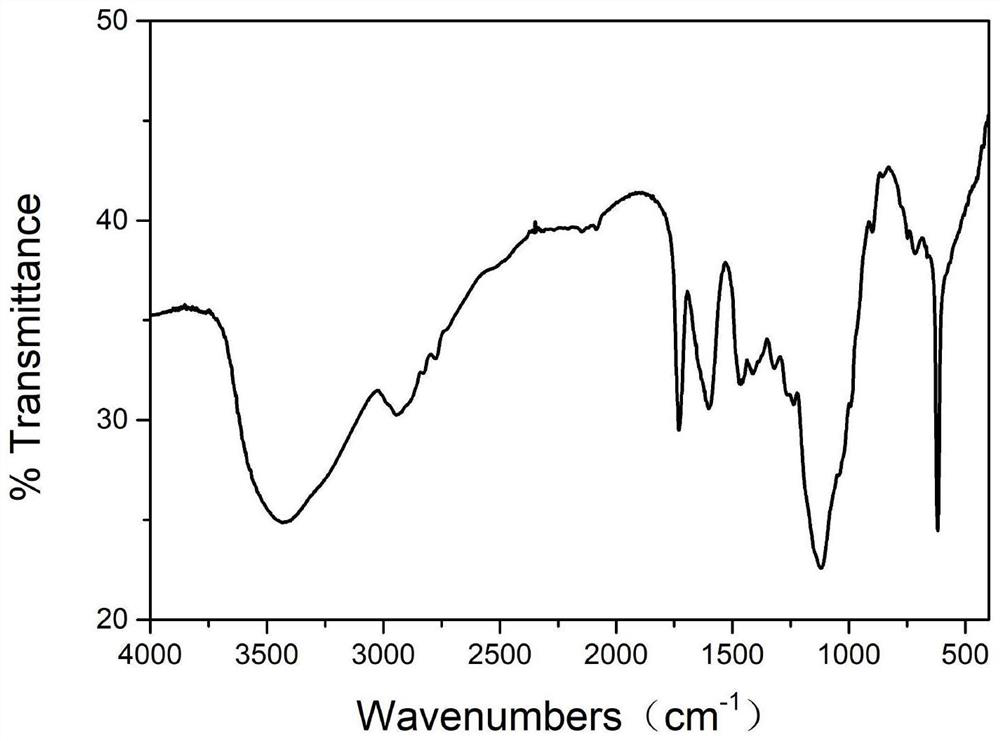
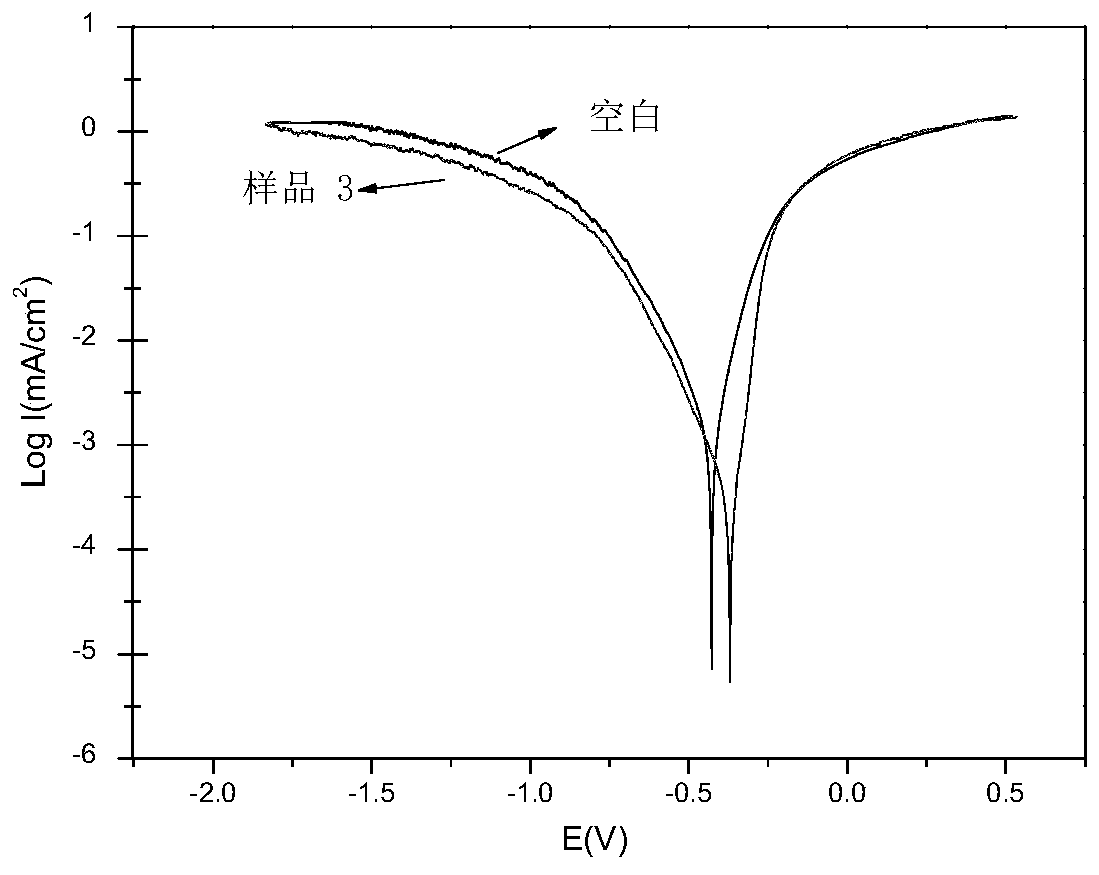
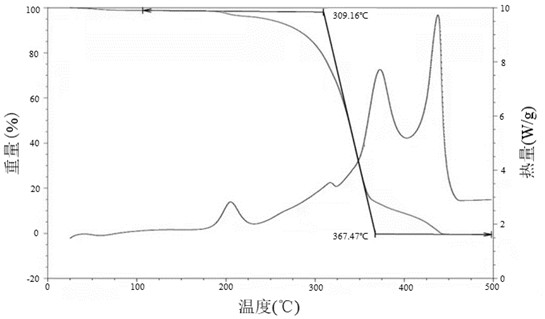
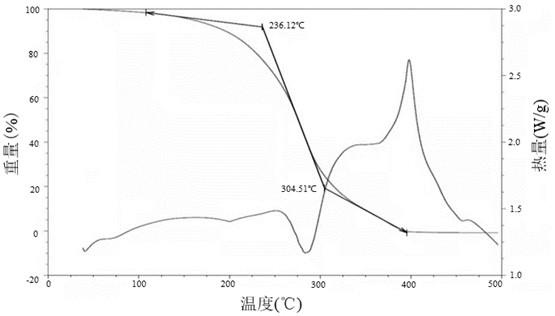
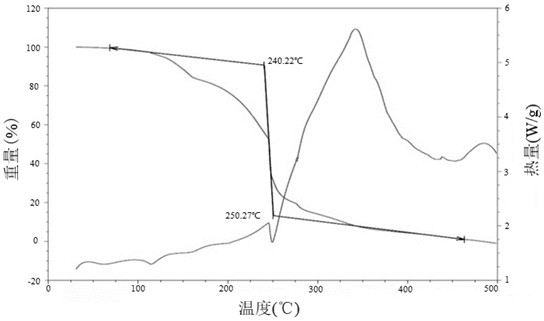
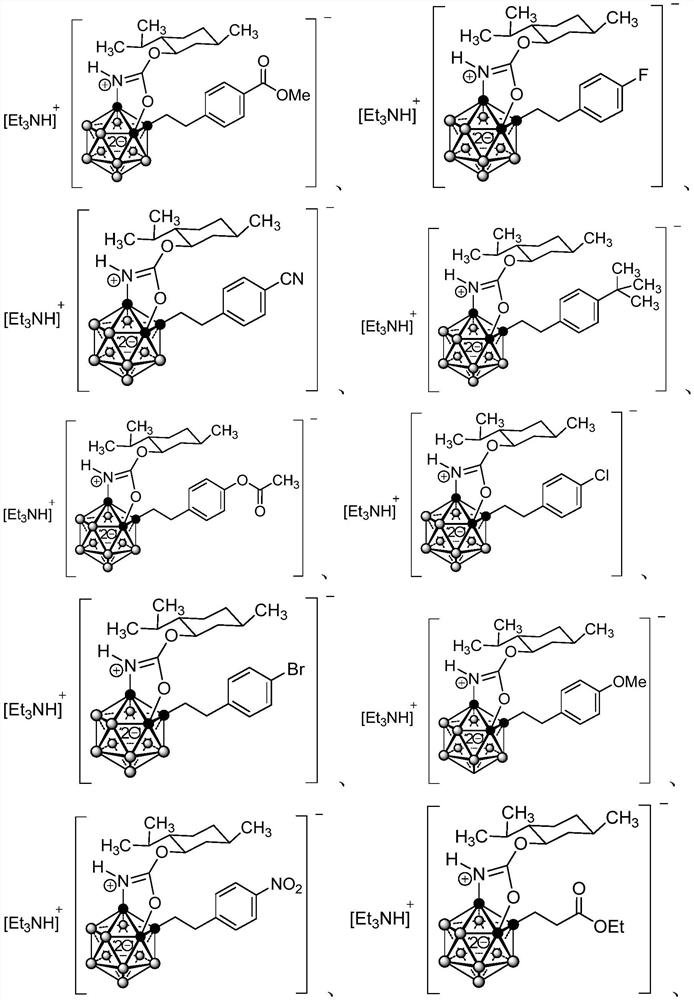
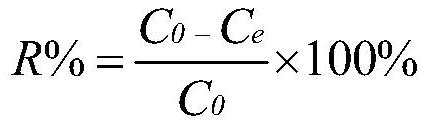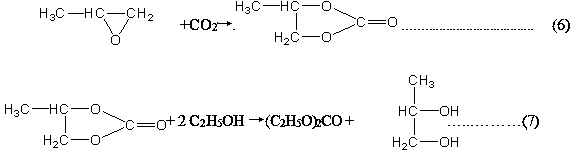Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
33results about How to "Short synthesis path" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
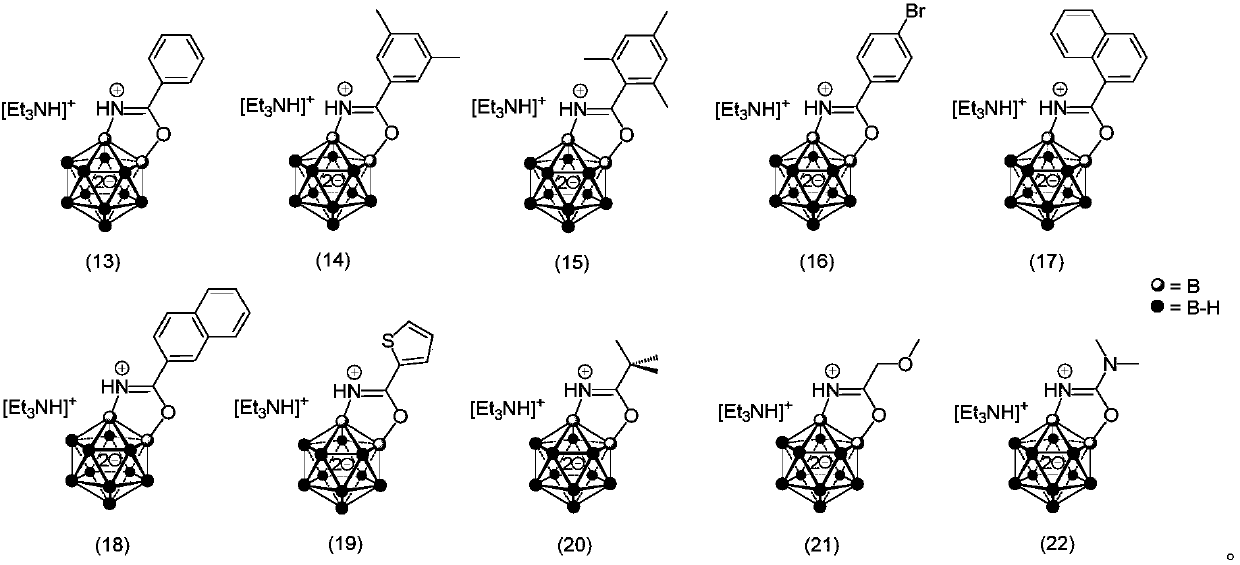
Patent Status
Application Year
Inventor
Nitrogen-containing organic phosphorous acid corrosion inhibitor and synthesis method thereof
The invention discloses a nitrogen-containing organic phosphorous acid corrosion inhibitor and a synthesis method thereof. The structural formula of the nitrogen-containing organic phosphorous acid corrosion inhibitor is shown by the formula I and the formula II; and the synthesis method of this type of compound comprises the following steps that aldehyde / ketone, melamine, hypophosphorous acid and hypophosphite are subjected to Mancini reaction to produce corresponding nitrogen-containing organic phosphonic acid, nitrogen-containing organic phosphonic acid is used as the acid corrosion inhibitor of carbon steel, galvanized steel and stainless steel, the dosage is 20-500 mg / L, the pH value of a corrosive medium is 0-7, the using temperature is 10-280DEG C, and metal materials can be protected effectively. The synthesis method of the nitrogen-containing organic phosphorous acid corrosion inhibitor has the following advantages that raw materials are easy to get, the price is low, the reaction condition is mild, the synthetic path is short, and the affect on environmental ecology is small.
Owner:GUANGDONG UNIV OF PETROCHEMICAL TECH
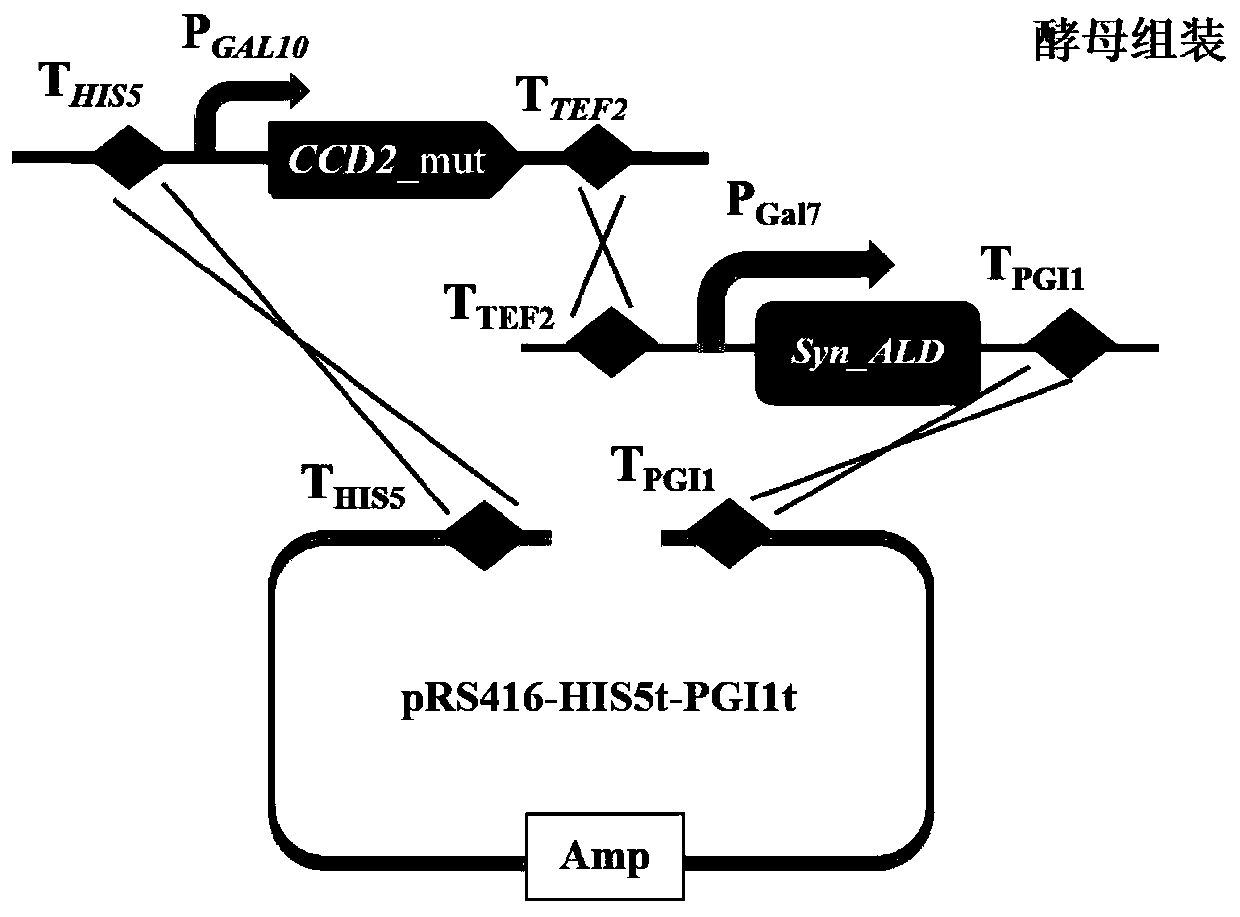
Saffron crocus sourced CCD2 mutant, coding sequence and application thereof, as well as recombinant yeast strain for producing crocetin
ActiveCN109810958AShort synthesis pathHigh catalytic efficiencyFungiMicroorganism based processesBiotechnologyZeaxanthin synthesis
The invention relates to the technical field of genetic engineering, and discloses a saffron crocus sourced CCD2 mutant, a coding sequence and application thereof, as well as a recombinant yeast strain for producing crocetin. The CCD2 mutant is provided with one or two or above of V120F, Y190K / A, R192V / F, E211A, E212A, T290V, K320A and S323F / A / T locus mutations. A rate-limiting enzyme CCD2 in thesynthesis route of the crocetin is transformed, the crocetin is directly synthesized from beta-carotene by widening the substrate spectrum of the CCD2, and the existing synthesis route of the crocetinis simplified and reconstructed, so that the problem that the total yield of multiple reactions is generally low is solved; and meanwhile, the efficiency of the CCD2 catalyzing an original substratezeaxanthin to synthesize the crocetin is improved, and the yeast strain of the high-yield crocetin is further obtained.
Owner:TIANJIN UNIV
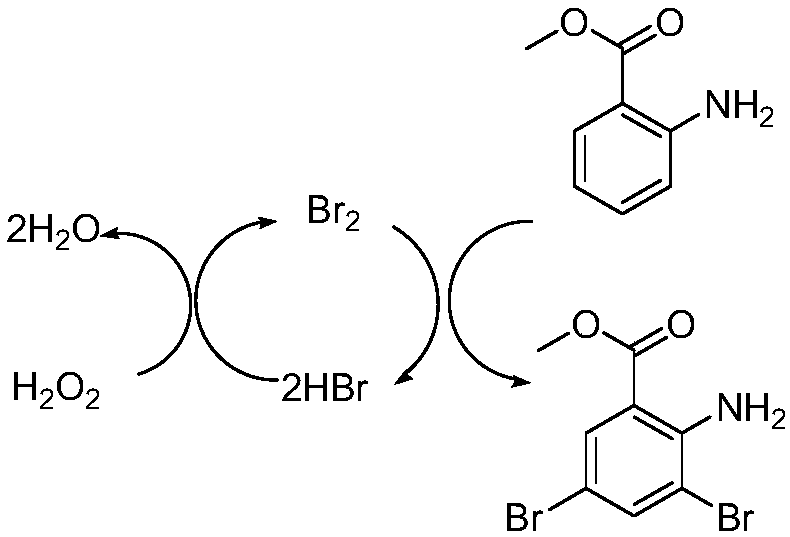
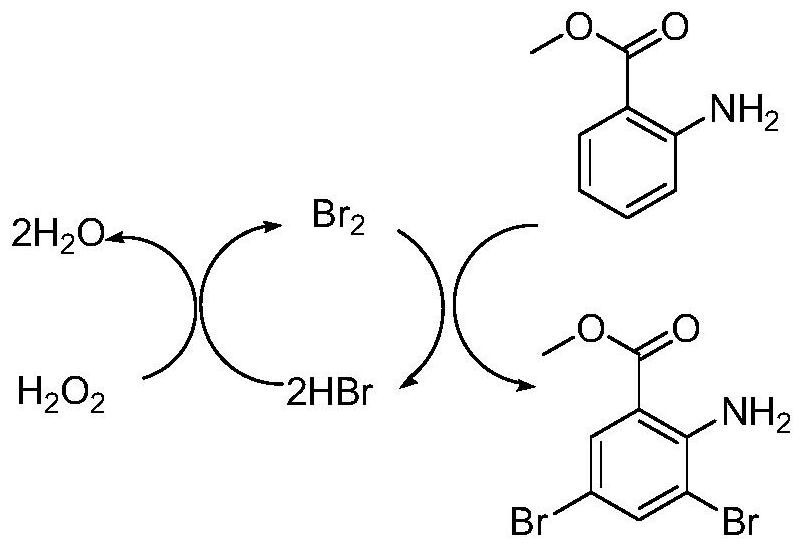
Preparation method for bromhexine hydrochloride
ActiveCN109535010ANo pollution in the processShort synthesis pathOrganic compound preparationAmino-carboxyl compound preparationOrganic synthesisBromhexine hydrochloride
The invention belongs to the field of organic synthesis and provides a preparation method for bromhexine hydrochloride. According to the invention, 2-aminobenzoic ester compounds are taken as raw materials and take part in bromination reaction, reduction reaction, condensation reaction and salt forming reaction, so as to acquire bromhexine hydrochloride. The preparation method has the advantages of short synthesis route, low cost, stable intermediate, no pollution to environment, high purity, high yield, reaching officinal standard and suitability for industrial expanded production.
Owner:GUANGZHOU YIPINHONG PHARMA +5
Borane compound used for antibacterial treatment and preparation method of borane compound
ActiveCN107266486AShort synthesis pathMild reaction conditionsAntibacterial agentsSenses disorderBenzeneAntibacterial activity
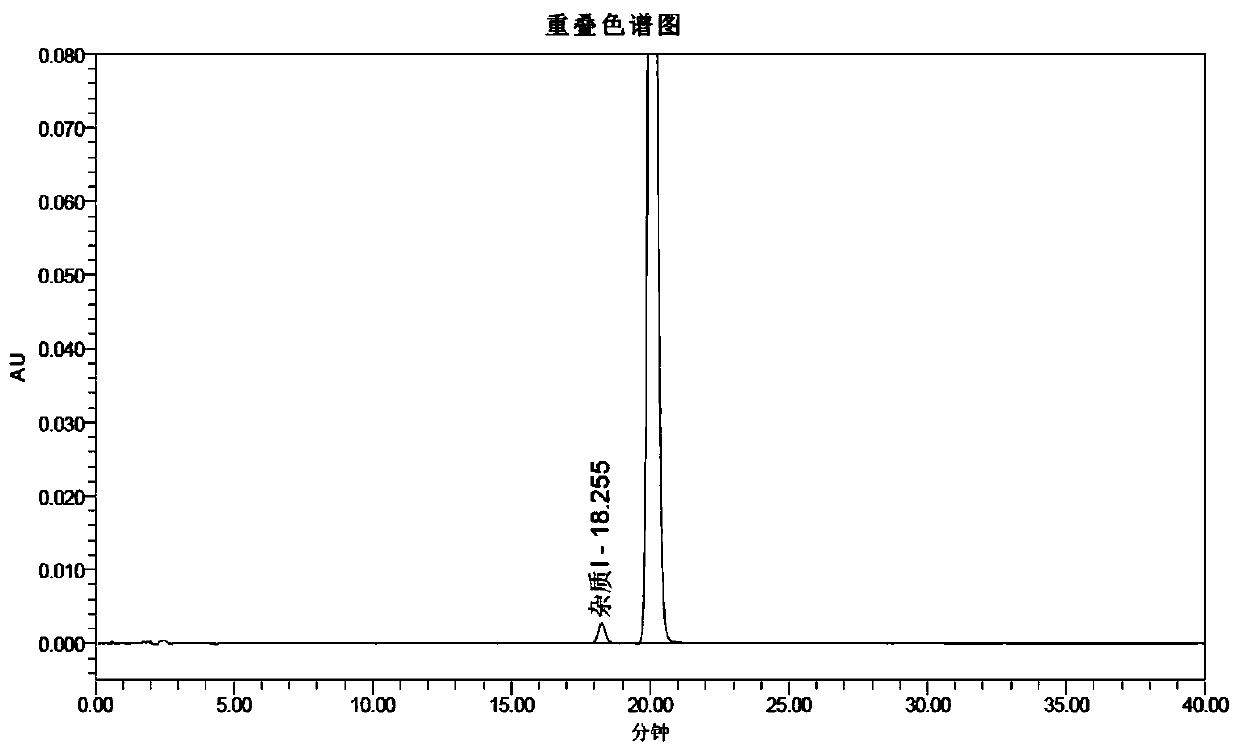
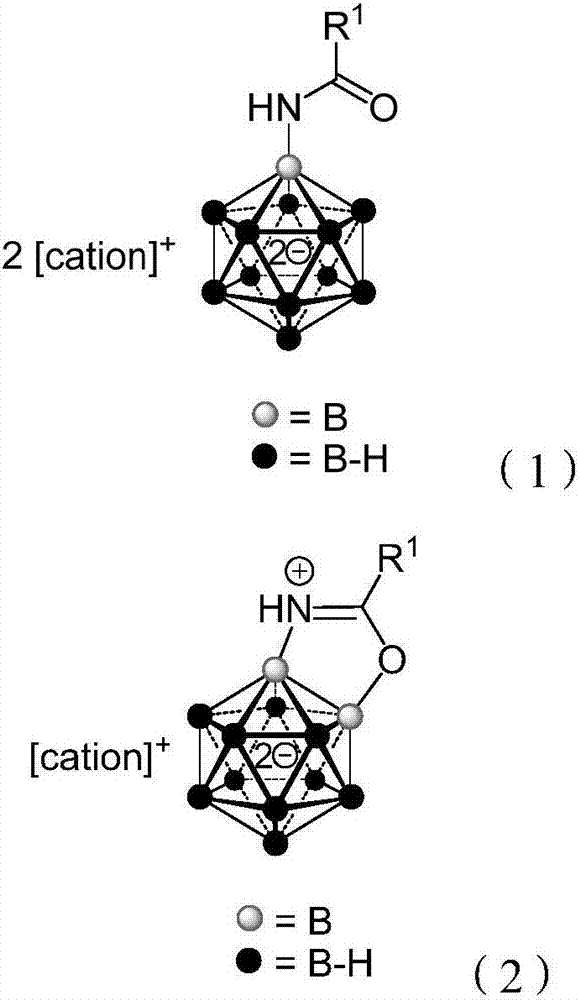
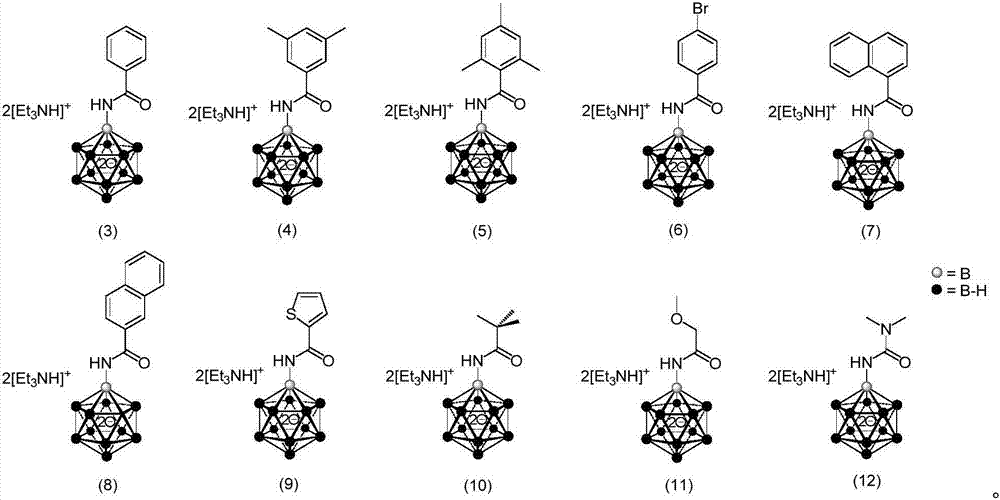
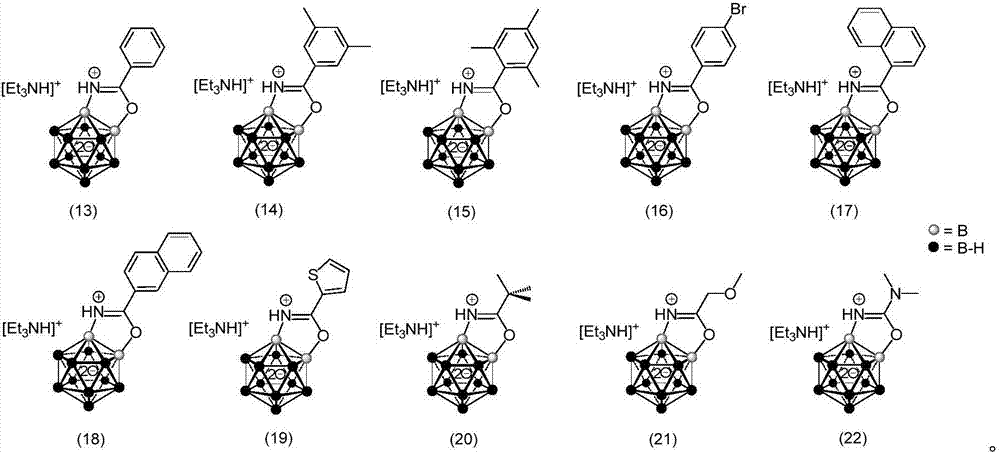
The invention provides a borane compound used for antibacterial treatment. The borane compound is a dodecaborane substituted amide or a dodecaborane-oxazole. The preparation method comprises: (1) taking a mono-aminated dodecaborane as a raw material and performing an acylation reaction with an acyl chloride compound under the effect of sodium hydride to obtain a dodecaborane substituted amide; and (2) performing oxidative coupling on the dodecaborane substituted amide obtained in the step (1) under the effect of (diacetoxyiodo)benzene to obtain a dodecaborane-oxazole. The invention also provides an application of the borane compound in preparing antibacterial agents. The borane compound has extremely high antibacterial activity on certain Gram negative bacteria, and has a huge prospect.
Owner:ZHEJIANG UNIV
Straw-based amphoteric dye adsorbent as well as preparation method and application thereof
InactiveCN112934188AShort selectivityShort adsorption timeOther chemical processesWater contaminantsCelluloseEthyl ester
The invention relates to the technical field of dye adsorbents, in particular to a straw-based amphoteric dye adsorbent as well as a preparation method and an application thereof. The preparation method comprises the following steps: by taking straw as a polymer skeleton, firstly reacting with haloacetic acid in an ethanol-water mixed system, grafting carboxyl on a cellulose molecular chain of the straw to prepare carboxymethyl straw cellulose, then carrying out graft copolymerization on the carboxymethyl straw cellulose and dialkylaminoethyl methacrylate, and grafting tertiary amino on a cellulose molecular chain of the straw, and forming the straw-based amphoteric adsorbent with both anions and cations. The method is simple to operate, mild in reaction condition and short in synthesis path, the used main raw material is waste straw, and the cost is low, so that the obtained product has good environmental friendliness, can be used as an adsorbent for treating anionic dyes and cationic dyes, has the characteristics of strong adsorption capacity, high decolorization rate, good selectivity, short adsorption time, wide application range and the like, is beneficial to industrial practical application, and has important significance in the field of environmental protection.
Owner:QINGDAO TECHNOLOGICAL UNIVERSITY
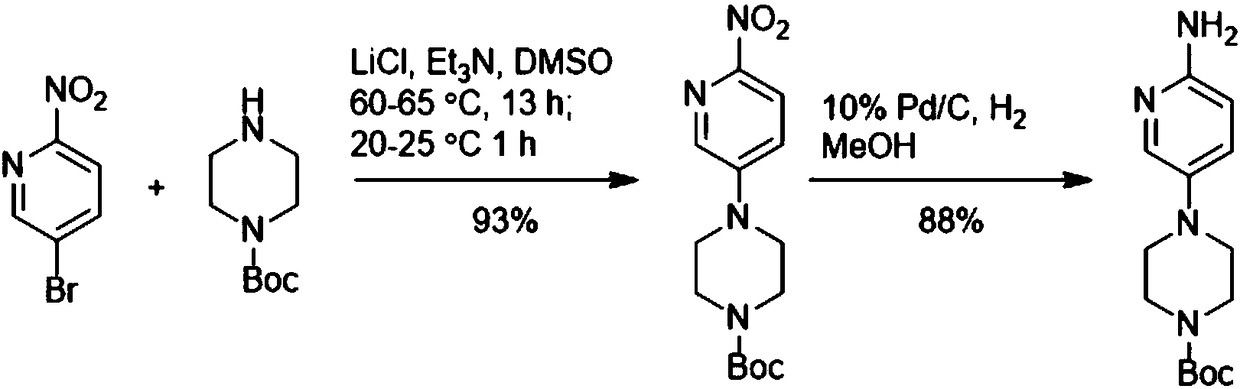
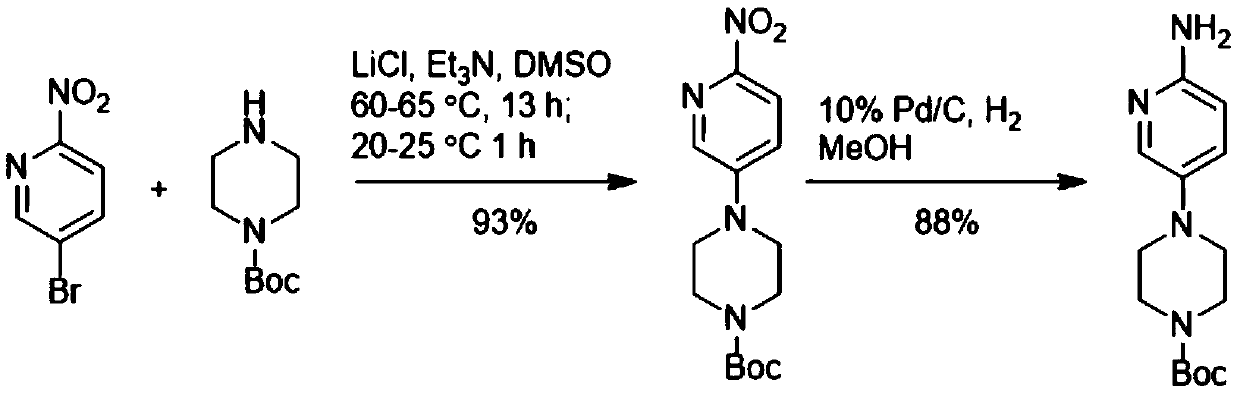
Method for preparing 4-(6-aminopyridin-3-yl) piperazine-1-carboxylate tert-butyl ester
The invention discloses a method for preparing 4-(6-aminopyridin-3-yl) piperazine-1-carboxylate tert-butyl ester. The method for preparing 4-(6-aminopyridin-3-yl) piperazine-1-carboxylate tert-butyl ester comprises the following steps: 2-aminopyridin, piperazine-1-formate tert-butyl ester and NSP-SA-NHS photo catalyst is added to the solvent, and the light reaction is carried out under the condition of the presence of an oxidizing agent to generate 4-(6-aminopyridin-3-yl) piperazine-1-carboxylate tert-butyl ester. According to the method, 4-(6-aminopyridin-3-yl) piperazine-1-carboxylate tert-butyl ester is synthesized by one step. On the one hand, the synthetic route of 4-(6-aminopyridin-3-yl) piperazine-1-carboxylate tert-butyl ester is effectively shortened, the generation of by-productsis effectively reduced and the yield of the target product is improved. On the other hand, only photo catalyst and oxidizing agent are used, so that the preparation method is safe and environment-friendly and low in cost.
Owner:深圳蓝新科技有限公司
Novel method for catalytically synthesizing acetanilide
InactiveCN107935877AEasy to prepareShort synthesis pathOrganic compound preparationCarboxylic acid amides preparationAcetic acidBoric acid
The invention provides a novel method for catalytically synthesizing acetanilide and relates to the technical field of preparation of the acetanilide. The novel method for catalytically synthesizing the acetanilide comprises the following step: enabling aniline and glacial acetic acid to react under the action of a catalyst, wherein the catalyst is a mixture of aluminum borate and manganese dioxide and the weight ratio of the aluminum borate to the manganese dioxide is (2 to 4) to 1. By adopting the novel method for catalytically synthesizing the acetanilide, the blank of a preparation methodfor catalytically synthesizing the acetanilide is filled; the preparation efficiency of the acetanilide is effectively improved, so that the yield of the acetanilide is improved; the novel method hasrelatively great industrial popularization value.
Owner:新乡市锦源化工有限公司
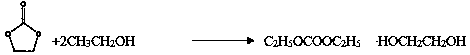
Production process of high-purity diethyl carbonate through direct catalysis of solid base catalyst
InactiveCN109369400ASimple processHigh selectivityOrganic compound preparationPreparation from organic carbonatesReactive distillationCatalytic distillation
The invention discloses a production process of high-purity diethyl carbonate through direct catalysis of a solid base catalyst and relates to a production process of chemical raw materials. The process disclosed by the invention comprises the following steps: performing atmospheric distillation on tower top liquid to obtain a high-purity diethyl carbonate solution by virtue of simple distillation, performing reduced pressure separation on tower bottoms to obtain lots of ethylene glycol, a tiny amount of diethyl carbonate and a small amount of ethylene carbonate, and directly taking the ethanol and ethylene carbonate as raw materials to circulate to a first reactive distillation column. A process route for synthesizing ethylene carbonate by taking ethylene carbonate and ethanol as raw materials is created. A technology of directly catalyzing the ethylene carbonate and ethanol to be simply separated in a catalytic distillation column by virtue of a multifunctional composite basic material is realized. The one-step production process for synthesizing diethyl carbonate is short in synthetic route, simple in process flow and high in product selectivity and yield, and is capable of effectively producing diethyl carbonate compared with the conventional process.
Owner:SHENYANG INSTITUTE OF CHEMICAL TECHNOLOGY
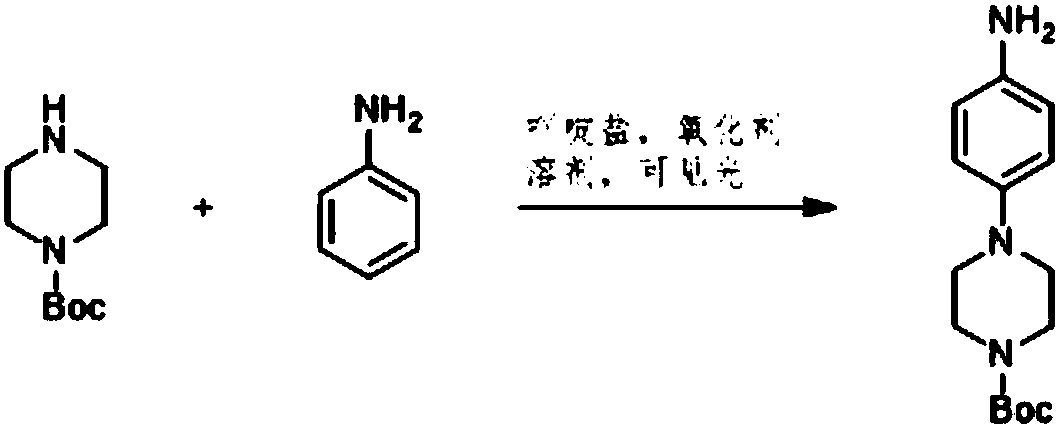
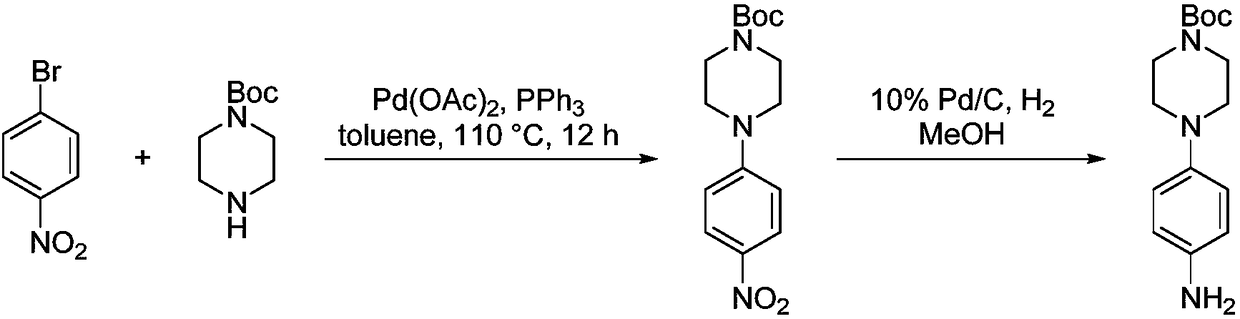
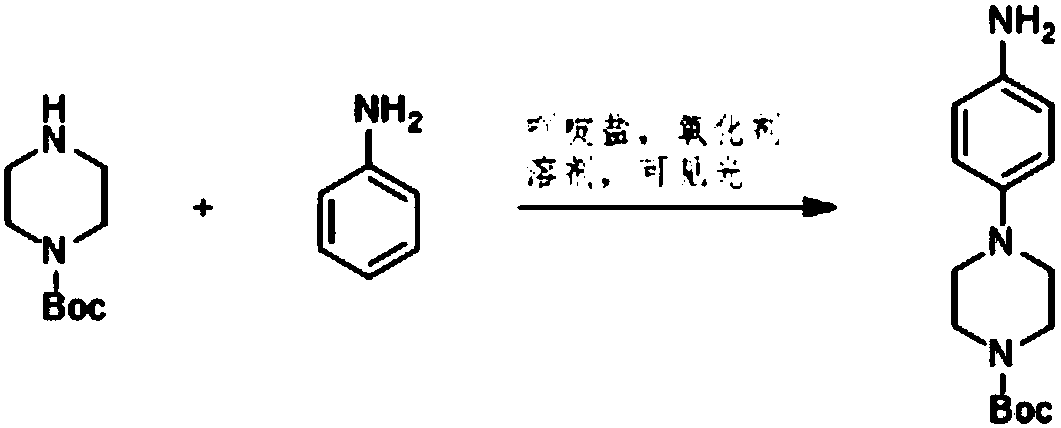
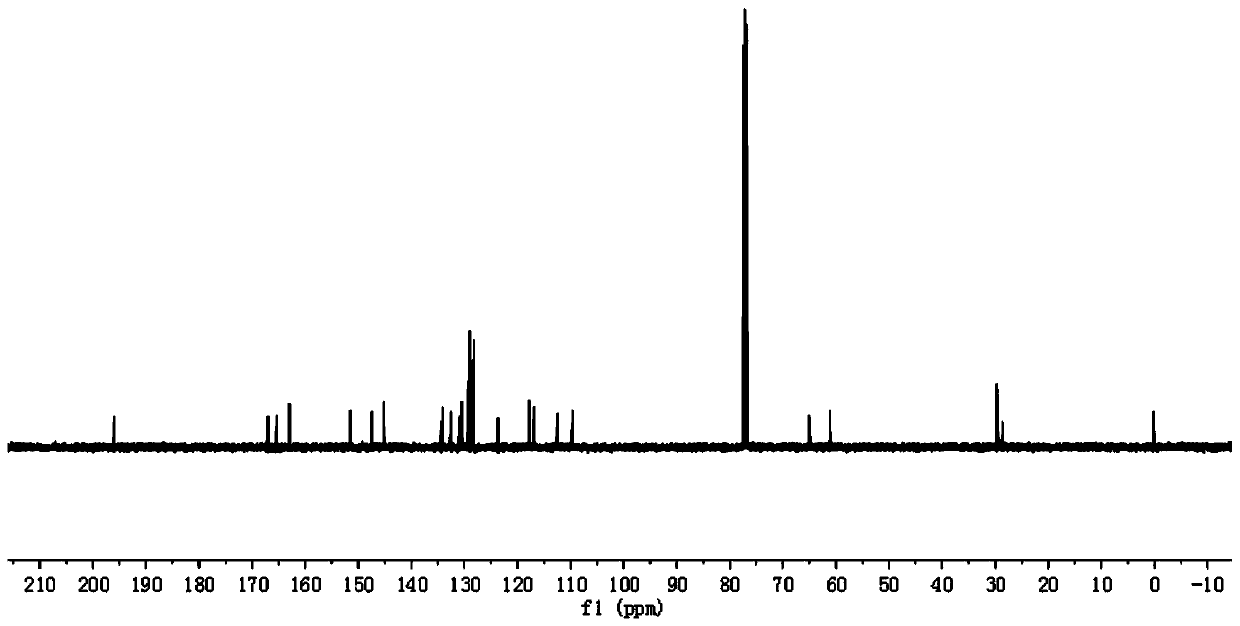
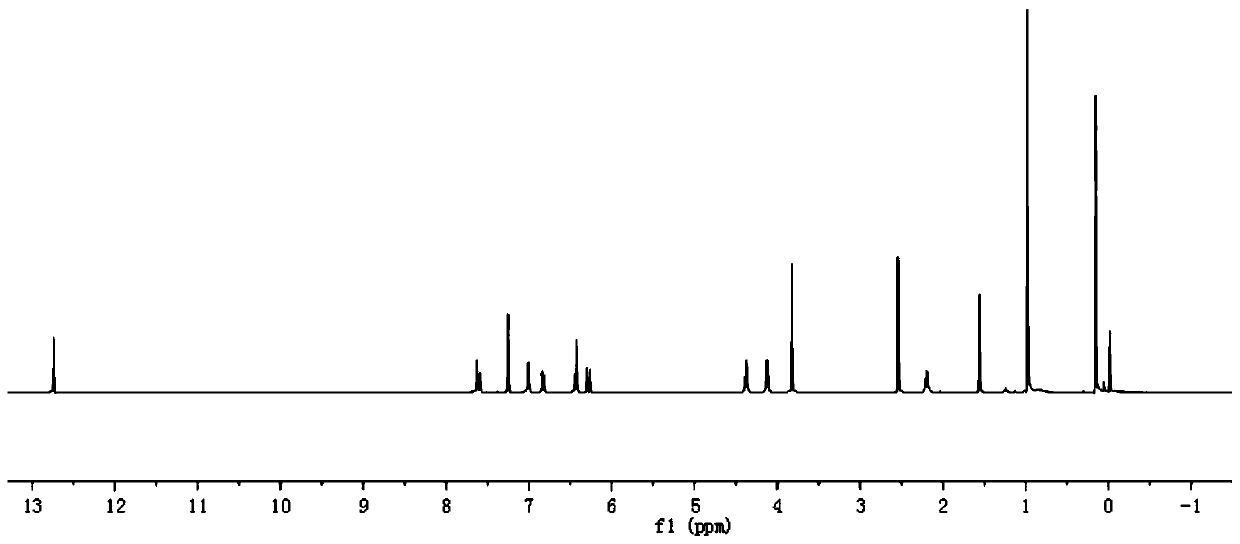
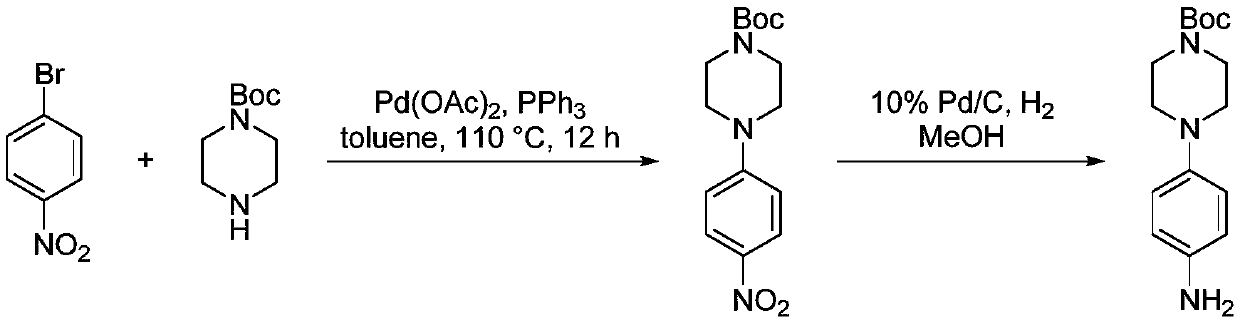
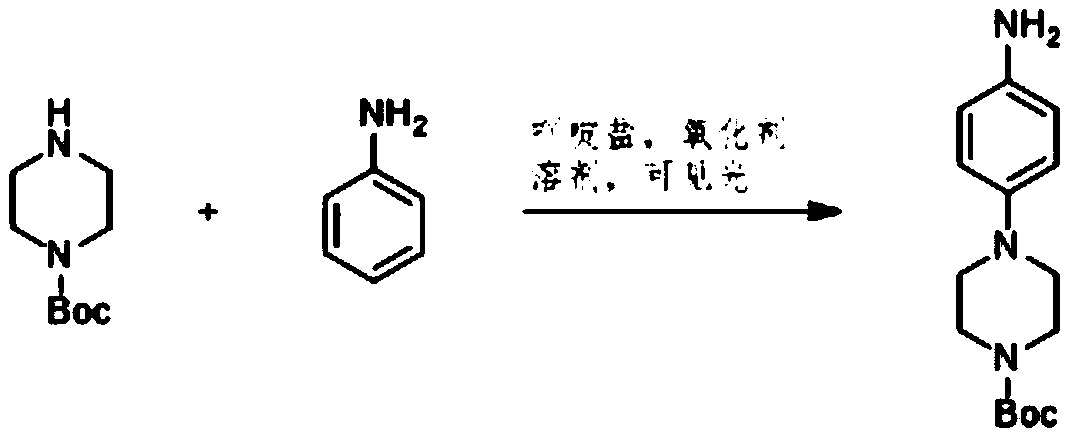
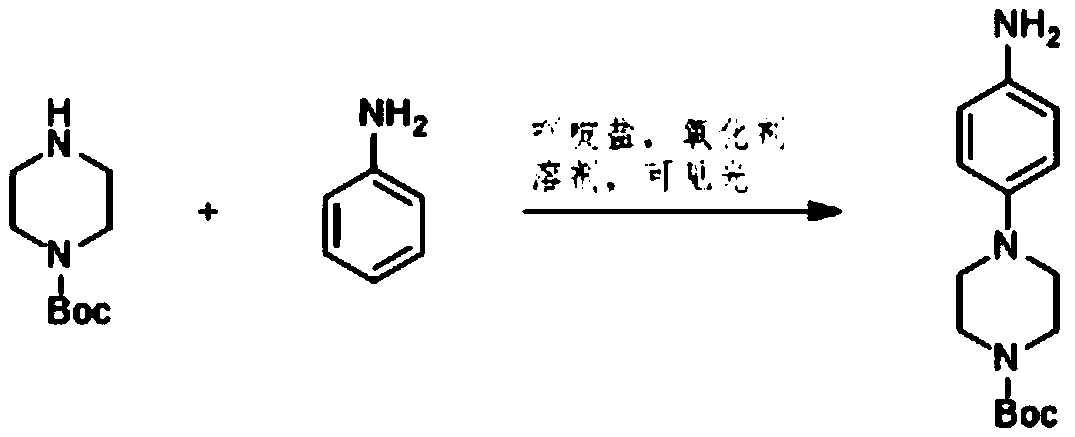
Preparation method of 4-(tert-butyl 1-piperazinecarboxylate-4-yl)aniline
ActiveCN108440451AShort synthesis pathReduce generationOrganic chemistryAcridineTert-Butyloxycarbonyl protecting group
The invention discloses a preparation method of 4-(tert-butyl 1-piperazinecarboxylate-4-yl)aniline. The preparation method of the 4-(tert-butyl 1-piperazinecarboxylate-4-yl)aniline comprises the following steps that aniline, piperazine-1-tert-butyl formate and acridine salt photocatalysts are added into a solvent; illumination reaction is performed under the condition of oxidizing agent existence;the 4-(tert-butyl 1-piperazinecarboxylate-4-yl)aniline is generated. The preparation method of the 4-(tert-butyl 1-piperazinecarboxylate-4-yl)aniline has the advantages that the 4-(tert-butyl 1-piperazinecarboxylate-4-yl)aniline is synthesized in one step; on one hand, the synthesis path of the 4-(tert-butyl 1-piperazinecarboxylate-4-yl)aniline is effectively shortened; the generation of by-products is effectively reduced; the target product yield is improved; on the other hand, only photocatalysts and oxidizing agents are used, so that the preparation method is safe and achieves an environment-friendly effect; the cost is low.
Owner:深圳蓝新科技有限公司
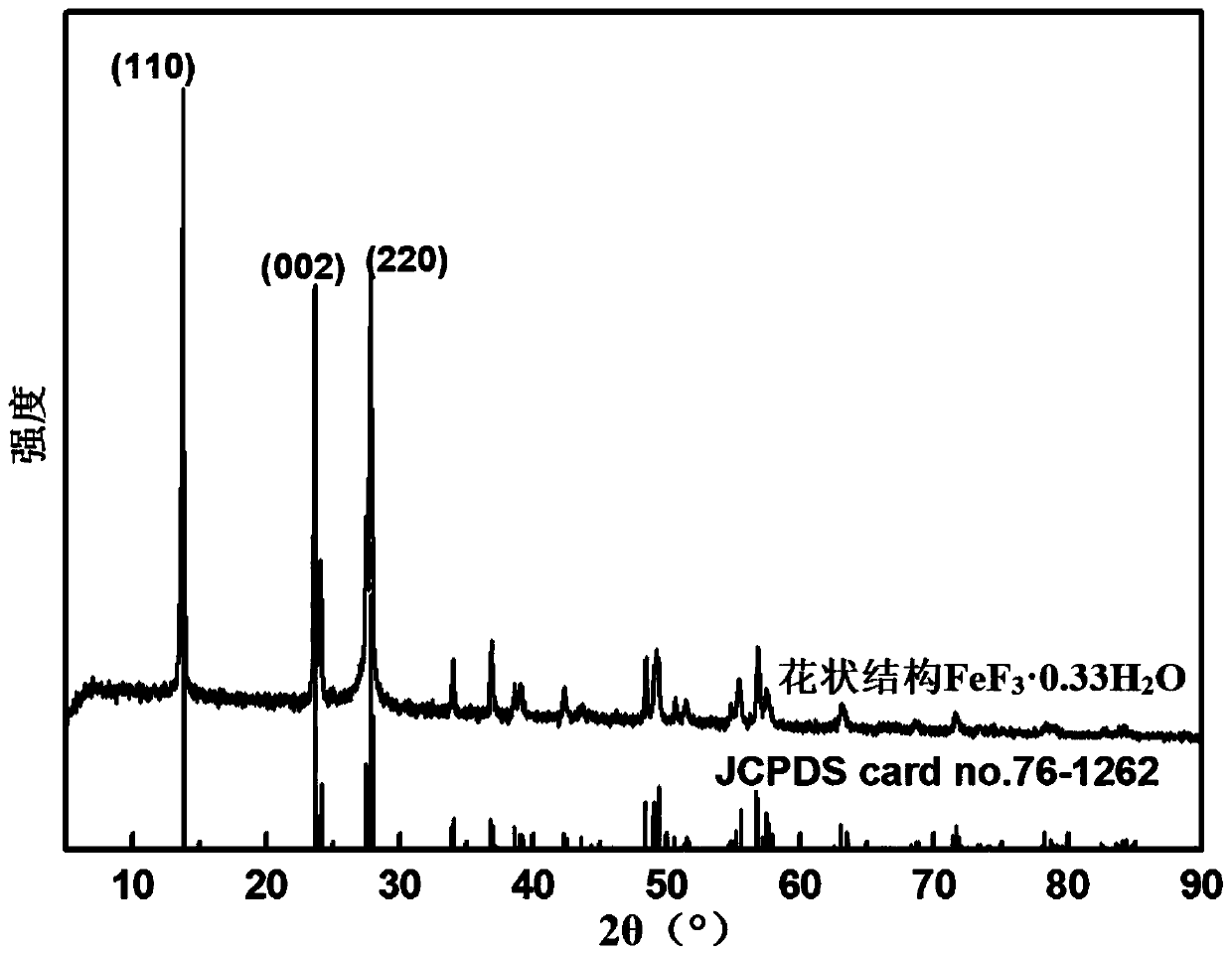
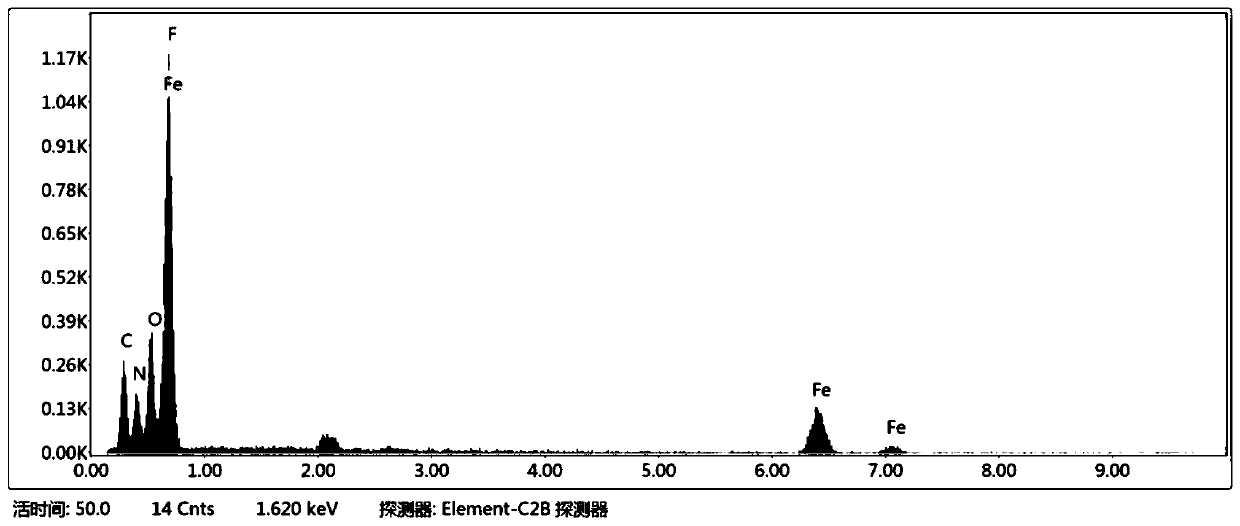
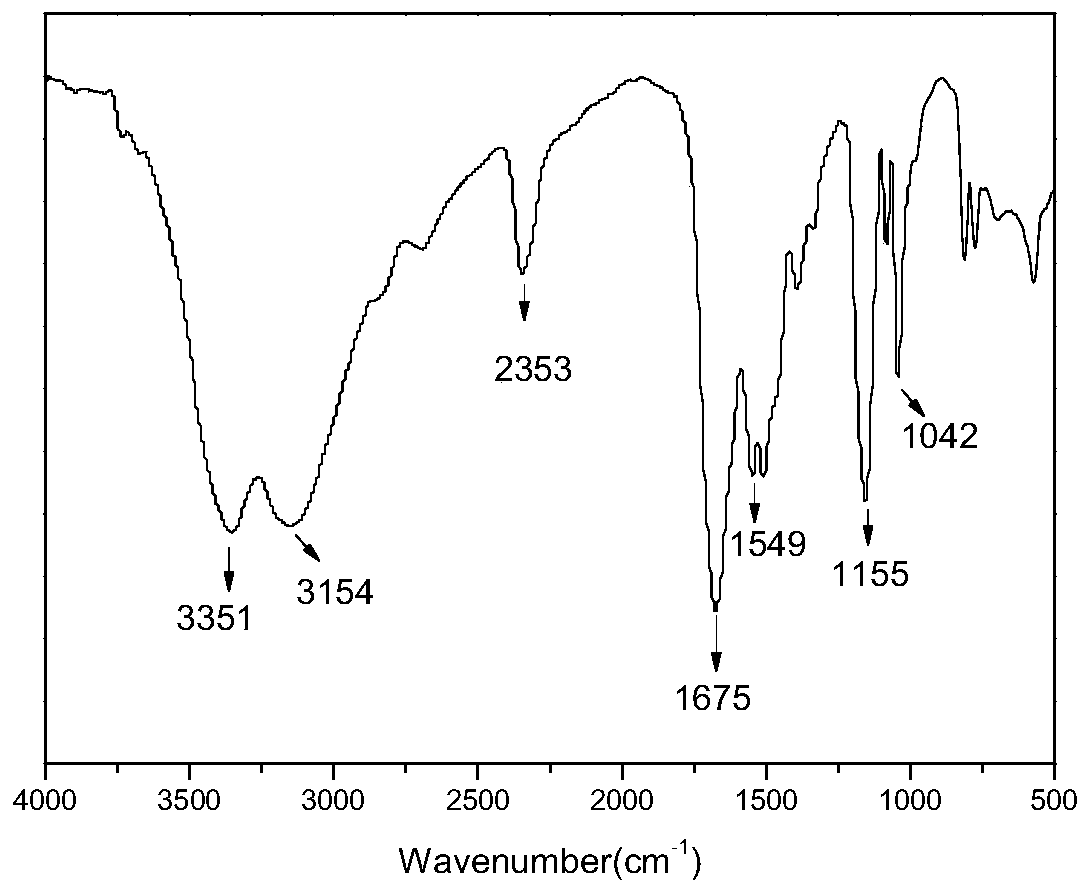
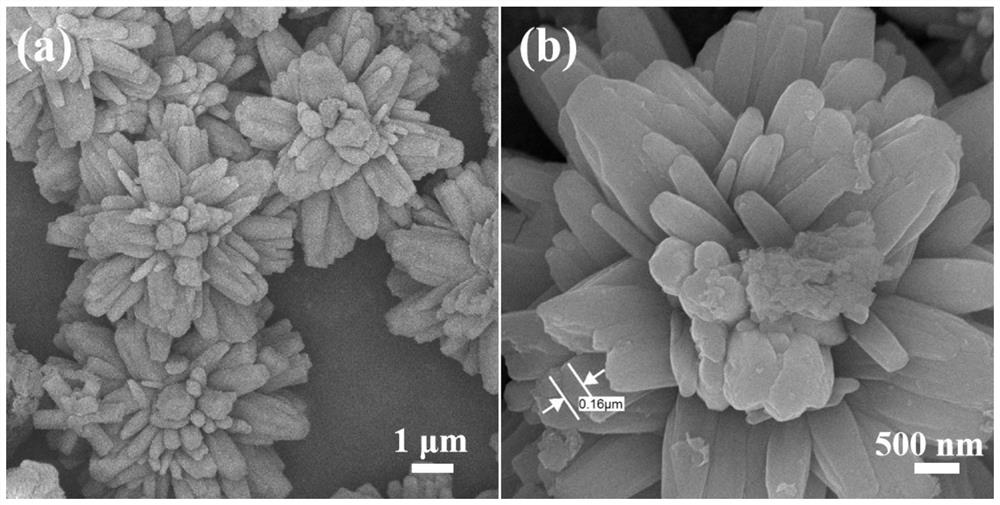
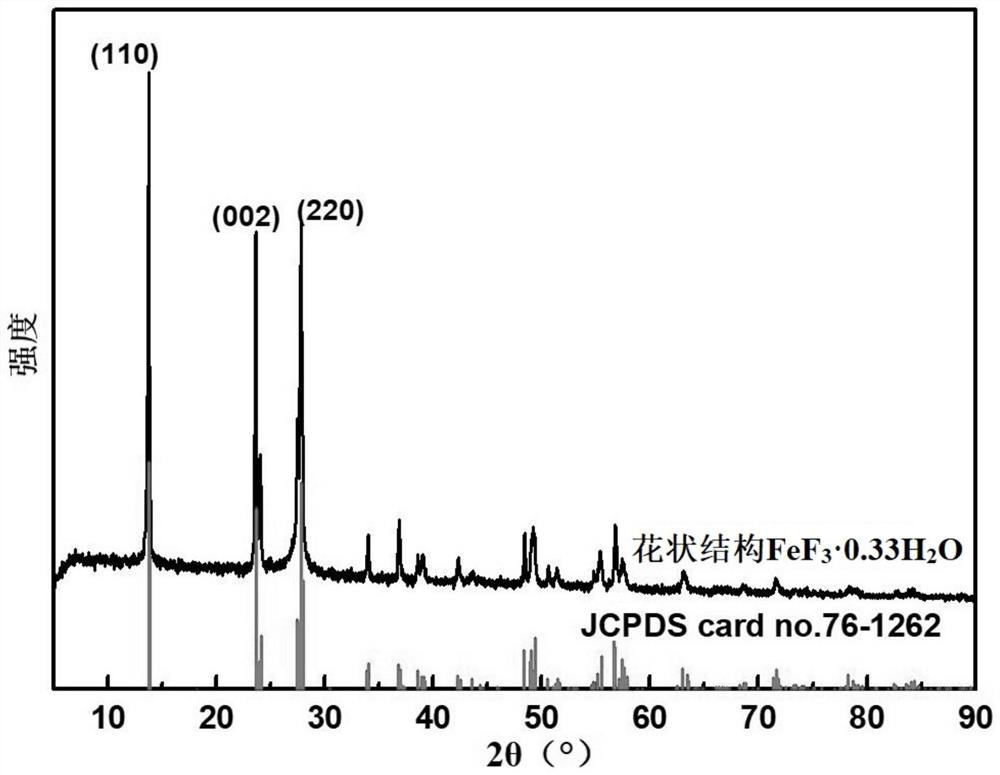
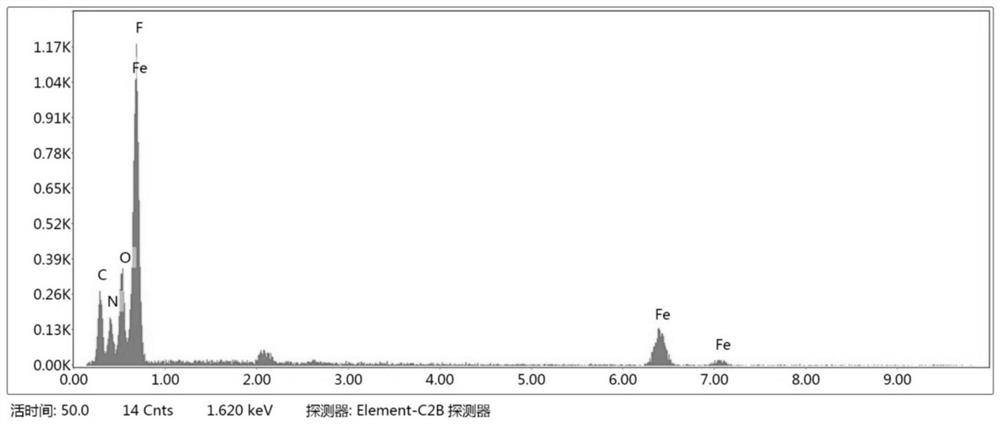
Method for preparing floriform metal fluoride nano material
The invention discloses a method for preparing a floriform metal fluoride nano material. The method comprises the following steps: 1) calcining a carbon nitride compound so as to obtain g-C3N4; 2) performing ultrasonic dispersion on g-C3N4 into an alcohol solvent so as to obtain a g-C3N4 dispersion; 3) adding a metal salt into the g-C3N4 dispersion, performing uniform mixing, further adding hydrofluoric acid, and performing uniform mixing so as to obtain a mixed solution; and 4) adding the mixed solution into a reaction kettle, performing a solvothermal reaction, further filtering the reactionliquid, and performing washing and drying on a solid obtained through filtration. The method disclosed by the invention can be adopted to prepare the floriform metal fluoride nano material and is cheap in raw material, easy in raw material obtaining, short in synthesis route, short in time, simple in operation and low in production cost, and in addition, the prepared floriform metal fluoride nanomaterial is small in particle size and thus is large in specific surface area, rich in active site and good in electrochemical activity and catalysis activity.
Owner:SOUTH CHINA NORMAL UNIVERSITY
Nitrogen-containing organic phosphorous acid corrosion inhibitor and synthesis method
The invention discloses a nitrogen-containing organic phosphorous acid corrosion inhibitor and a synthesis method thereof. The structural formula of the nitrogen-containing organic phosphorous acid corrosion inhibitor is shown by the formula I and the formula II; and the synthesis method of this type of compound comprises the following steps that aldehyde / ketone, melamine, hypophosphorous acid and hypophosphite are subjected to Mancini reaction to produce corresponding nitrogen-containing organic phosphonic acid, nitrogen-containing organic phosphonic acid is used as the acid corrosion inhibitor of carbon steel, galvanized steel and stainless steel, the dosage is 20-500 mg / L, the pH value of a corrosive medium is 0-7, the using temperature is 10-280DEG C, and metal materials can be protected effectively. The synthesis method of the nitrogen-containing organic phosphorous acid corrosion inhibitor has the following advantages that raw materials are easy to get, the price is low, the reaction condition is mild, the synthetic path is short, and the affect on environmental ecology is small.
Owner:GUANGDONG UNIV OF PETROCHEMICAL TECH
A front silver paste for solar cells with low contact resistance and high photoelectric conversion efficiency and preparation method thereof
ActiveCN114664475BImprove adhesionReduce contact resistanceOrganic compound preparationCarboxylic acid esters preparationSilicon solar cellMaterials science
A front silver paste for solar cells with low contact resistance and high photoelectric conversion efficiency and a preparation method thereof, and the technical field relates to a front silver paste for solar cells and a preparation method thereof. The purpose of the present invention is to solve the problem that the binder and thixotropic agent in the organic carrier used in the preparation of crystalline silicon solar cell conductive paste are more decomposed and residual ash after the cell is sintered, and the solvent is easy to precipitate, which eventually leads to the prepared solar cell. The problem of low sheet tension level and low photoelectric conversion efficiency of solar cells. In a front silver paste for solar cells with low contact resistance and high photoelectric conversion efficiency, the binder is poly5-hydroxy-2-ethyl pentenoate, and the thixotropic agent is pentaerythritol tetrakis(9-amino-10-hydroxyl) octadecanoic acid) ester, and the dispersant is poly-5-(N,N-dimethylamino)-1,4-pentanediol monotriethylamine ether. The present invention can obtain a front silver paste for solar cells with low contact resistance and high photoelectric conversion efficiency.
Owner:江苏聚盈新材料科技有限公司
Front silver paste for solar cell with low contact resistance and high photoelectric conversion efficiency and preparation method of front silver paste
ActiveCN114664475AGood bonding performanceLess residual sintering ashOrganic compound preparationCarboxylic acid esters preparationCrystalline siliconSilver paste
The invention discloses front silver paste for a solar cell with low contact resistance and high photoelectric conversion efficiency and a preparation method of the front silver paste, and relates to the technical field of front silver paste for a solar cell and a preparation method of the front silver paste. The invention aims to solve the problems of low tension level of a prepared solar cell and low photoelectric conversion efficiency of the solar cell due to the fact that a binder and a thixotropic agent in an organic carrier used for preparing the conductive paste of the crystalline silicon solar cell in the prior art are more in decomposed residual ash content after the cell is sintered and a solvent is easy to separate out. In the front silver paste for the solar cell with low contact resistance and high photoelectric conversion efficiency, a binding agent is poly (5-hydroxy-2-ethyl pentenoate), a thixotropic agent is pentaerythritol tetra (9-amino-10-hydroxy octadecanoic acid) ester, and a dispersing agent is poly (5-(N, N-dimethylamino)-1, 4-pentanediol monotriethylamine ether. The front silver paste for the solar cell, which is low in contact resistance and high in photoelectric conversion efficiency, can be obtained.
Owner:江苏聚盈新材料科技有限公司
Cinnamate derivatives and application of cinnamate derivatives as tyrosinase inhibitors and gels
PendingCN111440068AStrong inhibitory activityShort synthesis pathCosmetic preparationsGroup 4/14 element organic compoundsCinnamatesPerylene derivatives
The invention discloses cinnamate derivatives and application of the cinnamate derivatives as tyrosinase inhibitors and gels, and the structural general formula of the cinnamate derivatives is shown in the specification, in the formula, X and Y independently represent any one of O, S and NH, wherein R1 and R2 independently represent any one of H, OH, methoxy, allyl and acetyl; R3 and R4 respectively and independently represent any one of H, OH, methoxy, tert-butyl dimethyl siloxy and carbethoxy, and R3 and R4 are not tert-butyl dimethyl silyl at the same time; and n is an integer from 2 to 5.The cinnamate derivatives disclosed by the invention have obvious inhibitory activity on the activity of mushroom tyrosinase diphenolic enzyme and the content of tyrosinase and melanin in melanoma cells of B16F10 mice, and can be used for preparing the tyrosinase inhibitors. Meanwhile, the cinnamate derivatives can form stable gels in olive oil and can be used as micromolecular gels for cosmeticsand the like.
Owner:SHAANXI UNIV OF CHINESE MEDICINE
The preparation method of 4-(1-tert-butoxycarbonylpiperazin-4-yl)aniline
ActiveCN108440451BShort synthesis pathReduce generationOrganic chemistryAcridineTert-Butyloxycarbonyl protecting group
The invention discloses a preparation method of 4-(tert-butyl 1-piperazinecarboxylate-4-yl)aniline. The preparation method of the 4-(tert-butyl 1-piperazinecarboxylate-4-yl)aniline comprises the following steps that aniline, piperazine-1-tert-butyl formate and acridine salt photocatalysts are added into a solvent; illumination reaction is performed under the condition of oxidizing agent existence;the 4-(tert-butyl 1-piperazinecarboxylate-4-yl)aniline is generated. The preparation method of the 4-(tert-butyl 1-piperazinecarboxylate-4-yl)aniline has the advantages that the 4-(tert-butyl 1-piperazinecarboxylate-4-yl)aniline is synthesized in one step; on one hand, the synthesis path of the 4-(tert-butyl 1-piperazinecarboxylate-4-yl)aniline is effectively shortened; the generation of by-products is effectively reduced; the target product yield is improved; on the other hand, only photocatalysts and oxidizing agents are used, so that the preparation method is safe and achieves an environment-friendly effect; the cost is low.
Owner:深圳蓝新科技有限公司
A kind of preparation method of flower-like metal fluoride nanomaterial
ActiveCN110589771BShort synthesis pathShort timeFluoride preparationNickel halidesElectrochemistrySolvothermal reaction
The invention discloses a method for preparing a floriform metal fluoride nano material. The method comprises the following steps: 1) calcining a carbon nitride compound so as to obtain g-C3N4; 2) performing ultrasonic dispersion on g-C3N4 into an alcohol solvent so as to obtain a g-C3N4 dispersion; 3) adding a metal salt into the g-C3N4 dispersion, performing uniform mixing, further adding hydrofluoric acid, and performing uniform mixing so as to obtain a mixed solution; and 4) adding the mixed solution into a reaction kettle, performing a solvothermal reaction, further filtering the reactionliquid, and performing washing and drying on a solid obtained through filtration. The method disclosed by the invention can be adopted to prepare the floriform metal fluoride nano material and is cheap in raw material, easy in raw material obtaining, short in synthesis route, short in time, simple in operation and low in production cost, and in addition, the prepared floriform metal fluoride nanomaterial is small in particle size and thus is large in specific surface area, rich in active site and good in electrochemical activity and catalysis activity.
Owner:SOUTH CHINA NORMAL UNIVERSITY
A kind of preparation method of fluorescent silicon nanoparticle
ActiveCN106479487BShort synthesis pathOperational securityMethine/polymethine dyesNanoopticsQuantum yieldSilica nanoparticles
Owner:SUZHOU UNIV
Borane compound that can be used for antibacterial therapy and its preparation method
ActiveCN107266486BShort synthesis pathMild reaction conditionsAntibacterial agentsSenses disorderBenzeneAntibacterial activity
The invention provides a borane compound used for antibacterial treatment. The borane compound is a dodecaborane substituted amide or a dodecaborane-oxazole. The preparation method comprises: (1) taking a mono-aminated dodecaborane as a raw material and performing an acylation reaction with an acyl chloride compound under the effect of sodium hydride to obtain a dodecaborane substituted amide; and (2) performing oxidative coupling on the dodecaborane substituted amide obtained in the step (1) under the effect of (diacetoxyiodo)benzene to obtain a dodecaborane-oxazole. The invention also provides an application of the borane compound in preparing antibacterial agents. The borane compound has extremely high antibacterial activity on certain Gram negative bacteria, and has a huge prospect.
Owner:ZHEJIANG UNIV
A kind of preparation method of bromhexine hydrochloride
ActiveCN109535010BNo pollution in the processShort synthesis pathOrganic compound preparationAmino-carboxyl compound preparationBenzoic acidOrganic synthesis
The invention belongs to the field of organic synthesis and provides a preparation method of bromhexine hydrochloride. The present invention uses 2-aminobenzoate compounds as raw materials to obtain bromhexine hydrochloride after bromination reaction, reduction reaction, condensation reaction and salt-forming reaction, the synthetic route is short, the cost is low, the intermediate is stable, and it is environmentally friendly Pollution, high purity, high yield, in line with pharmaceutical standards, suitable for industrial expansion of production.
Owner:GUANGZHOU YIPINHONG PHARMA +5
Method for preparation of fluorescent silica nanoparticles
ActiveCN106479487AShort synthesis pathOperational securityMethine/polymethine dyesNanoopticsQuantum yieldSilica nanoparticles
The invention provides a method for preparation of fluorescent silica nanoparticles. The method includes: a), mixing indocyanine type fluorescent dye with an organic silicon source compound to obtain a precursor solution; b), allowing the precursor solution to react under constant temperature to obtain the fluorescent silica nanoparticles. The water-soluble fluorescent silica nanopartricles are prepared with the one-step method which is performed completely in water phase, so that the method is safe in operation, rapid and convenient, low in toxicity and safe and easy in obtaining raw materials; the water-soluble fluorescent silica nanoparticles are quite good in monodispersity, high in fluorescent property and quantum yield, good in stability, good in water solubility, capable of serving as fluorescence indicators widely applied to biological detection and analysis and hopeful to be used for optical related industrial production such as laser and organic light emitting diodes.
Owner:SUZHOU UNIV
Homogeneous phase ionic liquid catalyzed high-purity diethyl carbonate synthesizing technology
InactiveCN109456189AHigh selectivityHigh yieldOrganic compound preparationChemical industryAlcoholCatalytic distillation
The invention discloses a homogeneous phase ionic liquid catalyzed high-purity diethyl carbonate synthesizing technology, and relates to a catalyzed synthesizing technology. The technology is a catalytic distillation technology for preparing high-purity diethyl carbonate ionic liquid for a lithium battery through ethylene carbonate and alcohol ester exchange by repeatedly using a homogeneous phasecatalyst. In a reaction, a crude product of the initial reaction is a small amount of ethylene carbonate, ethyl alcohol, ethylene glycol, diethyl carbonate and ionic liquid, and a large amount of theethyl alcohol, the ethylene carbonate and the ionic liquid can be recovered and recycled for reproducing diethyl carbonate after the reaction is completed, finally the high-purity diethyl carbonate is obtained; in a reaction process, ethylene glycol is further generated, and the ethylene glycol is bulk chemicals, and can be used as a product for directly separating. In addition, the used catalystcan be repeatedly used, green, pollution-free, and high in catalytic activity. The production technology for diethyl carbonate one-step synthesis is short in synthetic route, simple in technologicalprocess, high in product selectivity and yield, and capable of more effectively producing the diethyl carbonate relative to an existing technology.
Owner:SHENYANG INSTITUTE OF CHEMICAL TECHNOLOGY
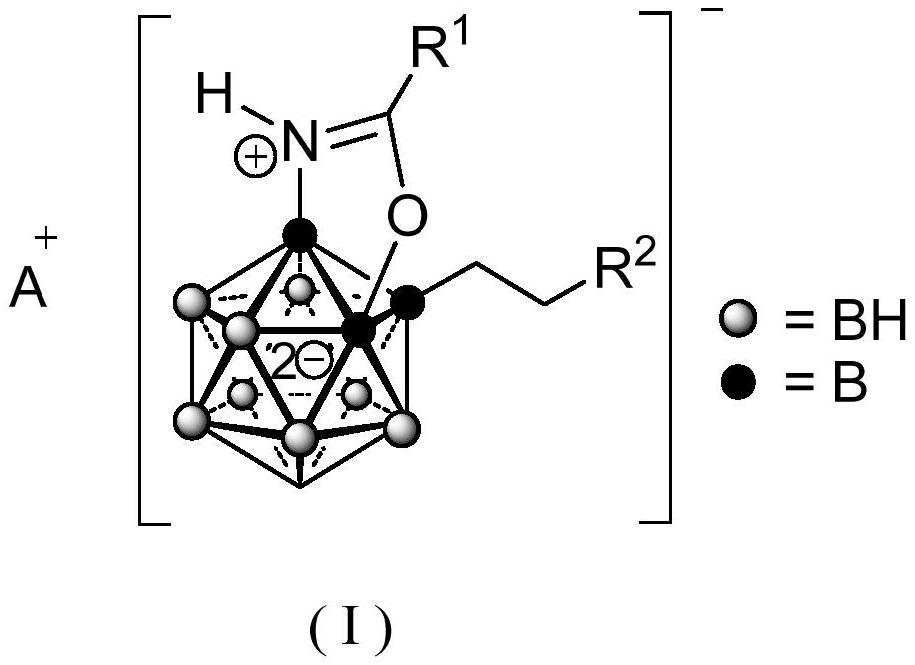
A 3-substituted oxazole-fused caged dodecaborane compound and its preparation method and application
ActiveCN111662315BShort synthesis pathMild reaction conditionsAntibacterial agentsOrganic compound preparationQuaternary ammonium cationAcyl group
The invention discloses a 3-substituted oxazole-fused caged dodecaborane compound, a preparation method and application thereof. The structure of the 3-substituted oxazole fused caged dodecaborane compound is as shown in formula (I), and the R 1 Be menthyl or camphoryl; The R 2 for H, C 6‑20 Aryl, C 1‑15 Alkyl, ‑C(O)O‑C 1‑5 Alkyl or ‑C(O)O‑C 6‑20 Aryl, the C 6‑20 Aryl is optionally replaced by 1, 2 or 3 H, F, Cl, Br, I, OH, NH 2 , NO 2 , CN, C 1‑5 Alkyl, C 1‑5 Alkoxy, -O-C(O)-C 1‑5 Alkyl, ‑C(O)‑NH‑C 1‑5 Alkyl, ‑NH‑C(O)‑C 1‑5 Alkyl, ‑C(O)O‑C 1‑5 Alkyl, C 6‑20 Aryl or 5-12 membered heteroaryl substituted; said A + For metal cations, quaternary ammonium cations or phosphorus cations. The preparation method utilizes menthyl and camphoryl as a directing group, selectively activates the 3-position B-H bond, and prepares the compound of formula (I); the stereoselectivity is high, and the yield is good, which increases the chance of discovering new antibacterial drug candidates . The compound of formula (I) provided by the invention has high-efficiency and broad-spectrum antibacterial effects.
Owner:ZHEJIANG UNIV
High-entropy rare earth co-doped nano low-heat-transfer powder material and preparation method thereof
ActiveCN114524440AWide infrared absorption bandSingle phaseChemical industryMetal boridesFiltrationSlurry
The invention provides a high-entropy rare earth co-doped nano low-heat-transfer powder material and a preparation method thereof.The preparation method comprises the following steps that 1, a rare earth source, a boron source and an intermediate are put into a high-pressure reaction kettle, hydrogen is introduced, the temperature is increased to 320-340 DEG C, full stirring is performed for activation, an obtained product is subjected to extraction layering, and precipitation is subjected to suction filtration, water washing and drying to obtain a rare earth-boron co-doped nano low-heat-transfer powder material; then carrying out wet grinding, carrying out spray granulation on the obtained slurry, and carrying out dry grinding on the obtained spherical powder to obtain a precursor; (2) putting the precursor into a rotary furnace for calcining, introducing a hydrogen-nitrogen mixed gas into the rotary furnace, heating to 900-1200 DEG C, preserving heat for 30-450 minutes, and cooling to obtain a primary product; and (3) removing impurities from the head product to obtain the high-entropy rare earth co-doped nano low-heat-transfer powder material. The powder of the high-entropy rare earth co-doped nano low-heat-transfer powder material is dispersed, fluffy and small in particle size, required temperature is low, and energy consumption is low.
Owner:TIANJIN BAOGANG RES INST OF RARE EARTHS
Method for preparing bis allyl alcohol compound 4-(3-methyl phenyl)-1,6-heptadiene-4-alcohol
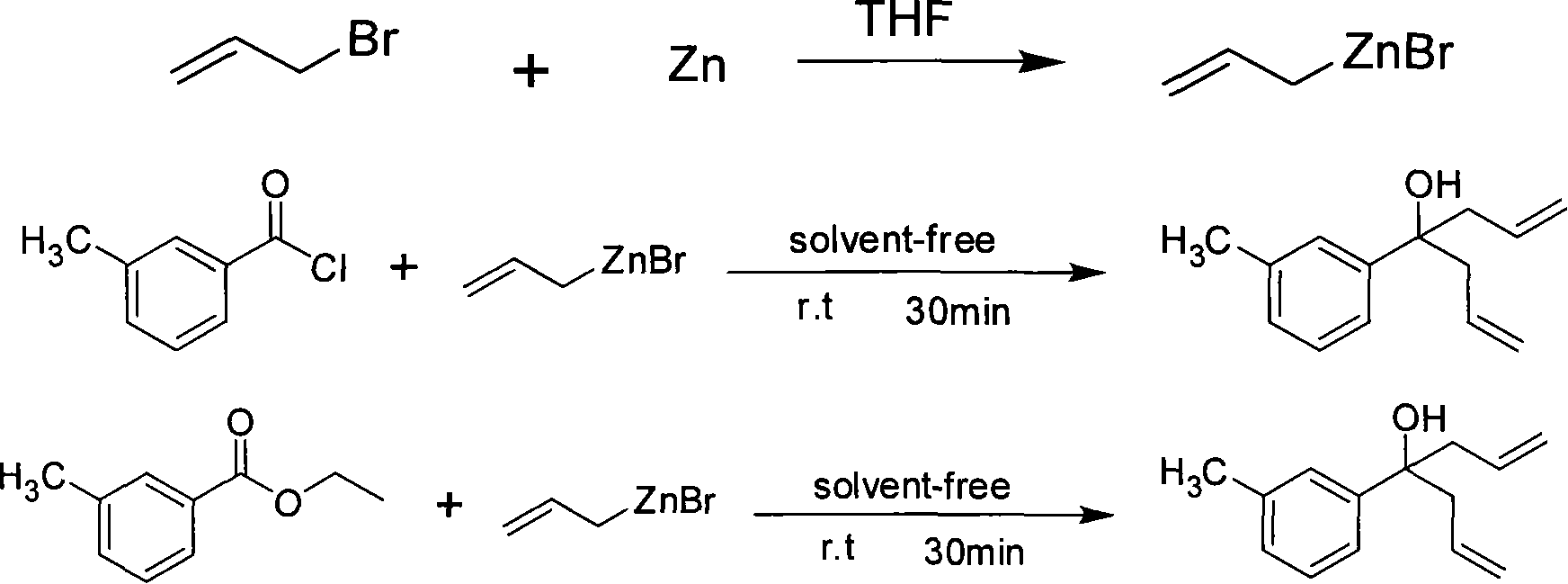
InactiveCN101250089AShort synthesis pathImprove efficiencyOrganic compound preparationHydroxy compound preparationDiethyl etherSolvent
The invention provides a method for synthesizing diallyl alcohol compound as 4-(3-methyl phenyl)-1, 6-heptadiene-4-alcohol, which uses allyl zine bromide and mtoluie chloride or mtoluie ethyl formate as raw materials to be reacted at room temperature, extracts via ether, dries and evoprates out solvent and separates via column chromatography to obtain the product. The invention has short synthesis route, high efficiency, high reaction yield (at least 92%), which can eliminate solvent and catalyst and use cheap and non-toxic metal zinc, thereby effectively avoiding the pollution of organic solvent and catalyst to protect environment. The invention has mild reaction conditions (room temperature), short reaction time, simple operation, reduced energy consumption, reduced synthesis cost and significant benefit for industrial production.
Owner:NORTHWEST NORMAL UNIVERSITY
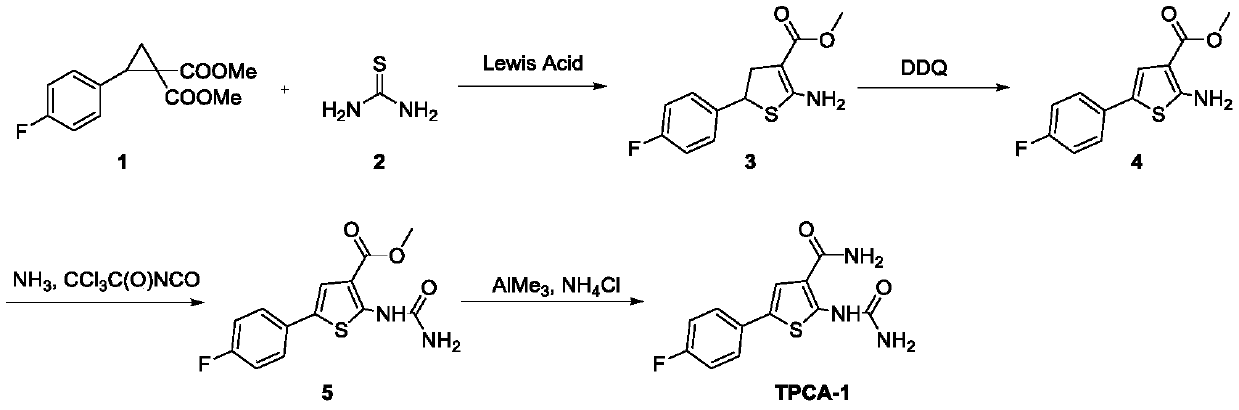
A kind of method of synthesizing thiophene inhibitor TPCA-1
The invention relates to a method for synthesizing a thiophenes inhibitor TPCA-1, and belongs to the field of organic chemical drug synthesis. The method comprises the following steps: taking thioureaand 2-(4-fluorophenyl) cyclopropane-1,1-dimethyl isophthalate as starting raw materials, and the TPCA-1 is obtained through a multi-step reaction including a ring enlargement reaction, an oxidation reaction, an addition reaction and an amidation reaction in sequence. In the used route of the method, the reaction raw materials are easily obtained, the yield is high, and the TPCA-1 can be obtainedsuccessfully.
Owner:HENAN NORMAL UNIV
Novel intermediate as well as preparation method and application thereof
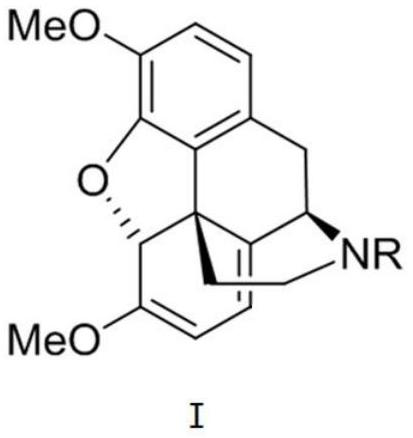
ActiveCN114502560AEfficient conversionHigh chemistryOrganic chemistry methodsBulk chemical productionBiomimetic synthesisDrugs synthesis
The invention relates to the field of drug synthesis, in particular to a novel intermediate as well as a preparation method and application thereof, the structural formula of the novel intermediate is as shown in formula I, and in the formula, R is a secondary amine protecting group. Based on a possible biogenic pathway of the morphine derivative, through a biomimetic synthesis strategy, an asymmetric transfer hydrogenation reaction and an intramolecular oxidation dearomatization Heck reaction in a process of preparing the intermediate are taken as key reactions of total synthesis, and efficient synthesis of the morphine derivative is realized. The novel intermediate provided by the invention is used for synthesizing the morphine derivative, and has the characteristics of obviously reducing the synthesis steps, improving the yield, reducing the emission of three wastes and saving the production cost.
Owner:SICHUAN UNIV
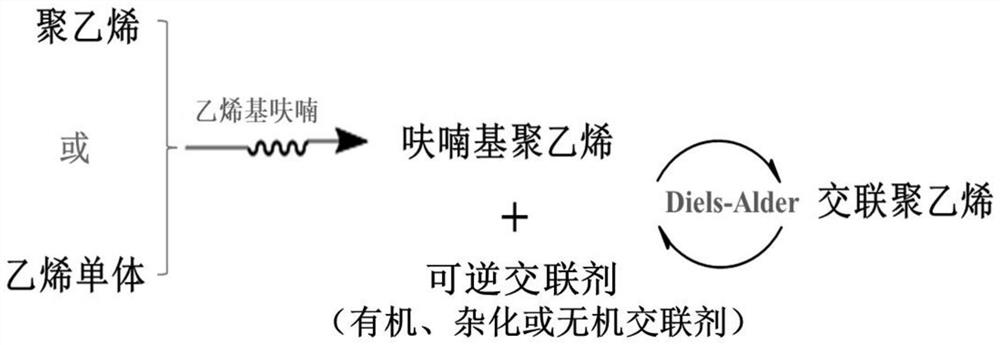
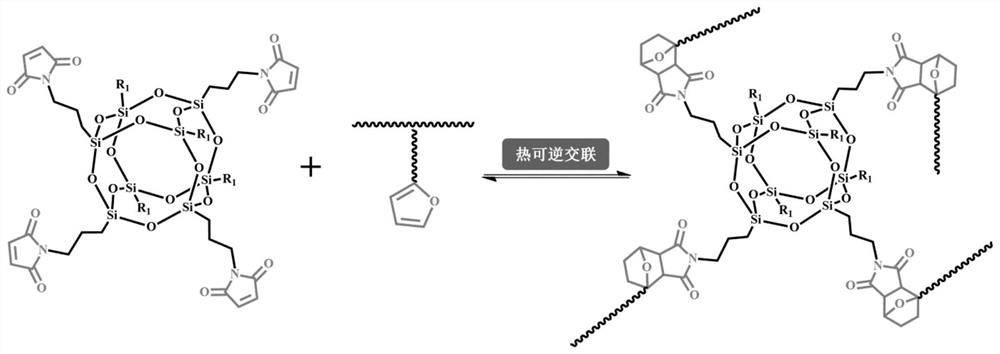
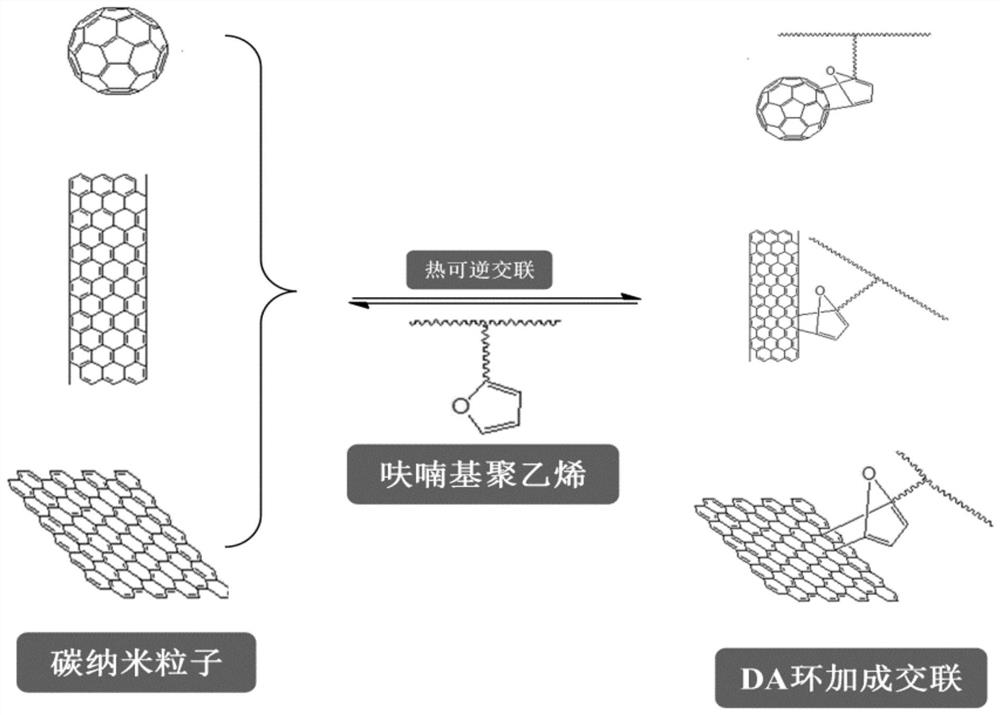
A kind of cross-linked polyethylene and its preparation method and application
Owner:SHANGHAI JIAOTONG UNIV
The preparation method of 4-(6-aminopyridin-3-yl)piperazine-1-carboxylic acid tert-butyl ester
The invention discloses a method for preparing 4-(6-aminopyridin-3-yl) piperazine-1-carboxylate tert-butyl ester. The method for preparing 4-(6-aminopyridin-3-yl) piperazine-1-carboxylate tert-butyl ester comprises the following steps: 2-aminopyridin, piperazine-1-formate tert-butyl ester and NSP-SA-NHS photo catalyst is added to the solvent, and the light reaction is carried out under the condition of the presence of an oxidizing agent to generate 4-(6-aminopyridin-3-yl) piperazine-1-carboxylate tert-butyl ester. According to the method, 4-(6-aminopyridin-3-yl) piperazine-1-carboxylate tert-butyl ester is synthesized by one step. On the one hand, the synthetic route of 4-(6-aminopyridin-3-yl) piperazine-1-carboxylate tert-butyl ester is effectively shortened, the generation of by-productsis effectively reduced and the yield of the target product is improved. On the other hand, only photo catalyst and oxidizing agent are used, so that the preparation method is safe and environment-friendly and low in cost.
Owner:深圳蓝新科技有限公司
A kind of high-entropy rare earth co-doped nanometer low heat transfer powder material and preparation method thereof
ActiveCN114524440BWide infrared absorption bandSingle phaseChemical industryMetal boridesSpray GranulationFiltration
Owner:TIANJIN BAOGANG RES INST OF RARE EARTHS
Malononitrile phorone fluorine-ion fluorescence probe and preparation method and application thereof
InactiveCN110272449AExcellent optical propertiesHigh sensitivityGroup 4/14 element organic compoundsFluorescence/phosphorescenceFluoProbesChemical structure
The invention discloses a malononitrile phorone fluorine-ion fluorescence probe and a preparation method and application thereof, and belongs to the technical field of analysis and detection. The chemical structural formula of the probe is shown in the description. The malononitrile phorone fluorine-ion fluorescence probe has the advantages of having a good optical property, being capable of highly selectively identifying fluorine ions, having large Stokes displacement and the long fluorescence emission wave length, being high in sensitivity and good in selectivity and the like; the probe is simple in synthesis route, high in yield, easy to separate, high in purity and capable of being used for identifying and detecting the content of fluorine ions in water environment, under the effect between fluorine and the probe, a macroscopic color change is generated, and whether or not the fluorine ions exist in the water environment is conveniently identified by naked eyes; the monitoring speed is relatively high, and interference of other ion elements in the water cannot cause influence. The raw materials are simple and easy to obtain, and the malononitrile phorone fluorine-ion fluorescence probe is low in cost and capable of easily achieving industrialized production, and has good application prospects in environment monitoring and a living body system.
Owner:YANGTZE NORMAL UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com