Cinnamate derivatives and application of cinnamate derivatives as tyrosinase inhibitors and gels
A technology of cinnamate and derivatives, applied in the preparation of carboxylate, preparations for skin care, preparation of organic compounds, etc., can solve the problem of insufficient tyrosinase inhibitory activity, unprovable safety, lack of cytotoxicity of compounds, etc. Cell experiments, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] Synthesis of compound 1: (E)-3-(2-acetyl-5-methoxyphenoxy)propyl-3-(3-methoxy-4-(prop-1-en-2-yloxy base) phenyl) acrylate
[0079]
[0080] 1. Weigh 1.66g (10.0mmol) paeonol (compound I-1) and 0.83g (6.0mmol) K 2 CO 3 Place in a round bottom flask, add 100mL of acetone as a solvent, stir at room temperature for 0.5h, then add 2.08g (15.0mmol) of 3-bromo-1-propanol (compound II-1), heat to 80°C for 8h under reflux, After the reaction was completed, it was cooled to room temperature, the solvent was removed under reduced pressure, and purified by silica gel column chromatography to obtain 2.691 g of crystalline compound III-1 with a yield of 65%, m.p.56°C.
[0081] 2. Add 1.96g (10mmol) of compound IV-1 and 15mL of thionyl chloride to a round bottom flask, connect the exhaust gas absorption device, raise the temperature to 80°C, and heat up to 90°C to recover thionyl chloride after no gas is produced. , to obtain compound V-1.
[0082] 3. Under the conditions of ic...
Embodiment 2
[0084]Synthesis of compound 2: (E)-3-(3-acetyl-4-hydroxyphenoxy)propyl-3-(4-acetoxy-3-methoxyphenyl)acrylate
[0085]
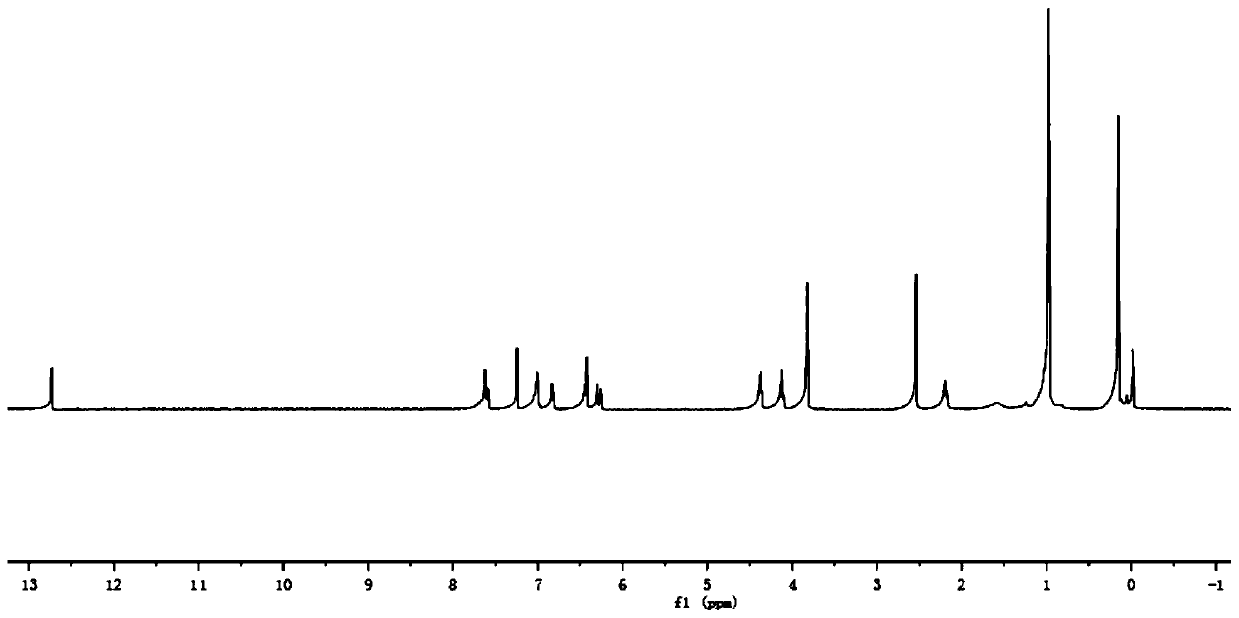
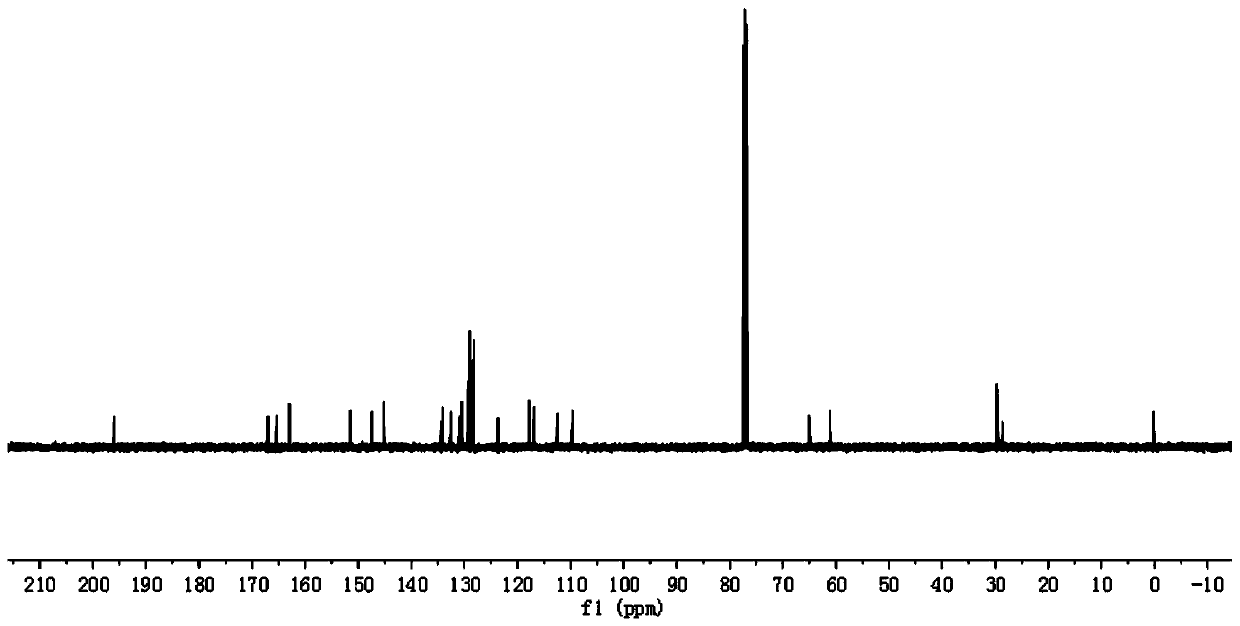
[0086] In step 1 of this example, the paeonol in step 1 of example 1 is replaced with equimolar 2,4-dihydroxyacetophenone (compound I-2), and the other steps are the same as step 1 of example 1 to obtain 3.52 g White crystal compound III-2, yield 85%. In step 3 of this example, compound III-1 in step 3 of example 1 was replaced with equimolar compound III-2, and the other steps were the same as step 3 of example 1 to obtain 4.71 g of white solid compound VI-2, namely compound 2. The yield is 65%, m.p.143~144℃, and the structural characterization data are: 1 H NMR (400MHz, CDCl 3 )δ: 12.74(s,1H),7.66(s,1H),7.64(d,J=1.2Hz,1H),7.63(t,1H),7.17(s,1H),7.13(d,J=1.9 Hz,1H),7.10(dd,J=3.8,1.8Hz,2H),7.06(s,1H),7.04(s,1H),6.45(d,J=2.5Hz,1H),6.44-6.42(m ,2H),6.40(s,1H),6.36(s,1H),4.40(t,J=6.2Hz,2H),4.14(t,J=6.1Hz,2H),3.88(s,6H),2.55 (s,3H),2.23(s,3H)2.19-2.16(m,2H...
Embodiment 3
[0088] Synthesis of compound 3: (E)-3-(4-allyl-2-methoxyphenoxy)propyl-3-(4-acetoxy-3-methoxyphenyl)acrylate
[0089]
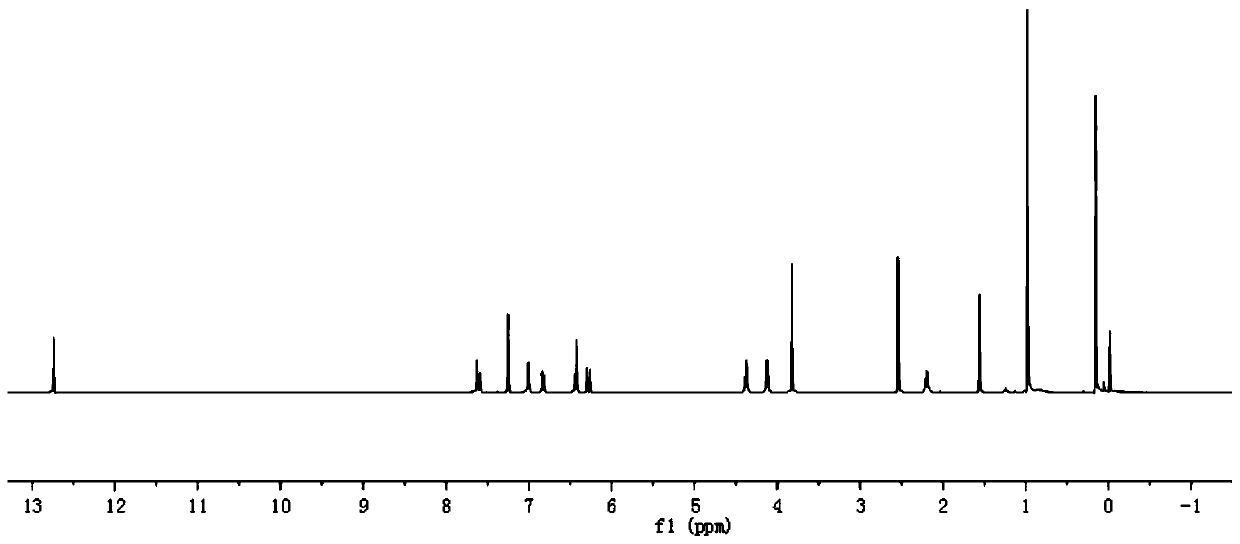
[0090] In step 1 of this example, equimolar eugenol (compound I-3) was used to replace paeonol in step 1 of example 1, and the other steps were the same as step 1 of example 1 to obtain 2.58g of white crystal compound III-3 , yield 88%. In step 3 of this example, compound III-1 in step 3 of example 1 was replaced with equimolar compound III-3, and other steps were the same as step 3 of example 1 to obtain 3.78g of white crystal compound VI-3, namely compound 3. The yield is 68%, m.p.79~80℃, and the structural characterization data are: 1 H NMR (400MHz, CDCl 3 )δ: 7.64(d, J=16.0Hz, 1H), 7.26(s, 1H), 7.10(s, 2H), 7.05(d, J=8.0Hz, 1H), 6.84(d, J=8.7Hz, 1H), 6.71(d, J=6.1Hz, 1H), 6.38(d, J=16.0Hz, 1H), 5.95(d, J=16.8, 6.7Hz, 1H), 5.08(d, J=17.0Hz, 2H), 4.42(t, J=5.1Hz, 2H), 4.14(t, J=6.3Hz, 2H), 3.86(d, J=7.8Hz, 3H), 3.33(d, J=6.7Hz, 1H) ,2.24(dd,J=8.1,4....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com