating tablet production method
InactiveCN101361719AOrganic active ingredientsPharmaceutical product form changeOrally disintegrating tabletCompressibility
The invention relates to a medical prescription for producing bromhexine hydrochloride orally disintegrating tablets by using powder direct compression process, a technology and a powder feeder used for a novel tablet machine. Compared with the conventional technique of the powder direct compression process, the orally disintegrating tablets produced by the invention has mouth-feel of cool-refreshing and fine-smooth, smaller tablet weight difference, good content uniformity, fast disintegration and dissolution, high dissolution speed and dissolution rate, strong hardness and good compressibility of the tablets.
Owner:HONGYI SCI & TECH CO LTD NANCHANG
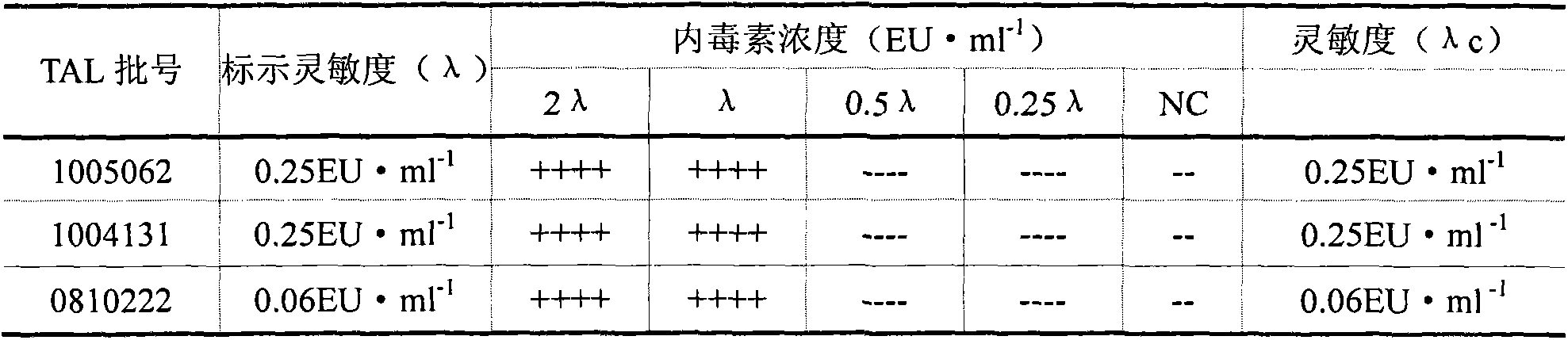
Method for detecting content of bacterial endotoxin of bromhexine hydrochloride raw material
InactiveCN103837673AAccurate contentReduce inspection costsPreparing sample for investigationBiological testingInterference factorLimit value
The invention aims at providing a sensitive and reliable method, and particularly relates to a method for quickly detecting the content of bacterial endotoxin of a bromhexine hydrochloride raw material. The method comprises the following steps of measuring bromhexine hydrochloride powder, dissolving the bromhexine hydrochloride powder in DMSO (dimethyl sulfoxide) to have the pre-interference experiment and find out the maximal dilution times; preparing a bromhexine hydrochloride test solution according to 1600 times of the maximal dilution times to have the interference experiment with two TAL (tachypleus amebocyte lysate) with the sensitivity of 0.25EU / ml from two different factories, finally confirming whether the interference factor exists or not, and determining the limit value of the bacterial endotoxin; diluting the bromhexine hydrochloride solution according to the non-interfered maximal valid dilution times, and then examining the bacterial endotoxin.
Owner:张嵩
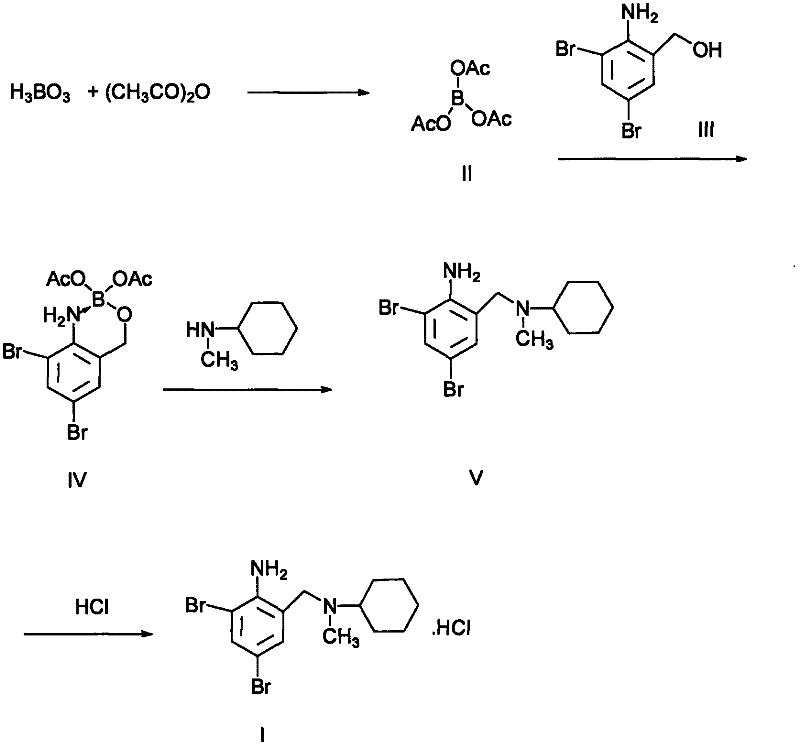
Novel preparation method for bromhexine hydrochloride
InactiveCN102531922ASimple processMild reaction conditionsAmino preparation from aminesAcetic anhydrideObstructive chronic bronchitis
The invention discloses a preparation method for bromhexine hydrochloride. The preparation method comprises the following steps of: reacting acetic anhydride with boric acid to generate triacetic acid boric acid ester; reacting 2-amino-3,5- dibromo benzylalcohol with triacetic acid boric acid ester to generate 2-amino-3,5-dibromo benzylalcohol boron chelate; reacting 2-amino-3,5-dibromo benzylalcohol boron chelate with N- methylcyclohexylamine to generate N-(2-amino-3,5-dibromobenzyl)-N-methylcyclohexylamine (bromhexine); and reacting bromhexine with hydrogen chloride to generate crude bromhexine hydrochloride, and refining to obtain refined bromhexine hydrochloride. The bromhexine hydrochloride is suitable for treating chronic bronchitis and other respiratory diseases with phlegm which is difficult to cough out.
Owner:陈学峰
Compound cefaclor preparation and preparation method
InactiveCN102114019AGood water solubilityGreat tasteAntibacterial agentsOrganic active ingredientsSolubilitySuspending Agents
The invention relates to a preparation of a medicine and a preparation method, in particular to a compound cefaclor preparation and a preparation method, and belongs to the technical field of pharmacy. The preparation is characterized by being prepared by adding a proper amount of pharmaceutical excipients into 25 to 35 weight parts of medicine, namely cefaclor and 0.5 to 1.5 weight parts of bromhexine hydrochloride, wherein the cefaclor is calculated based on anhydrous cefaclor, and the bromhexine hydrochloride is calculated based on bromhexine; and the excipients consist of a suspending agent, a flocculating agent, a flavoring agent and a flow aid. The invention provides a formula capable of improving the dissolubility and suspension stability of medicines and the mouthfeel of finished products, and a preparation technology.
Owner:汤明昌 +1
Bromhexine hydrochloride compound and medicine composition thereof
ActiveCN102775316AImprove stabilityNot easy to changeOrganic active ingredientsAmino compound purification/separationPowder diffractionBromhexine hydrochloride
The invention relates to a bromhexine hydrochloride compound and a medicine composition thereof. The bromhexine hydrochloride is measured by the powder X-ray diffraction measurement method; and the characteristic diffraction peaks of the X-ray powder diffraction atlas represented by a diffraction angle of 2(theta)+ / -0.2 degrees are displayed at 6.78 degrees, 10.39 degrees, 13.40 degrees, 13.90 degrees, 16.75 degrees, 17.11 degrees, 17.74 degrees, 18.38 degrees, 27.80 degrees, 28.81 degrees, 30.06 degrees, 31.53 degrees and 35.78 degrees.
Owner:江西璟瑞药业有限公司
Bromhexine hydrochloride solution composition for inhalation and preparation method thereof
InactiveCN105456187AImprove stabilitySimple prescriptionOrganic active ingredientsDispersion deliveryInhalationMedicine
The invention provides a bromhexine hydrochloride solution composition for inhalation and a preparation method thereof. A bromhexine hydrochloride solution for inhalation is prepared from bromhexine hydrochloride, tartaric acid and water for injection. The bromhexine hydrochloride solution for inhalation has the advantages of being simple in prescription composition, high in stability, high in safety of drug application, suitable for industrial production and the like.
Owner:HEBEI RENHE YIKANG PHARMA
Pharmaceutical composition containing dexchlorpheniramine and preparation thereof
The invention discloses a combination containing r-chlorpheniramine, which is formed by the r-chlorpheniramine and the medicine salt and the other or a plurality of active ingredients or pharmaceutical carriers selected from non-steroidal anti-inflammatory drug, ephedrine alkaloids, coffeine, dextromethorphan hydrobromide, carbetapentane citrate, glyceryl guaiacolate, bromhexine hydrochloride, artificial cow-bezoar, amantadine hydrochloride, aminophylline and zinc gluconate. The orally taken preparation developed from the combination comprises granule, tablet, capsule, dispersible tablet, chewable tablet, effervescent tablet, orally disintegrating tablet, buccal tablet, dry suspension and certain solid formulation.
Owner:FUKANGREN BIO PHARMA
Production method of bromhexine hydrochloride
ActiveCN103333074AReduce wasteImprove product quality stabilityOrganic compound preparationAmino compound preparationAcetic acidAlcohol
The invention relates to a production method of bromhexine hydrochloride. The production method is characterized by comprising the following steps of: 1) carrying out a condensation reaction between 3,5-dibromo-2-aminobenzalcohol and N-methyl cyclohexane in the presence of glacial acetic acid as a catalyst, thereby generating N-methyl-N-cyclohexyl-2-amino-3,5-dibromobenzylamine; 2) salifying the generated N-methyl-N-cyclohexyl-2-amino-3,5-dibromobenzylamine with hydrochloric acid to obtain bromhexine hydrochloride; and 3) decoloring and refining the obtained bromhexine hydrochloride in an alcoholic solution to obtain a pure product, wherein an azeotropic water-carrying agent is added to the condensation reaction in the step 1), and water in the reaction mixed solution in the reaction process is removed immediately through distillation.
Owner:ZHEJIANG WANBANG PHARMA
Bromhexine hydrochlorie injection, its preparation method and application
ActiveCN102293741BImprove stabilityReduce usageOrganic active ingredientsPharmaceutical delivery mechanismMedicineD-Glucose
The invention provides a bromhexine hydrochlorie injection, which contains bromhexine hydrochlorie and glucose and does not contain ethanol. Without ethanol, the injection can be used together with antibiotic drugs and has good stability. In accord with national regulations for pharmaceutical preparations, the injection has stable and reliable quality, and therefore can be used safely. The invention also provides a preparation method of the bromhexine hydrochlorie injection, comprising the steps of: preparing an isotonic water solution of glucose, then predissolving the bromhexine hydrochlorie in the isotonic water solution, and conducting an ultrasonic treatment. According to the method of the invention, the use of ethanol in the process of preparing a liquid preparation of bromhexine hydrochlorie can be avoided successfully, and possible adverse reactions during clinical application are avoided. With a process simple for operation, the method provided in the invention improves the stability of the injection.
Owner:HANKANG BIOCHEMICAL & PHARMA CO LTD
Method for synthesizing bromhexine hydrochloride
ActiveCN104628577AReduce pollutionFew reaction stepsOrganic compound preparationAmino compound preparationBenzaldehydeCyclohexylamine
The invention relates to a method for preparing bromhexine hydrochloride. The method comprises the steps of synthesizing bromhexine free alkali from 2-amino-3,5-dibromo benzaldehyde and N-methyl cyclohexylamine in the presence of a catalyst and a reducer in one step, and enabling the produced bromhexine free alkali to undergo salt forming reaction with a hydrogen chloride salt forming reagent, thereby preparing bromhexine hydrochloride, wherein the catalyst is a sulfonic acid type catalyst Amberlyst<R>15(H). According to the method, the number of reaction steps is greatly reduced, the production cycle is shortened, the reaction yield is increased, and the production cost is reduced; the method disclosed by the invention has no need of using virulent reagents, is mild in reaction conditions and little in environmental pollution, and is free from special requirements on equipment.
Owner:SHANGHAI DINGYA PHARM CHEM CO LTD
Cefaclor composition particles and preparation method thereof
ActiveCN102525949AImprove antibacterial propertiesNot very stableAntibacterial agentsOrganic active ingredientsSolubilityDisease
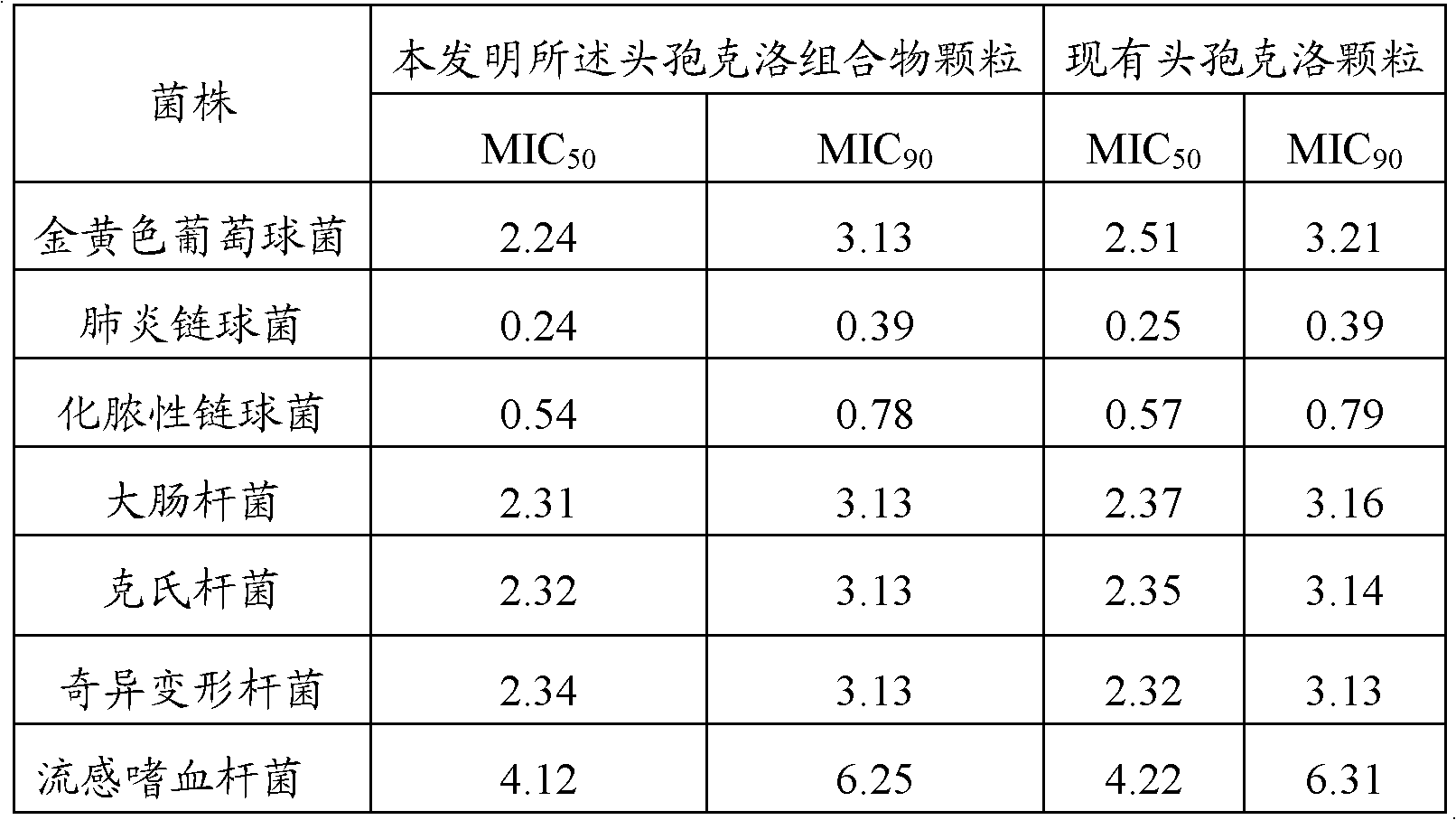
The invention relates to the technical field of medicine, and discloses cefaclor composition particles and a preparation method thereof. The cefaclor composition particles disclosed by the invention are prepared from the following raw materials in parts by weight: 250 parts of cefaclor, 8 parts of bromhexine hydrochloride, 1,490 parts of sucrose, 20 parts of acesulfame, 2.5 parts of essence and 250 parts of sorbitol. The cefaclor composition particles disclosed by the invention comprise sorbitol which can be taken as a medicament dispersing agent for enhancing the medicament solubility and can be used for enhancing the antibacterial action of cefaclor. As proved by a test, the cefaclor composition particles disclosed by the invention have enhanced antibacterial action and high stability and can be widely applied to treatment of bacterial infection diseases such as respiratory tract infection, bone and joint infection, pelvic infection, abdominal infection and the like.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD
Stable bromhexine hydrochloride liquid preparation composition and preparation method thereof
ActiveCN109620799AEnsure safetyAvoid problemsOrganic active ingredientsInorganic non-active ingredientsCyclodextrinBuffer solution
The invention discloses a stable bromhexine hydrochloride liquid preparation composition and a preparation method thereof. The liquid preparation composition is prepared from bromhexine hydrochloridewith the pharmaceutically-acceptable dose, an osmotic pressure modifier with the pharmaceutically-acceptable dose, a stabilizer with the pharmaceutically-acceptable dose, a pH value buffer solution with the pharmaceutically-acceptable dose, and injection water. The stabilizer is a chemically-modified derivative of cyclodextrin or salt of the derivative. The chemically-modified derivative of the cyclodextrin or the salt of the derivative is selected as the stabilizer, by means of the properties of the chemically-modified derivative of the cyclodextrin or the salt of the derivative, the stabilizer is used for helping solubilization of the bromhexine hydrochloride, the bromhexine hydrochloride can be kept stable within the pH range of 1-14, bromhexine cannot be separated out, and the safety of clinical medication is guaranteed.
Owner:CHENGDU XINJIE HIGH TECH DEV CO LTD
Bromhexine hydrochloride soluble powder for livestock and poultry, and preparation method and application thereof
PendingCN111789817AImprove solubilityImprove poor solubilityAntibacterial agentsPowder deliveryBiotechnologyCyclodextrin
The invention belongs to the field of livestock and poultry medicines, and discloses bromhexine hydrochloride soluble powder for livestock and poultry and a preparation method and application thereof.The soluble powder is prepared by uniformly mixing bromhexine hydrochloride, a cosolvent and a soluble filler, and can be used as a medicine for treating respiratory tract infection of the livestockand poultry. Through reasonable proportioning, beta-cyclodextrin and a stabilizer do not need to be added, and it can still be guaranteed that the prepared bromhexine hydrochloride soluble powder hasgood solubility and stability; the cosolvent and the bromhexine hydrochloride are uniformly mixed, so that the solubility of the bromhexine hydrochloride is further increased, the problem of poor solubility of the bromhexine hydrochloride is solved, and the bromhexine hydrochloride soluble powder is suitable for the livestock suffering from respiratory infection.
Owner:HEBEI KEXING PHARMA
Veterinary drug for treating respiratory tract infection of poultry and preparation method thereof
InactiveCN101670061AImprove efficacyGood treatment effectAntibacterial agentsAmphibian material medical ingredientsDiseaseRespiratory disease
The invention discloses a veterinary drug for treating respiratory tract infection of poultry and a preparation method thereof. The veterinary drug is prepared mainly from the following materials by weight part: 30-120 parts of radix stemonae, 10-120 parts of scutellaria baicalensis, 30-210 parts of giant knot weed, 10-60 parts of indigo naturalis, 10-60 parts of artificial bezoar, 10-40 parts ofborneol, 10-90 parts of realgar, 10-60 parts of liquorice, 30-120 parts of platycodon root, 90-210 parts of cyrtomium fortunei, 30-120 parts of blackberry lily, 10-210 parts of dried toad, 150-420 parts of radix isatidis and 1-10 parts of bromhexine hydrochloride. The veterinary drug is prepared by grinding, screening and evenly mixing the materials, and grinding the materials by a superfine pulverizing technology into submicron powder and packing. The veterinary drug is mainly used for treating respiratory diseases of poultry caused by the bacterial viruses such as infectious bronchitis of chicken, infectious laryngotracheitis, newcastle disease and influenza. The invention has the advantages of rapid drug effect, favorable effect and little remaining charge, has simple preparation methodand is easy for promotion and application.
Owner:ZHENGZHOU HOUYI PHARMA
Preparation method of bromhexine hydrochloride
PendingCN111470983ASave man hoursReduce pollutionOrganic compound preparationAmino preparation by functional substitutionMethylanilineCyclohexylamines
The invention belongs to the field of pharmaceutical chemicals, and particularly relates to a bromhexine hydrochloride synthesis method. The synthesis method comprises following steps: step A, carrying out a reduction reaction between 2-amino-3,5-dibromobenzaldehyde and a reducing agent to generate 2-amino-3,5-dibromobenzyl alcohol; step B, reacting 2-amino-3,5-dibromobenzyl alcohol obtained in the step A with thionyl chloride to generate 2,4-bromo-6-chloromethyl aniline; and step C, carrying out an amination reaction between 2, 4-bromo-6-chloromethyl aniline obtained in the step B and N-methyl cyclohexyl amine, and then carrying out a salt forming reaction with an HCl salifying reagent to obtain bromhexine hydrochloride. According to the preparation method, thionyl chloride is only used as a reactant; in addition, the steps are simple, the intermediates obtained in the steps A and B do not need to be purified and directly used for the next step of reactions, therefore, the working hours required by the whole production route are remarkably shortened, the final yield is increased by 5% or above, and the method is particularly suitable for large-scale production.
Owner:INCREASEPHARM TIANJIN INST CO LTD
Preparation method for compound tilmicosin enteric-coated granules
ActiveCN104586875AIncrease payEffective infectionAntibacterial agentsOrganic active ingredientsOral glucoseDisease
The invention belongs to the technical field of veterinary medicines, and specifically relates to a preparation method for compound tilmicosin enteric-coated granules. The preparation method sequentially comprises the steps of: preparing materials, mixing the materials, granulating, preparing a coating solution, coating and the like, wherein the raw materials of the granules are tilmicosin, colistin, gatifloxacin, a sweetening agent, a flavouring agent, bromhexine hydrochloride, oral glucose, corn starch, saccharose powder, dextrin, talcum powder and starch slurry; the raw materials of the coating solution are HPMC, Tween-80, polyethylene glycol 6000, talcum powder and ethanol. The method disclosed by the invention enables the tilmicosin effective ingredient not to be broken in gastric acid, and to safely arrive at intestinal tracts to be absorbed; the obtained compound tilmicosin enteric-coated granules are capable of preventing and treating respiratory diseases such as mycoplasma diseases, pleuropneumonia and swine plague, capable of reducing the occurrences of bacterial diseases such as diarrhoea and salmonellosis, and capable of increasing the feed conversion.
Owner:ZHENGZHOU DOURIN VETERINARY TECH
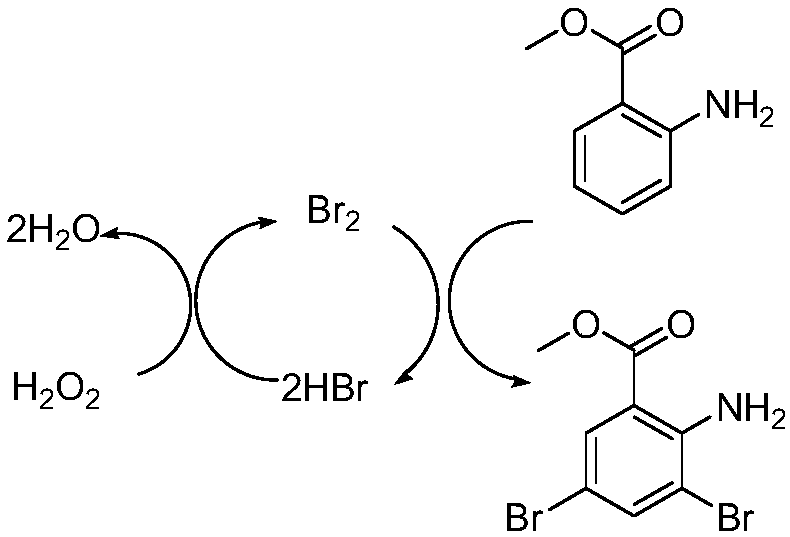
Preparation method for bromhexine hydrochloride
ActiveCN109535010ANo pollution in the processShort synthesis pathOrganic compound preparationAmino-carboxyl compound preparationOrganic synthesisBromhexine hydrochloride
The invention belongs to the field of organic synthesis and provides a preparation method for bromhexine hydrochloride. According to the invention, 2-aminobenzoic ester compounds are taken as raw materials and take part in bromination reaction, reduction reaction, condensation reaction and salt forming reaction, so as to acquire bromhexine hydrochloride. The preparation method has the advantages of short synthesis route, low cost, stable intermediate, no pollution to environment, high purity, high yield, reaching officinal standard and suitability for industrial expanded production.
Owner:GUANGZHOU YIPINHONG PHARMA +5
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com