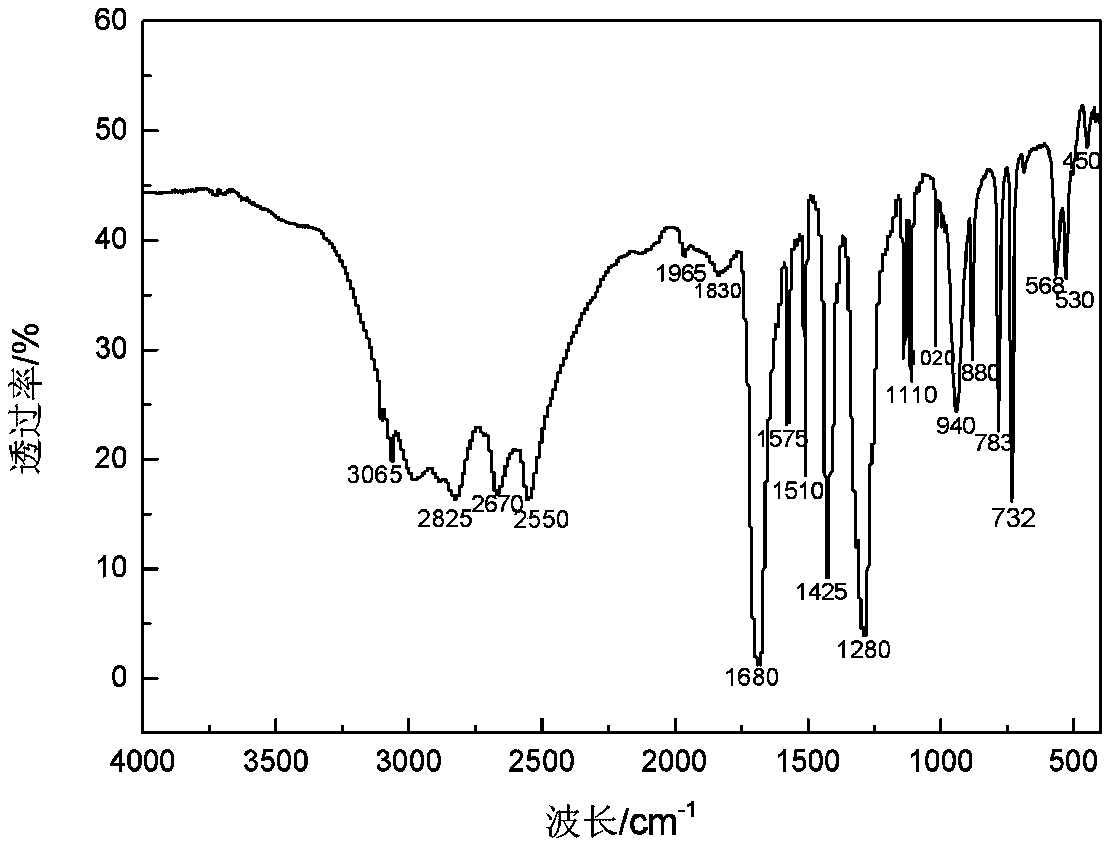
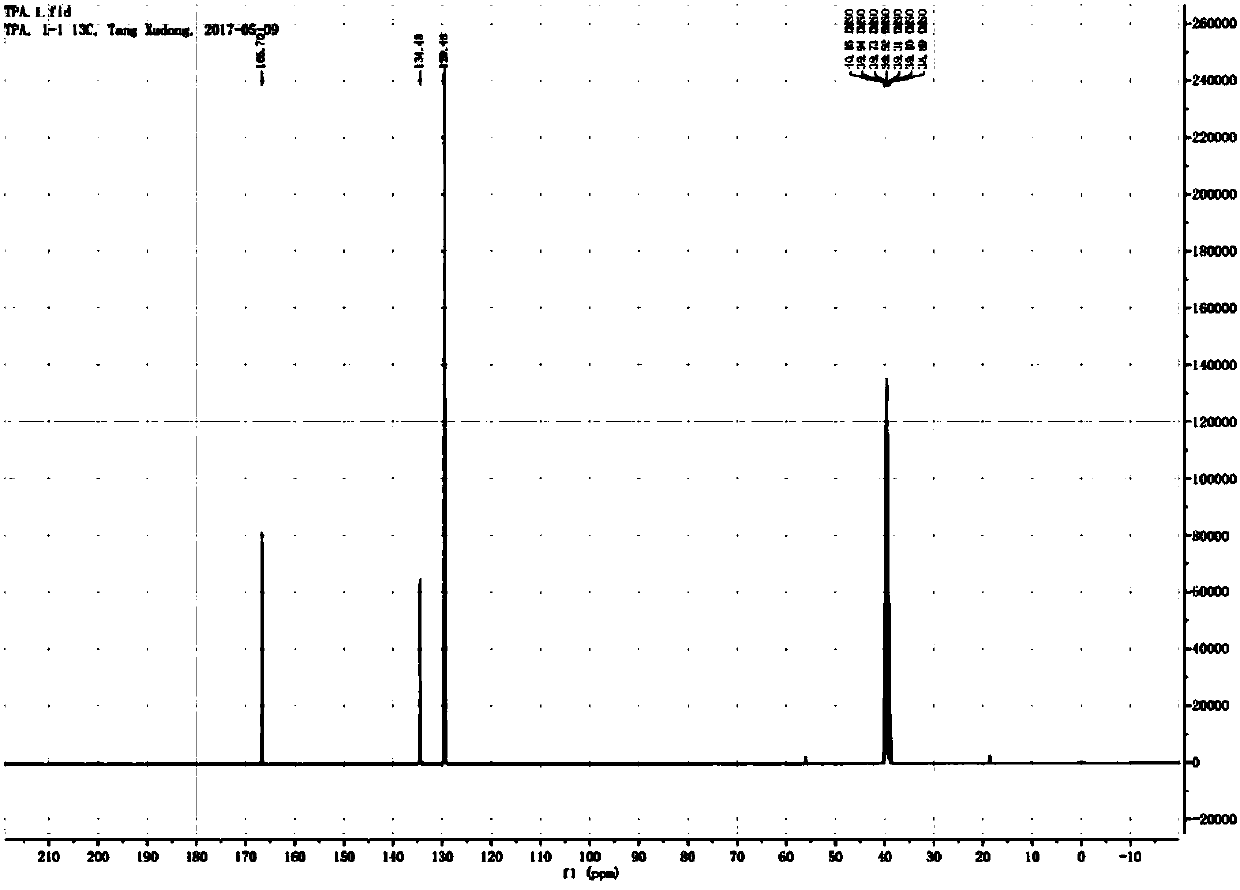
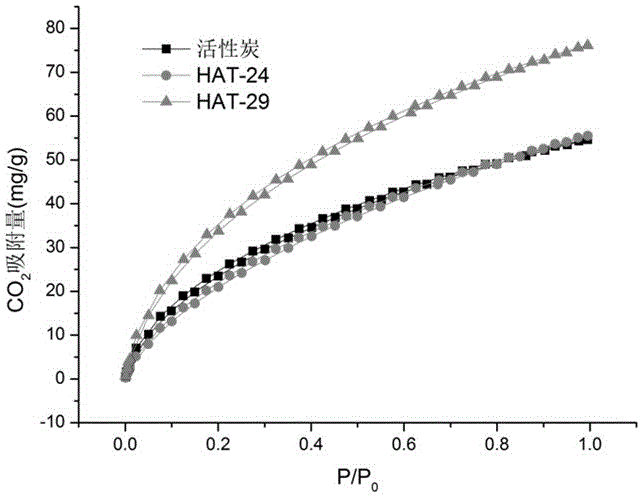
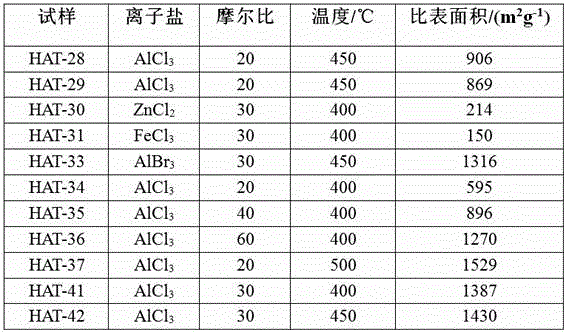
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
39results about How to "No by-products" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
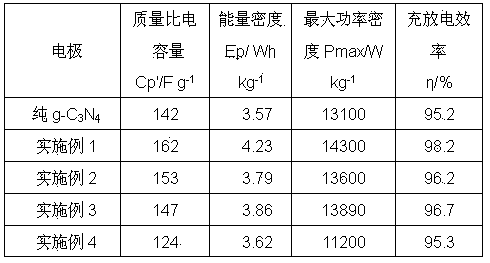
A method for preparing a g-C3N4/carbon quantum dot composite electrode
ActiveCN103745836AImprove performanceNot easy to decomposeHybrid/EDL manufactureCapacitanceElectron transfer
Provided is a method for preparing a g-C3N4 / carbon quantum dot composite electrode. The method comprises: adding carbon quantum dots into ethanol in order to prepare carbon quantum dot ethanol solution; mixing urea with the carbon quantum dot ethanol solution, performing ultrasonic dispersion on the mixed solution and then transferring the mixed solution to a crucible; warming the mixed solution to 350 to 700 degree centigrade with a muffle furnace step by step, maintaining the temperature for one to three hours and then decreasing the temperature of the mixed solution to room temperature; and grinding the obtained substance with ethanol and filtering the same so as to obtain g-C3N4 / carbon quantum dot composite material. The method has advantages of simpleness, no by-products, raw material easy to obtain, and low price. The obtained composite material is stable in performance, uneasy to decompose, and nontoxic. The method may increase the electron transfer rate of the g-C3N4 material, has good conductive performance, enhances electrode specific area, improves the electron adsorption capability of an electrode surface, and effectively increases the specific capacitance of a capacitor.
Owner:BOHAI UNIV
Method for synthesizing cyclohexanone-oxime and caprolactam
ActiveCN104341318ALow yieldImprove conversion rateLactams preparationOximes preparationGas phaseCyclohexanone oxime
The invention relates to a method for synthesizing cyclohexanone-oxime and caprolactam, particularly a method for synthesizing cyclohexanone-oxime and caprolactam by performing catalytic hydrogenation on nitrocyclohexane used as a raw gas phase. The method has the advantages of high controllability, low cost, environmental protection, higher conversion ratio of raw materials and higher yield, and has industrialization application prospects. The reaction process is simple, no coproduct ammonium sulfate is generated in the reaction process, and no wastewater or waste gas is generated to pollute the environment, and thus, the method is an environment-friendly production technique.
Owner:XIANGTAN UNIV
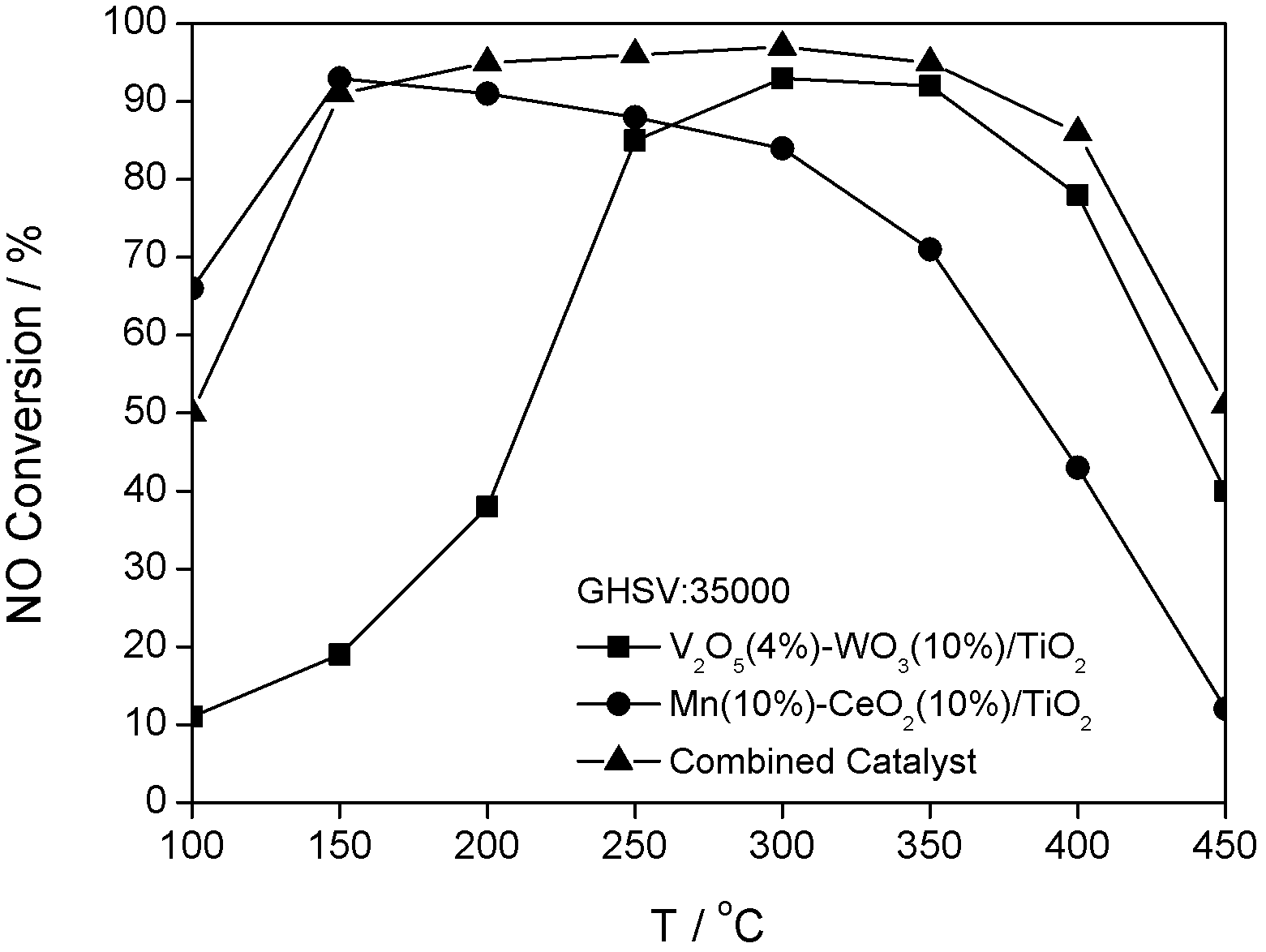
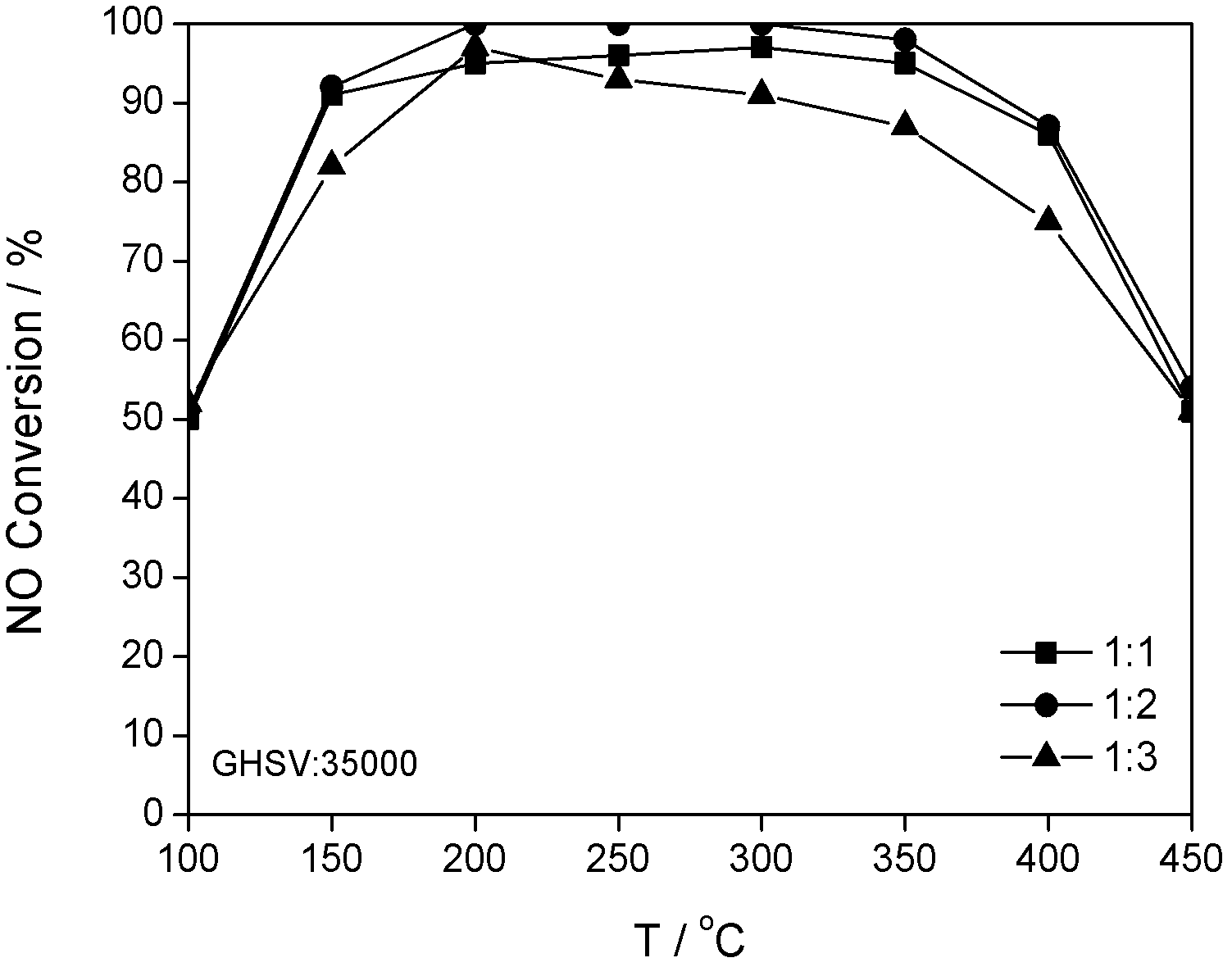
Combined catalyst for improving denitration performance and application thereof
InactiveCN102600832AEfficient removalHigh and low temperature - high temperature catalytic activityDispersed particle separationMetal/metal-oxides/metal-hydroxide catalystsMass ratioEngineering
The invention discloses a combined catalyst for improving denitration performance and an application thereof, which belongs to the field of catalysts. In the catalyst, industrial anatase crystal form TiO2 is taken as a carrier; V2O5-WO3 / TiO2 is put at the front section of MnOx-CeO2 / TiO2, and is called front section; MnOx-CeO2 / TiO2 is called back section; the front section refers to a section where an air current main body is contacted firstly; the back section refers to a section where the air current main body is contacted secondly; the mass ratio of the front section to the back section is 1:1-1:3; V2O5 accounts for 2-4 percent of the mass of the carrier TiO2; WO3 accounts for 8-10 percent of the mass of the carrier TiO2; Mn accounts for 10-20 percent of the mass of the carrier TiO2; CeO2 accounts for 8-10 percent of the mass of the carrier TiO2; and MnOx is taken as the general name of MnO2 and Mn2O3. The combined catalyst can be applied to an integral catalyst for industrial application. The combined catalyst has a wider active temperature window; particularly, the removing efficiency of NOx by NH3-SCR is greatly increased under the exhaust low-temperature wording condition of a diesel engine; and moreover, the same NOx removing efficiency as the conventional catalyst can be obtained at a medium-high temperature section.
Owner:BEIJING UNIV OF CHEM TECH
Prepn. of water soluble chitosan with controllable molecular weight
There is a method of preparing the controlled molecular weight, water-soluble shell glycan, which fully dissolve all kinds of non-acetyl shell glycans into the hydrochloric acid, then slowly add the oxidant-sodium hypochlorite-hydrogen peroxide to the solution, then make them react under the room temperature to break away the 1,4-beta-D-indican bond, and produce the low molecular weight, water-soluble shell glycan. The invention's process is simple, and the prepared low molecular weight, water-soluble shell glycan had good water solubility and retains the main-chain structure of the shell glycan, and can be used as not only the materials of oral medicines and health products, but also the additive of the cosmetics and the food trades.
Owner:SHANGHAI JIAO TONG UNIV
Anti-aging composition comprising NADH and ceramide, skincare product as well as preparation method and application thereof
InactiveCN109350557ASimple preparation processAchieve anti-aging anti-free radical effectCosmetic preparationsToilet preparationsAnti ageingChemistry
The invention discloses an anti-aging composition comprising NADH and ceramide, a skincare product as well as a preparation method and application thereof, which relates to the field of skincare products. The composition comprises the following main components: 1 to 100 parts by weight of NADH, and 1 to 50 parts by weight of ceramide. All components of the anti-aging composition comprising the NADH and ceramide have a synergistic effect, so that an effect for resisting the aging free radicals can be comprehensively realized in various aspects. The skincare product adopting the anti-aging composition is simple in preparation process, free from byproducts, safe, effective and low in production cost.
Owner:HOBOOMLIFE BIO TECH SHENZHEN CO LTD
Modified zirconium silver-carrying powder and preparation method thereof
InactiveCN1281689CImprove discoloration problemDissolution inhibitionInorganic pigment treatmentMaterials scienceCrystallization
The invention relates to a modified silver-loaded zirconium phosphate powder, which is prepared by the following method: fully mix the silver-loaded zirconium phosphate powder and a crystallization accelerator of 3 to 20 wt% of its weight, and mix it uniformly in a place where the volume content of oxygen is more than 5v%. It is obtained by firing at 800-1100°C for 1-5 hours in an oxygen atmosphere. Described crystallization accelerator is MgO or ZnO or can generate the compound of this oxide compound when roasting, and B 2 o 3 or a mixture of compounds capable of forming this oxide upon firing, B 2 o 3 The molar ratio to MgO or ZnO is 0-0.3. The modified zirconium phosphate silver-loaded powder can be stored in the air for a long time, and does not change color when mixed with resin, which fundamentally improves the problem of discoloration of the zirconium phosphate silver-loaded powder when it is used in industries such as textiles, plastics, and papermaking; and the present invention The preparation method provided has simple procedures, easy industrial production, no by-products and no pollution to the environment. At present, it has been applied in Shanghai, Hunan and other enterprises, and the product quality is excellent.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Germanium dioxide/graphene composite and preparation method thereof
ActiveCN106099066ANo pollution in the processSimple and fast operationCell electrodesSecondary cellsAir atmosphereEvaporation
The invention relates to the technical field of lithium ion battery anode materials, in particular to a germanium dioxide / graphene composite. The composite is formed by wrapping germanium dioxide submicron particles by graphene nanosheets, and the diameter of the germanium dioxide submicron particles ranges from 400 nm to 900 nm. Germanium dioxide powder and graphene oxide are dissolved in distilled water together, then water is completely evaporated at the temperature of 25 DEG C to 45 DEG C, the powder obtained after evaporation is calcined in an air atmosphere for 1-3 h at the temperature of 200 DEG C to 300 DEG C, and the composite is obtained. The prepared germanium dioxide / graphene composite is used as a lithium ion battery anode material and has high charge and discharge specific capacity, good circulating stability and fast charge and discharge performance, the preparation method is simple, pollution is avoided, the reaction temperature is low, the obtained product is high in purity, and no by-product is generated.
Owner:SHANGQIU NORMAL UNIVERSITY
Stone oil sulphonic acid, its salt, preparation method and uses thereof
InactiveCN101402592AWide variety of sourcesSolving non-sulfonatable problemsDrilling compositionSulfonic acid preparationDistillationEther
The invention discloses petroleum sulfonic acid and salt thereof as well as a preparation method and application thereof. The petroleum sulfonic is prepared by the following method: causing petroleum fractions and nonylphenol polyethenoxy ether to carry out sulfonation reaction to obtain the petroleum sulfonic acid, wherein the distillation range of the petroleum fractions is more than 280 DEG C under normal atmosphere, is preferably between 280 and 600 DEG C. The petroleum sulfonate is prepared by the method comprising the following steps: 1) preparing the petroleum sulfonic acid by the method for preparing the petroleum sulfonic acid; and 2) neutralizing the petroleum sulfonic acid obtained in step 1) with an alkali solution to pH value of between 7 and 9, and then obtaining the petroleum sulfonate. The petroleum sulfonate prepared by the method can reduce oil water interfacial tension to between 10<-2> and 10<-3> mN / m, has good universality for improving recovery ratio for different crude oil systems, and can be used as an oil displacement agent in oil extraction.
Owner:BEIJING TIANYI GERUN NEW ENERGY TECH DEV
Method for preparing terephthalic acid through degradation of waste PET
InactiveCN108047023ALarge specific surface areaDisaggregation Velocity BlockPreparation from carboxylic acid esters/lactonesRenewable resourceCompanion animal
The invention specifically relates to a method for preparing terephthalic acid through degradation of waste PET, belonging to the technical field of utilization of chemical renewable resources. According to the method, waste PET materials and water are used as raw materials and subjected to a catalytic hydrothermal reaction to prepare terephthalic acid and glycol; under proper degradation conditions, the degradation rate of PET polyester reaches 100.0%, and the yield of terephthalic acid reaches 99.75%; and the purity of treated terephthalic acid is close to 100%. The provided method for preparing terephthalic acid through degradation of waste PET compensates for the defects of low yield of terephthalic acid and a great number of by-products in the prior art to a certain extent.
Owner:CHANGCHUN UNIV OF TECH
Fused aza-heterocyclic aromatic hydrocarbon porous framework of two-dimensional lamellar structure, and preparation method and application thereof
ActiveCN105949443AImprove reaction efficiencyNo by-productsOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsLamellar structureNitrogen
The invention specifically relates to a fused aza-heterocyclic aromatic hydrocarbon porous framework of a two-dimensional lamellar structure, and a preparation method and application thereof, belonging to the technical field of functional materials. According to the invention, aza-heteropolycyclic aromatic hydrocarbon is synthesized on the basiss of cyclization of a diamino group and a diketo group for formation of phenazine and is used as a monomer; and then aza-heteropolycyclic aromatic hydrocarbon is subjected to the Scholl reaction under the catalysis of a Lewis acid salt so as to allow aza-heteropolycyclic aromatic hydrocarbon to undergo homopolymerization at high temperature and low pressure, so a conjugated polymer material of a microporous structure and with two-dimensional lamellar layers for complex catalysis of metal is produced. The two-dimensional material has the characteristics of a high specific surface area, a full conjugate plane structure, heat stability, resistance to acid and alkali, excellent catalytic activity, high recovery rate, etc. The method provided by the invention is simple to operate and controllable in process; and the prepared material can be used for catalyzing a cycloaddition reaction of CO2 with epoxypropane compounds so as to immobilize CO2 and has good application prospects.
Owner:FUDAN UNIV
Modified zirconium silver-carrying powder and preparation method thereof
InactiveCN1640946AImprove discoloration problemDissolution inhibitionInorganic pigment treatmentColor changesOxygen content
The modified silver-carrying zirconium phosphate powder is prepared through the process of mixing silver-carrying zirconium phosphate powder with crystallization promoter in the amount of 3-20 wt% of the zirconium phosphate powder, and roasting at 800-1100 deg.c inside atmosphere with oxygen content over 5 vol% for 1-5 hr. The crystallization promoter is mixture of MgO or ZnO or compound capable of producing MgO or ZnO in roasting and B2O3 or compound capable of producing B2O3 in roasting with molar ratio of B2O3 to MgO or ZnO 0-0.3. The modified silver-carrying zirconium phosphate powder may be maintained in the air for long time and has no color change during mixing with resin.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Preparation method of buffalo milk fat natural flavoring base material
InactiveCN107028148AUniform reaction systemEasy to operateFood scienceReaction systemFood processing
The invention provides a preparation method of a buffalo milk fat natural flavoring base material, and belongs to the technical field of food processing. The preparation method comprises the following steps of using raw buffalo milk or buffalo milk fat obtained through degreasing the raw buffalo milk as an enzymolysis substrate, performing pasteurization, and performing enzymolysis sequentially through flavored protease and lipase so as to obtain uniform semi-solid buffalo milk fat natural flavoring base material. The preparation method is simple in operating sequences and uniform in reaction systems; the product namely the flavoring base material is rich and full in flavor, rich in micromolecular peptide, amino acids and fatty acid, free from bad flavor of bitter taste and the like, and stable in quality; and the flavoring base material can be wholly applied, can also be used in a manner that after the flavoring base material is centrifuged, milk fat phases are taken to be independently used, and the flavoring base material is widely applied, and is suitable for dairy products and other various healthy foods of which the milk fragrance flavor needs to be improved.
Owner:GUANGXI ZHUANG AUTONOMOUS REGION BUFFALO INST
Method for producing dodecanoic lactam
ActiveCN109516941ASimple operation and controlImprove conversion rateLactams preparationCatalyst activation/preparationSolventBeckmann rearrangement
The invention discloses a method for producing dodecanoic lactam. The method comprises the following steps: carrying out an oximation reaction in the presence of a solvent a by taking cyclododecanoneand Raschig liquid as raw materials; performing pressure distillation on an oil phase obtained by liquid separation to remove and recover the solvent, dissolving the obtained cyclododecone oxime in amolten state with a solvent b, carrying out a Beckmann rearrangement reaction under catalysis of a rare-earth metal modified magnetic solid super-acid catalyst c, and refining, thereby obtaining the product dodecanoic lactam.
Owner:WANHUA CHEM GRP
Synthetic method of zinc polyaspartate
ActiveCN110862540AReduce usageAvoid it happening againWater/sewage treatment by substance additionZinc hydroxideImide
The invention discloses a production method of zinc polyaspartate. The method comprises the following concrete steps: controlling the pH value of a system by using ammonia water, hydrolyzing polysuccinimide (PSI) to obtain polyaspartic acid (PASP), dissolving zinc hydroxide / zinc oxide into soluble Zn(NH3)4(OH)2 in the presence of an ammonium salt, and reacting the soluble Zn(NH3)4(OH)2 with polyaspartic acid to generate zinc polyaspartate. According to the method, while PSI is hydrolyzed into PASP, zinc polyaspartate is generated from PASP, and the two steps of reactions are carried out at thesame time, so working hours are shortened, and energy consumption is reduced; the method adopts a direct reaction of PASP with a zinc salt and avoids the use of a calcium polyaspartate intermediate,so procedures and working hours are shortened, energy is saved, energy consumption is lowered, and the generation of a large amount of a calcium salt is avoided; and the method has the advantages of no byproduct, no waste generation, environmental protection, and easiness in realization of industrial production.
Owner:SHANDONG TAIHE WATER TREATMENT TECH CO LTD
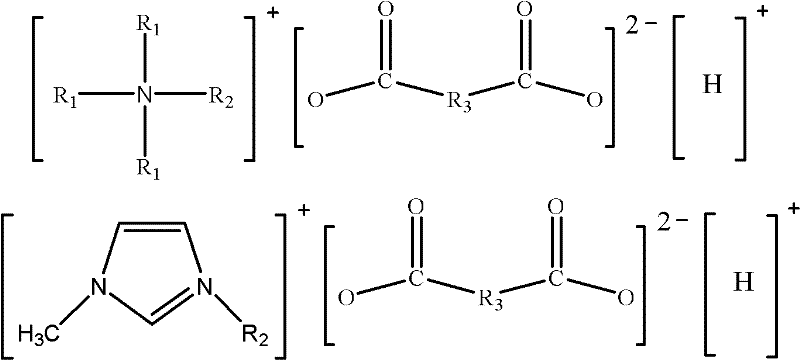
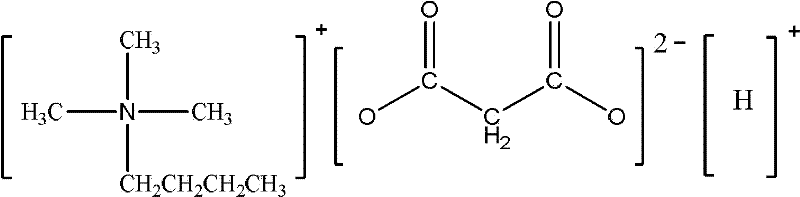
Dicarboxylic hydrogen salt ionic liquid with asymmetric chemical structure and weak acidity, and preparation method thereof
InactiveCN102516092ALow melting pointLow viscosityOrganic compound preparationDispersed particle separationChemical structureQuaternary ammonium cation
The invention relates to a dicarboxylic hydrogen salt ionic liquid with an asymmetric chemical structure and a weak acidity. The cation of the ionic liquid is a tetraalkyl quaternary ammonium cation with the asymmetric structure or an N,N-dialkylimidazole cation with the asymmetric structure, and the anion is a dicarboxylic hydrogen radical anion. The ionic liquid of the invention, which has a low viscosity and a low melting point, can truly meet industrial application requirements. According to the invention, chlorinated tetraalkyl quaternary ammonium with the asymmetric structure or chlorinated N,N-dialkylimidazole with the asymmetric structure and potassium hydrogen dicarboxylate are adopted as raw materials, the target ionic liquid is obtained through one-step synthesis by adopting a microwave process, and the ionic liquid which has the advantages of simple preparation process, high product yield and purity, no byproduct, and low synthetic cost is very suitable for the industrialized production. The dicarboxylic hydrogen salt ionic liquid with the asymmetric chemical structure and the weak acidity of the invention can be used to rapidly and efficiently absorb SO2. The invention discloses a preparation method of the ionic liquid.
Owner:南京大学扬州化学化工研究院 +1
High-molecular-weight low-alkalinity light stabilizer as well as preparation and application thereof
InactiveCN110845729AGood extraction resistancePrevent migration and volatilizationBulk chemical productionSide productOrganic chemistry
The invention belongs to the field of light stabilizers and preparation thereof, and particularly relates to a high-molecular-weight low-alkalinity light stabilizer and preparation and application thereof. The structure of the light stabilizer is shown as a compound I. The light stabilizer has high molecular weight, good extraction resistance, narrow molecular weight distribution and excellent light stability, volatilization and migration in the using process can be effectively prevented, the molecular weight is about 2000-10000, and the molecular weight distribution is below 2.0. The preparation method of the light stabilizer is simple, mild in synthesis condition, easy to operate, high in product yield, free of by-products, clean and environmentally friendly.
Owner:SHENYANG RES INST OF CHEM IND
Polyether modified organosilicon polymer and preparation method and application thereof
ActiveCN113912851AImprove heat resistanceImprove tensile propertiesPolyether adhesivesSilane compoundsEndcapping
The invention provides a polyether modified organosilicon polymer and a preparation method and application thereof. The preparation method of the polyether modified organosilicon polymer comprises the following steps of: S1, carrying out hydrosilylation chain extension reaction on double-end allyl terminated polypropylene glycol and a double-end hydrogen-containing silane compound under the action of a first Pt catalyst to obtain macromolecular double-end allyl terminated polypropylene glycol ether; and S2, carrying out hydrosilylation reaction on the macromolecular double-end allyl terminated polypropylene glycol ether obtained in the step S1 and trimethoxy hydrogen-containing silane under the action of a second Pt catalyst to obtain the polyether modified organosilicon polymer. The polyether modified organosilicon polymer provided by the invention is prepared by adopting two-step hydrosilylation reaction in a specific sequence, and is mild in condition, high in yield, free of byproducts, economical and environment-friendly.
Owner:江西晨光新材料股份有限公司 +1
Stone oil sulphonic acid, its salt, preparation method and uses thereof
InactiveCN101402592BWide variety of sourcesSolving non-sulfonatable problemsDrilling compositionSulfonic acid preparationDistillationEther
The invention discloses petroleum sulfonic acid and salt thereof as well as a preparation method and application thereof. The petroleum sulfonic is prepared by the following method: causing petroleum fractions and nonylphenol polyethenoxy ether to carry out sulfonation reaction to obtain the petroleum sulfonic acid, wherein the distillation range of the petroleum fractions is more than 280 DEG C under normal atmosphere, is preferably between 280 and 600 DEG C. The petroleum sulfonate is prepared by the method comprising the following steps: 1) preparing the petroleum sulfonic acid by the method for preparing the petroleum sulfonic acid; and 2) neutralizing the petroleum sulfonic acid obtained in step 1) with an alkali solution to pH value of between 7 and 9, and then obtaining the petroleum sulfonate. The petroleum sulfonate prepared by the method can reduce oil water interfacial tension to between 10<-2> and 10<-3> mN / m, has good universality for improving recovery ratio for different crude oil systems, and can be used as an oil displacement agent in oil extraction.
Owner:BEIJING TIANYI GERUN NEW ENERGY TECH DEV
g‑c for supercapacitors 3 no 4 Preparation method of carbon quantum dot composite electrode material
ActiveCN103745836BImprove performanceNot easy to decomposeHybrid/EDL manufactureCapacitanceUltrasonic dispersion
A preparation method of g-C3N4 / carbon quantum dot composite electrode material for supercapacitors, adding carbon quantum dots to ethanol to prepare carbon quantum dot ethanol solution; mixing urea with carbon quantum dot ethanol solution, after ultrasonic dispersion Transfer to a crucible, use a muffle furnace to gradually raise the temperature to 350°C-700°C for 1h-3h, then drop to room temperature, add ethanol to grind and filter the obtained material to obtain the g-C3N4 / carbon quantum dot composite material. The advantages are: the preparation method is simple, there is no by-product, the raw material is easy to obtain, and the price is low; the obtained composite material has stable performance, is not easy to decompose, and is non-toxic; it can increase the electron transfer rate of the g-C3N4 material, has good electrical conductivity, and enhances the electrode The specific surface area enhances the ability of the electrode surface to absorb electrons, effectively increasing the specific capacitance of the capacitor.
Owner:BOHAI UNIV
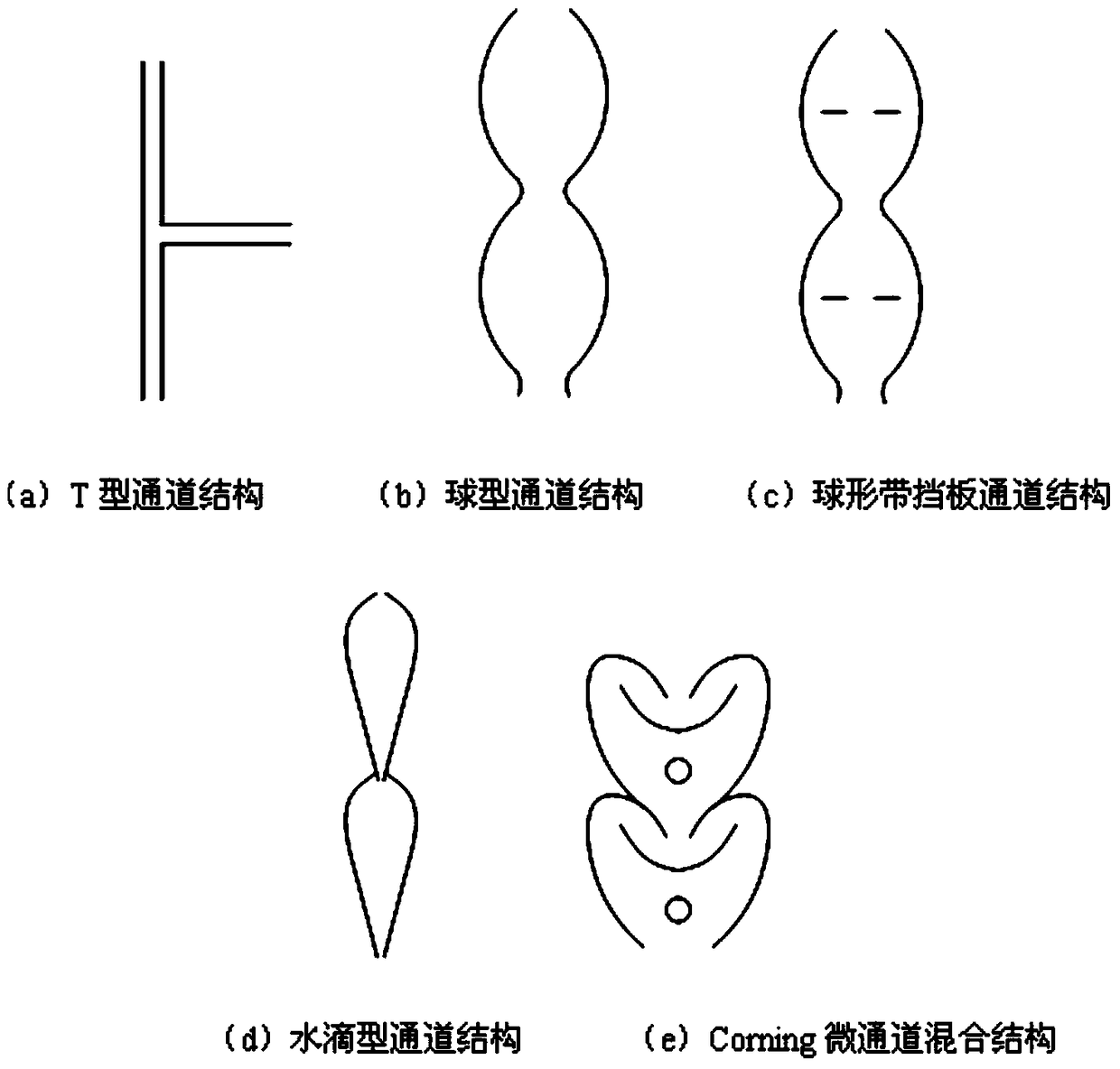
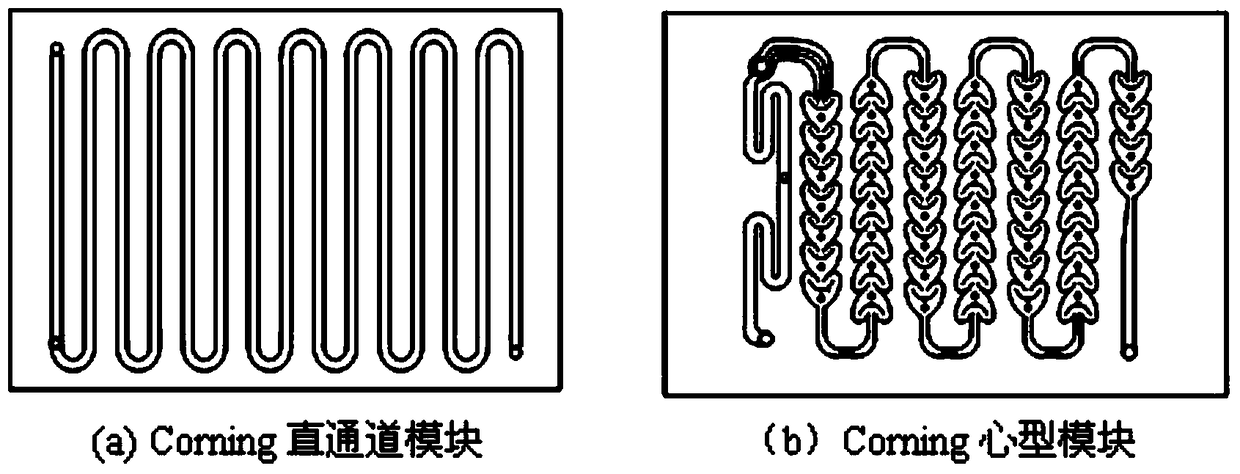
A kind of method that utilizes microchannel reactor to carry out olefin addition reaction
ActiveCN106397106BAdequate responseAtom utilization is highPreparation by halogen additionPhotochemistryAddition reaction
The present invention discloses a method for carrying out addition on olefin and fluorine gas by using a microchannel reactor, wherein C2-C9 olefin or fluoroolefin reacts with F2 in a microchannel reaction module to obtain the corresponding fluoroalkane. According to the present invention, with the method, the purity of the prepared fluoroalkane is high, and the method has the cost advantage.
Owner:ZHEJIANG RES INST OF CHEM IND CO LTD +1
Preparation method of fluorine resin
The invention discloses a preparation method of fluorine resin, comprising the following steps of: mixing 33-62 parts by weight of perfluoroalkyl iodide and 0.3-0.8 parts by weight of initiator, and then heating to 60-100 DEG C, wherein the initiator is benzoyl peroxide or azobisisobutyronitrile; dropping 7-14 parts by weight of olefin, wherein the olefin is one or two of styrene, octylene, pinene and limonene; reacting at 60-100 DEG C for 3-5 hours; then adding 75-100 parts by weight of solvent, 28-33 parts by weight of acetic acid and 65-70 parts by weight of zinc powder, wherein the solvent is isopropanol or xylene; reacting at 60-100 DEG C for 3-5 hours to obtain a product; and filtering, water-washing, dehydrating and separating the product to obtain a target product. A continuous adding manner is adopted in the method, the reaction is easy to control, no byproduct is generated, the obtained product is easy to separate, and has high selectivity, low frictional factor (0.05-0.1), a water repellence angle of over 110 DEG and high oil repellency up to the first grade, does not freeze at 35 DEG C below zero, and does not decompose at 350 DEG C; and the fluorine resin can be used in the fields such as deep-sea drilling, ignitable ice exploiting, astronautical technology, equipment lubrication under the working condition of permanent freezing layers, and the like.
Owner:JINZHOU DPF TH CHEM CO LTD
Recycling method of waste lithium iron phosphate positive electrode material
ActiveCN111392706AImprove conductivityGood atom economyPositive electrodesWaste accumulators reclaimingPhosphateAtom economy
The invention relates to a recycling method of a waste lithium iron phosphate positive electrode material. The method comprises the following steps: adding phosphate into the waste lithium iron phosphate positive electrode material, then carrying out supercritical carbon dioxide extraction, and filtering to obtain a solid intermediate A; heating the solid intermediate A to obtain a solid intermediate B; and adding a polymer containing a benzene ring into the solid intermediate B, and calcining to obtain regenerated lithium iron phosphate. The method is simple in process, low in environmental pressure, low in manufacturing cost, good in atom economy, controllable in quality and easy to industrialize, and the final product is good in performance.
Owner:GUANGDONG GUANGHUA SCI TECH +1
A flue gas denitrification device for co waste heat boiler
ActiveCN104415660BCompact layoutSmall footprintDispersed particle separationAmmonia storageEngineering
Owner:美斯顿(天津)催化剂有限公司
Method for preparing arginine ketoglutaric acid by spraying method
PendingCN111925305AOptimize the production processNo by-productsOrganic compound preparationCarboxylic acid salt preparationKetoglutaric AcidChemistry
The invention relates to the field of arginine ketoglutaric acid preparation processes, in particular to a method for preparing arginine ketoglutaric acid by a spraying method. The method comprises the following steps: 1, starting up for inspection; 2, draining water from the system; 3, air supply testing; 4, adjusting the temperature; 5, adjusting the pressure; 6, spray testing; 7, high-pressurefeeding; 8, detecting the temperature; 9, dehumidifying and refluxing; and 10, centralized material collection. The beneficial effects are that: production is carried out by adding a set pressure spraying mode, the production process is optimized, byproducts are avoided, the production yield is high, production pollution is avoided, all the used raw materials are fermented and produced by themselves, the product yield is high, the product quality is good, and compared with a traditional water reaction recrystallization method, a one-time spraying method is adopted in the production process forproduction, the production process is less in pollution, the yield is higher, and the product quality is greatly improved.
Owner:JING JING PHARMA
Decolorizing method of (meth)acrylic acid
ActiveCN102260161BImprove efficiencyReduce energy consumptionCarboxylic compound separation/purificationMeth-Temperature and pressure
The invention discloses a decolorizing method of (meth)acrylic acid. The decolorizing method is characterized by employing a falling film crystallization technology. The used technology comprises the following steps: a) adding 90-95wt% of acrylic acid or methacrylic acid mother liquor into a crystallizer in accordance with the capacity of the crystallizer; b) starting a freezer, and then operating the crystallizer under required temperature and pressure conditions; c) circulating the acrylic acid or methacrylic acid mother liquor at a certain rate, and cooling for crystallization; d) melting the crystallized part, and cutting the residual liquid in a certain ratio; e) adding 75wt% of polymerization inhibitor (150+ / -20 ppm) when the melting of the crystallized part is soon finished; f) completely melting the crystal, cutting the residual liquid in a certain ratio, adding the remaining 25wt% of polymerization inhibitor (50+ / -20 ppm), and returning the obtained product to a raw material tank; and g) repeating the above steps from a) to f) for secondary crystallization, and conveying the obtained product into a finished product tank. The decolorizing method has the advantages of high efficiency, low energy consumption, no byproduct generation, no pollution and the like.
Owner:SHANGHAI HUAYI NEW MATERIAL
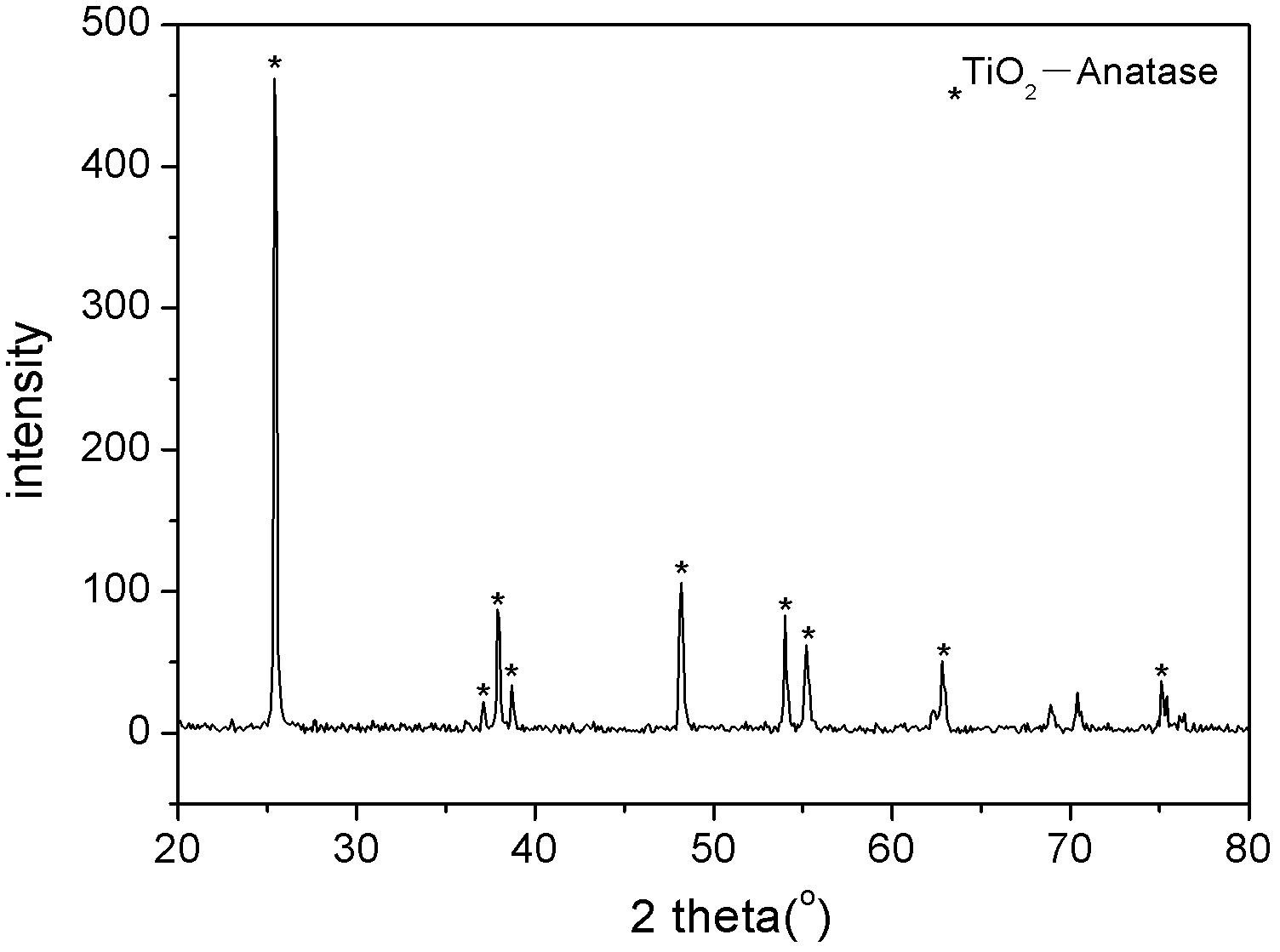
A bundle of tiof 2 low temperature preparation method
InactiveCN105148953BPromote degradationReduce reflectivityPhysical/chemical process catalystsTetraWavelength range
Owner:XIAN UNIV OF SCI & TECH
Preparation method of 3-nitrophthalic anhydride
The invention relates to a preparation method of 3-nitrophthalic anhydride. The method includes the following steps: placing 3-nitrophthalic acid in a reaction solvent, adding a catalyst, and heatingfor reaction; and removing the reaction solvent and the catalyst from an obtained substance under negative pressure to obtain the 3-nitrophthalic anhydride. In the preparation method, the 3-nitrophthalic acid raw material can be easily obtained, the 3-nitrophthalic anhydride is synthesized by direct catalytic dehydration in one step, and the preparation method has the advantages of simple process,low cost, high yield and no by-products.
Owner:JIANGSU YONGAN CHEM CO LTD
A kind of production method of laurolactam
ActiveCN109516941BImprove conversion rateNo by-productsLactams preparationCatalyst activation/preparationLaurolactamBeckmann rearrangement
A production method of laurolactam, in the presence of a solvent a, the oximation reaction is carried out with cyclododecanone and Raschig liquid as raw materials; the oil phase obtained by liquid separation is removed by pressure distillation and the solvent is recovered, and the obtained Molten cyclododecanone oxime is dissolved in solvent b, and undergoes Beckmann rearrangement reaction under the catalysis of rare earth metal-modified magnetic solid superacid catalyst c, and the laurolactam product is obtained after refining.
Owner:WANHUA CHEM GRP CO LTD
A kind of silicon calcium phosphate nanopowder, preparation method and application
ActiveCN109455720BEfficient synthesisHigh purityMaterial nanotechnologyTissue regenerationCalcium biphosphateFluid phase
The invention relates to a silicon calcium phosphate nanopowder, a preparation method and an application. The preparation method includes: mixing the calcium source with the first liquid phase dispersant to prepare a calcium source dispersion system, then adding the silicon source dropwise, and dispersing evenly to obtain a calcium-silicon mixed liquid; mixing the phosphorus source with the second liquid phase dispersant , to be prepared into a phosphorus source dispersion system; the phosphorus source dispersion system is added dropwise to the calcium-silicon mixed solution, and the mixed system containing the calcium-silicon mixed solution and the phosphorus source dispersion system is ultrasonically treated during the dropping process, and the addition ends Afterwards, ultrasonic treatment is continued; the mixed system after ultrasonic treatment is filtered, washed, and dried to obtain a silicon calcium phosphate precursor; the silicon calcium phosphate precursor is calcined to obtain silicon calcium phosphate nanopowder. The silicon calcium phosphate nanometer powder obtained by the method has high purity, nanoscale particle size, low degree of agglomeration, good dispersion, low calcining temperature, cheap raw materials, simple operation, no by-products, and easy industrial production.
Owner:上海积力威尔生物科技合伙企业(有限合伙)
Method for recovering triethylamine hydrochloride from glyphosate reaction liquid produced by glycine process
InactiveCN112538019AReduce environmental costsSignificant environmental benefitsAmino compound purification/separationGroup 5/15 element organic compoundsSolventSpray dried
The invention discloses a method for recovering triethylamine hydrochloride from glyphosate reaction liquid produced by a glycine process. The method comprises the following steps: S1, removing waterand hydrochloric acid from the glyphosate reaction liquid to obtain a solid mixture; S2, adding a solvent into the solid mixture, conducting heating, and carrying out phase transfer to obtain a solvent phase, wherein a solid phase is also obtained in the step and is used for synthesizing glyphosate; S3, cooling the solvent phase to obtain a crude triethylamine hydrochloride product; and S4, centrifuging the obtained crude triethylamine hydrochloride product, and carrying out spray drying to obtain a finished triethylamine hydrochloride product. According to the invention, on the premise of ensuring the content of triethylamine hydrochloride in the finished triethylamine hydrochloride product, the yield of triethylamine hydrochloride is increased.
Owner:溧阳市凯明化工技术咨询服务有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com