Novel preparation method for bromhexine hydrochloride
A technology of bromhexine hydrochloride and boric acid, applied in the chemical industry, can solve the problems of harsh reaction conditions, serious environmental pollution, poor reaction quality and the like in the synthesis process, and achieves good sealing and environmental protection effects, high reaction quality and few reaction steps. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
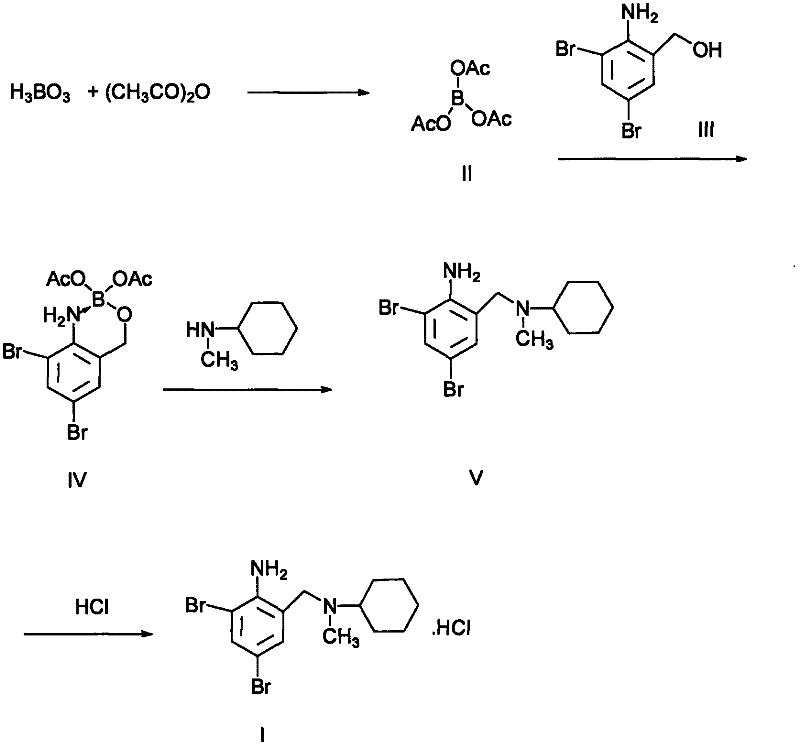
[0022] 33 grams of boric acid, 170 milliliters of acetic anhydride, and 1 gram of anhydrous zinc chloride were mixed evenly. Heating to reflux under stirring for 1 hour, cooling, and suction filtration yielded 78 grams of triacetate borate.
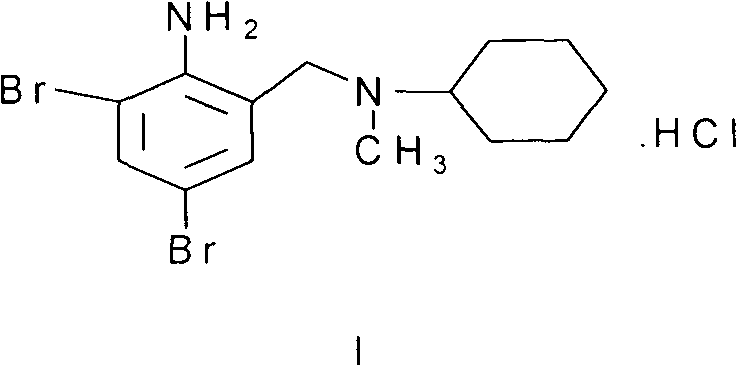
[0023] 37 grams of 2-amino-3,5-dibromobenzyl alcohol and 27 grams of borate triacetate were mixed uniformly and stirred at room temperature for 5 hours. Add 50 ml of N-methylcyclohexylamine and 80 ml of diethylene glycol to the reactant, and heat at 150-155° C. for 2 hours. After the reaction was completed, it was cooled to room temperature. Add 200 ml of ice water and adjust the pH to about 8 with acetic acid. The reaction solution was extracted three times with 300 ml of ethyl acetate, and the oil layer was separated. After drying over anhydrous sodium sulfate and distillation, 40 g of a light green oily substance was obtained, which was bromhexine base, and the yield was 80%.
[0024] Dissolve 40 grams of bromhexine base in 500 mil...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com