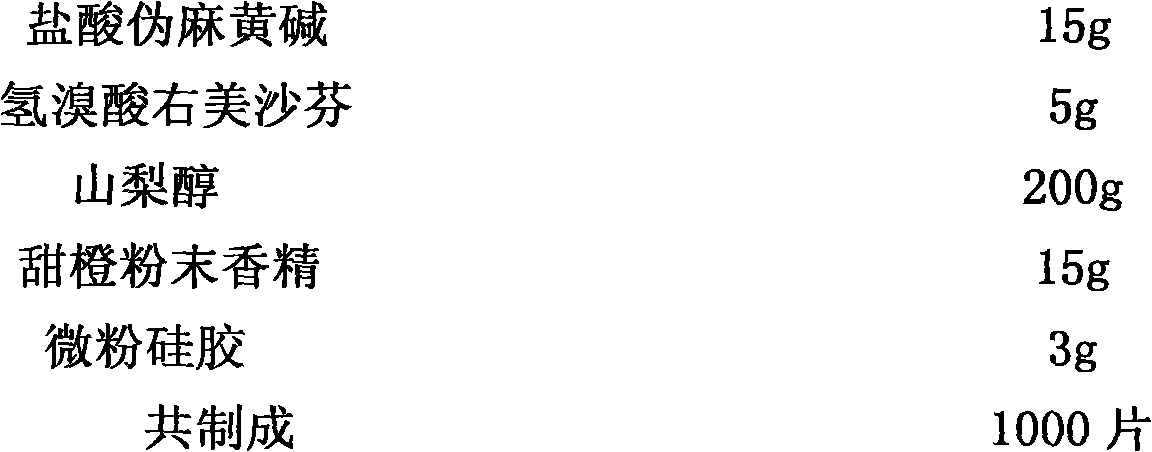Pharmaceutical composition containing dexchlorpheniramine and preparation thereof
A technology of dexchlorpheniramine and chlorpheniramine, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
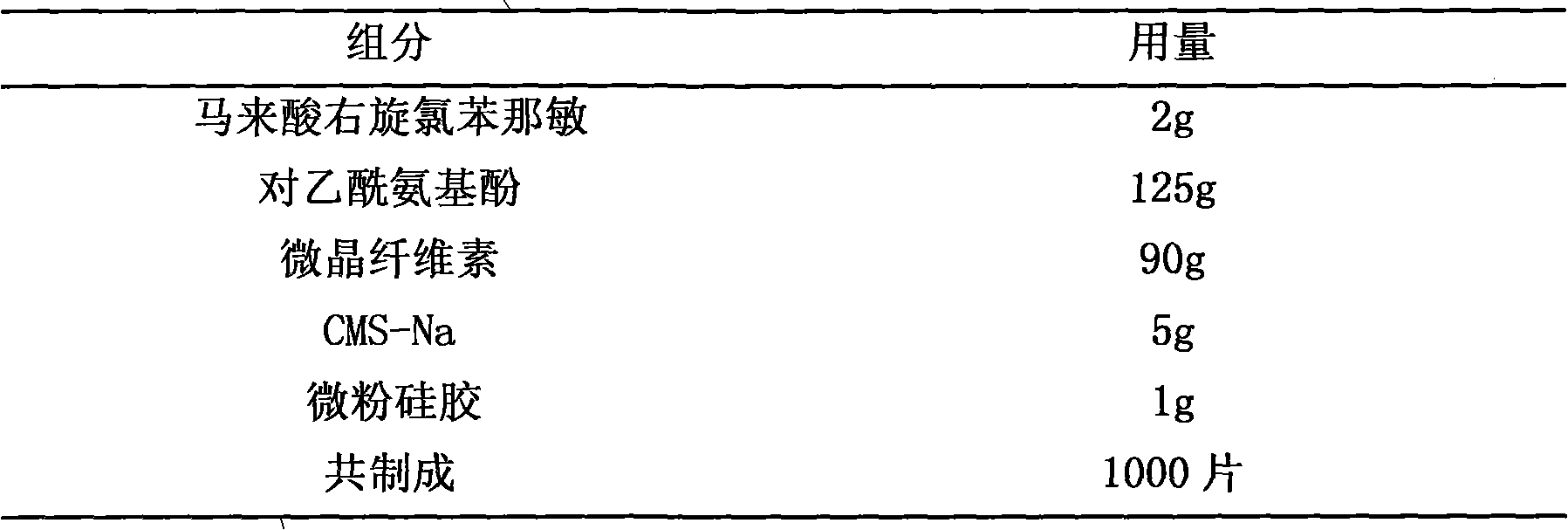
[0016] Embodiment 1, dexchlorpheniramine maleate+paracetamol tablet
[0017]
[0018] Preparation:
[0019] Pass acetaminophen and microcrystalline cellulose through an 80-mesh sieve, mix well, and set aside; take another appropriate amount of 50% ethanol solution, add dexchlorpheniramine maleate to dissolve, and use this solution as a binder to prepare Soft material, granulate with 24 mesh sieve, dry at 50°C, granulate with 20 mesh sieve, add micropowder silica gel and CMS-Na, mix well, and press a suitable die to form a tablet. If the above-mentioned tablets are coated, coated tablets can be obtained, which can be film-coated tablets, enteric-coated tablets, etc.
Embodiment 2
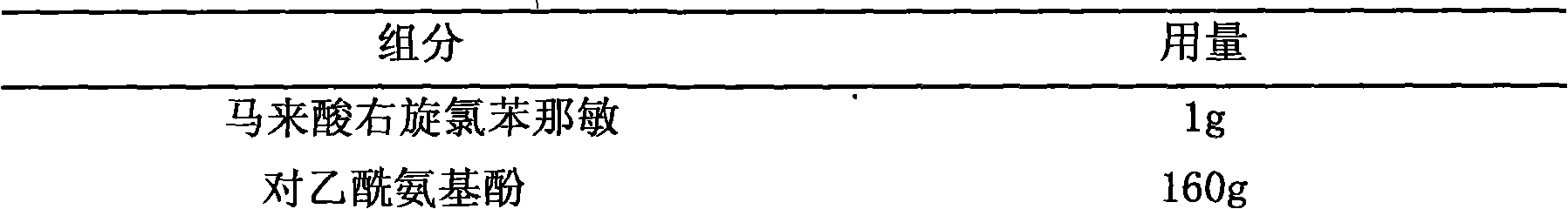
[0020] Embodiment 2, dexchlorpheniramine maleate + acetaminophen + pseudoephedrine hydrochloride + dextromethorphan hydrobromide chewable tablets
[0021] prescription:
[0022]
[0023]
[0024] Preparation:
[0025] Pass acetaminophen, pseudoephedrine hydrochloride, and dextromethorphan hydrobromide through an 80-mesh sieve, mix them with sorbitol evenly, take an appropriate amount of 50% ethanol solution, add dexchlorpheniramine maleate to dissolve it, and use it as Binder soft material, granulated with 24 mesh sieve, dried at 50°C, granulated with 20 mesh sieve. Add sweet orange powder essence and micro-powder silica gel, mix evenly, and press into tablets to obtain the product.
Embodiment 3
[0026] Embodiment 3, dexchlorpheniramine maleate+pseudoephedrine hydrochloride dispersible tablet
[0027]
[0028] Preparation:
[0029] Pass pseudoephedrine hydrochloride and microcrystalline cellulose through an 80-mesh sieve respectively, and mix them uniformly by the method of equal increments, and set aside; take an appropriate amount of 50% ethanol solution, add dexchlorpheniramine maleate to dissolve it, and use it as an adhesive Soft materials, granulated with a 16-mesh sieve, dried, granulated with a 20-mesh sieve, added other flavoring agents, sweeteners, glidants, disintegrating agents, etc., mixed evenly, and compressed into tablets to obtain the product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com