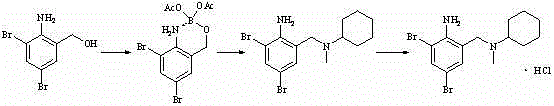
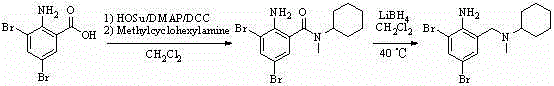
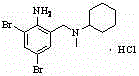
Method for synthesizing bromhexine hydrochloride
A technology of bromhexine hydrochloride and bromhexine hydrochloride, applied in the field of chemical medicine synthesis, can solve the problems such as unobtainable starting materials or intermediates, harsh reaction conditions, unstable intermediates and the like, shortening the production cycle and reducing the number of reaction steps. , the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The preparation of embodiment 1 bromhexine hydrochloride
[0032]
[0033] (1) Preparation of bromhexine free base
[0034] Take 6.0 g (0.0215 mol) of 2-amino-3,5-dibromobenzaldehyde and 2.7 g (0.0239 mol) of N-methylcyclohexylamine, add 100 mL of tetrahydrofuran, stir at 25°C to mix evenly, Then add 0.5g Amberlyst? 15 (H), 1.7g sodium borohydride (0.0447mol), continue to stir, use TLC method (HSGF254 silica gel plate, developer: V 石油醚 :V 乙酸乙酯 =9:1) Monitor the reaction process, after the reaction is terminated, filter the mixture, add 30mL saturated brine to the filtrate, let stand for layers, extract with ether (3×50mL), combine the organic phases, wash with water, wash with saturated brine, no Dry over sodium sulfate, filter, concentrate to remove the solvent, column chromatography (petroleum ether: ethyl acetate = 9:1) to obtain 4.7 g of light yellow oily liquid, yield 58%.
[0035] (2) Preparation of bromhexine hydrochloride
[0036] Add 4.7g of bromhexine i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com