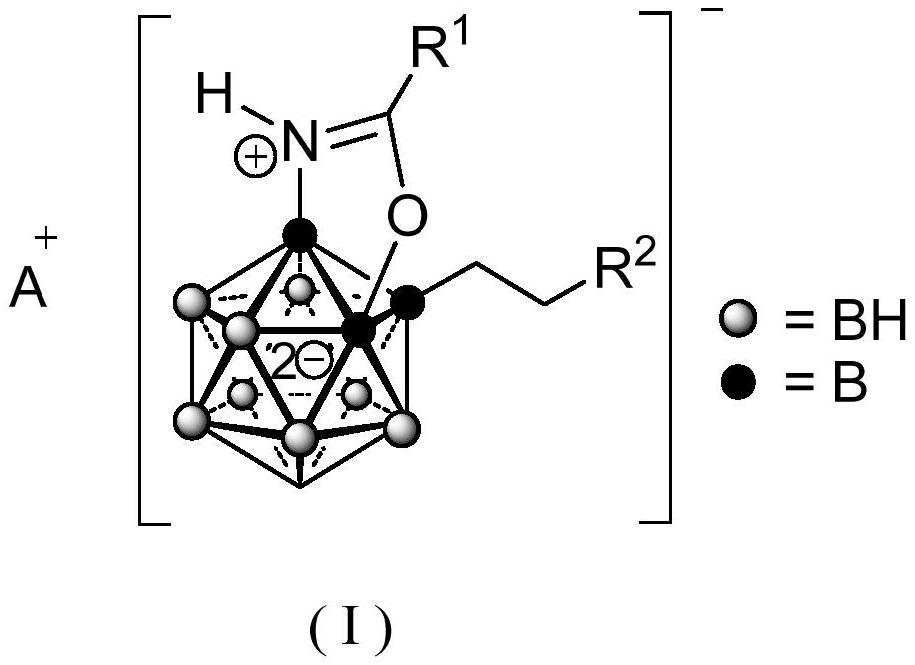
A 3-substituted oxazole-fused caged dodecaborane compound and its preparation method and application
A technology of compounds and compositions, applied in the field of 3-substituted oxazole-fused caged dodecaborane compounds and their preparation, capable of solving unexplored problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
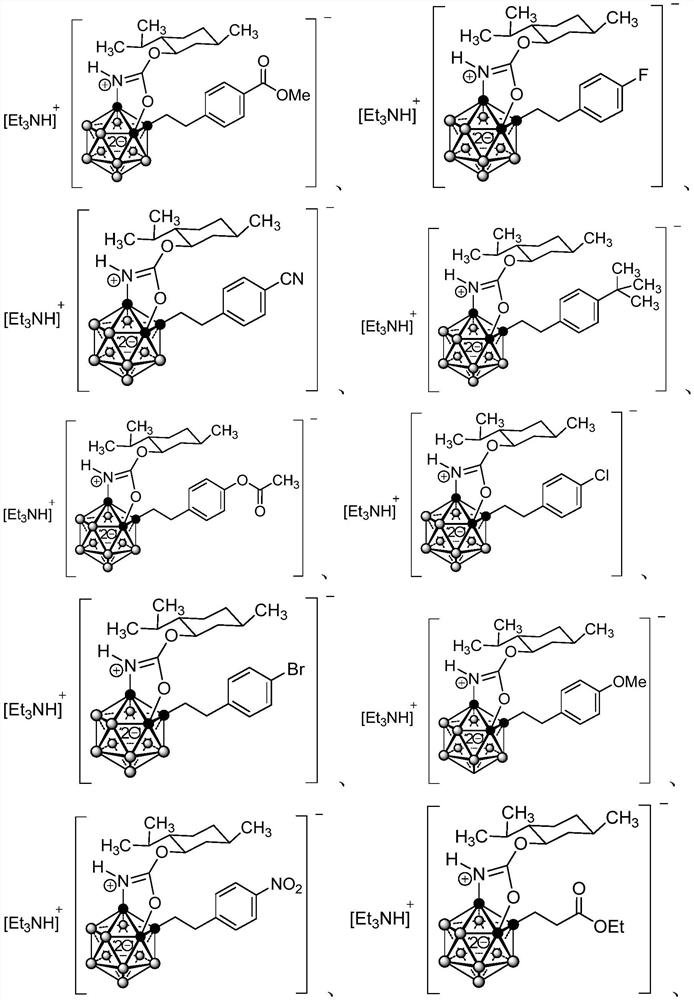
Image
Examples
Embodiment 1
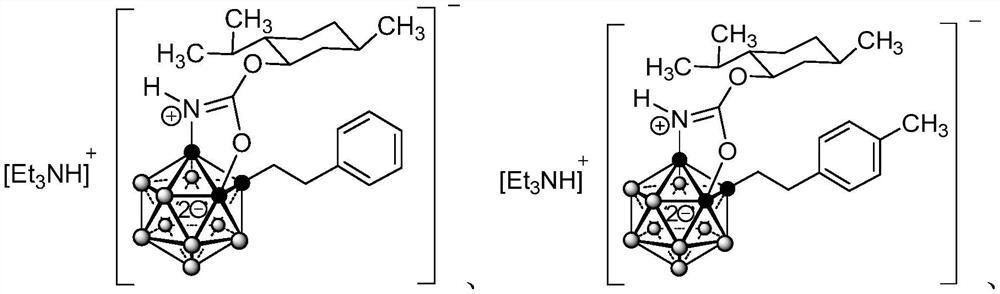
[0109] Example 1 RV-02
[0110]
[0111] In the glove box, under the protection of inert nitrogen gas, prepare a dry 20mL small glass reaction bottle, add a stirring bar, add [Et 3 NH][B 12 h 11 NH 3 ] (0.80 mmol, 1 equiv) and NaH (2.5 mmol, 3.1 equiv). Ultra-dry THF (6 mL) was then added. Stir at 25°C for 30 minutes until stopped by hydrogen evolution. Menthyl chloroformate (0.9 mmol, 1.1 equiv) was dissolved in ultra-dry THF (1 mL) and added dropwise to the mixture via a plastic syringe over 30 minutes. The mixture was stirred for a further 2 hours at 25°C.
[0112] After the reaction was completed, the reaction bottle was moved outside to a fume hood. with [Et 3 NH]Cl solution (10mL H 2 O+2equiv[Et 3 NH]Cl) quenched the reaction; the pH at this point was about 7-8. Mixture with CH 2 Cl 2 / MeCN=4:1 (8×10 mL) extraction. The resulting organic layer was washed with MgSO 4 Dry, then filter and concentrate by rotary evaporation. The resulting mixed residue was...
Embodiment 2
[0123] Example 2 RV-03
[0124]
[0125] RV-03 was prepared according to the preparation method of Example 1, except that styrene was replaced by p-methylstyrene.
[0126] 1 H { 11 B}NMR (400MHz, CD 3 COCD 3 , 23°C): δ8.15 (br, 1H, NH anion), 8.09-7.70 (br, 1H, cation NH), 7.06-6.89 (overlap m, 4H, phenyl H), 4.78-4.66 (m, 1H , methyl CH), 3.50(q, J=7.4Hz, 6HN–CH 2 cation), 2.59-2.46(m,2H,B–CH 2 –CH 2 ),2.22(s,3Hphenyl-CH 3 ), 2.14-2.08 (overlap with solvent residual m, 1H, methyl CH), 2.03-1.88 (m, 1H, methyl CH), 1.74-0.39 (extensive overlap with m, 16H, 7-methyl CH and 9BH), 1.43 (t, J=7.3Hz, 9H, cation CH 3 ), 0.92-0.82 (overlap m, 6H, 2 methyl CH 3 ), 0.79 (d, J=6.9Hz, 3H, methyl CH 3 ),0.58-0.51(m,2H,B–CH 2 –CH 2 ).
[0127] 13 C{ 1 H}NMR (100MHz, CD 3 COCD 3 ,23°C): δ169.75 (C=N), 145.47, 134.26, 129.28, 128.45 (characteristic peak of phenyl), 82.93 (methyl CH), 48.13 (cation CH 2 ), 47.97 (methyl CH), 41.62, 36.94 (2 methyl CH 2 ), 34.52 (B–CH 2 –...
Embodiment 3
[0130] Example 3 RV-04
[0131]
[0132] RV-04 was prepared according to the preparation method of Example 1, except that styrene was replaced by methyl 4-vinylbenzoate.
[0133] 1 H { 11 B}NMR (400MHz, CD 3 COCD 3 ,23℃):δ8.17(br,1H,NH anion),7.85(d,J=7.7Hz,2H,phenyl H),7.24(d,J=7.7Hz,2H,phenyl H),4.80 -4.62(m, 1H methyl CH), 3.83(s, 3H, –COOCH 3 ),3.48(q,J=7.4Hz,6H,N–CH 2 cation), 2.67-2.56(m,2H,B–CH 2 –CH 2 ), 2.16-2.07 (overlap with solvent residual m, 1H, methyl CH), 1.96-1.86 (m, 1H, methyl CH), 1.77-0.41 (extensive overlap with m, 16H, 7CH and 9BH), 1.42 (t , J=7.2Hz, 9H, cation CH 3 ), 0.91 (d, J=8.2Hz, 3H, methyl CH 3 ), 0.87 (d, J=8.3Hz, 3H, methyl CH 3 ), 0.78 (d, J=6.7Hz, 3H, methyl CH 3 ),0.65-0.50(m,2H,B–CH 2 –CH 2 ). Only one N–H signal was clearly detected.
[0134] 13 C{ 1 H}NMR (100MHz, CD 3 COCD 3 ,23°C): δ169.59 (C=N), 167.43 (C=O), 155.05, 129.98, 128.75, 127.57 (characteristic peak of phenyl), 82.45 (methyl CH), 51.97 (COOCH 3 ), 48.11...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com