Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
48results about How to "Good sensitivity and specificity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
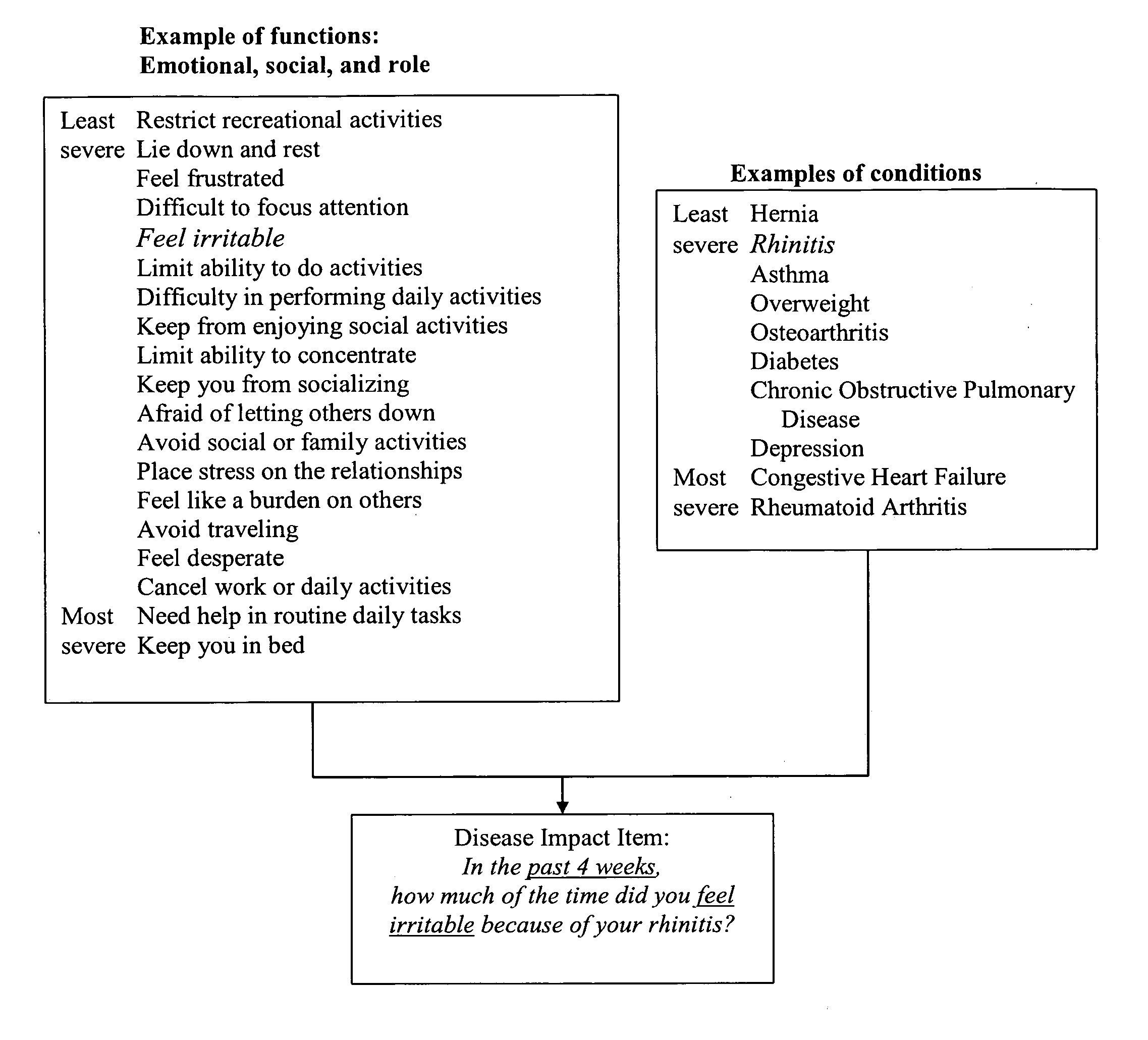
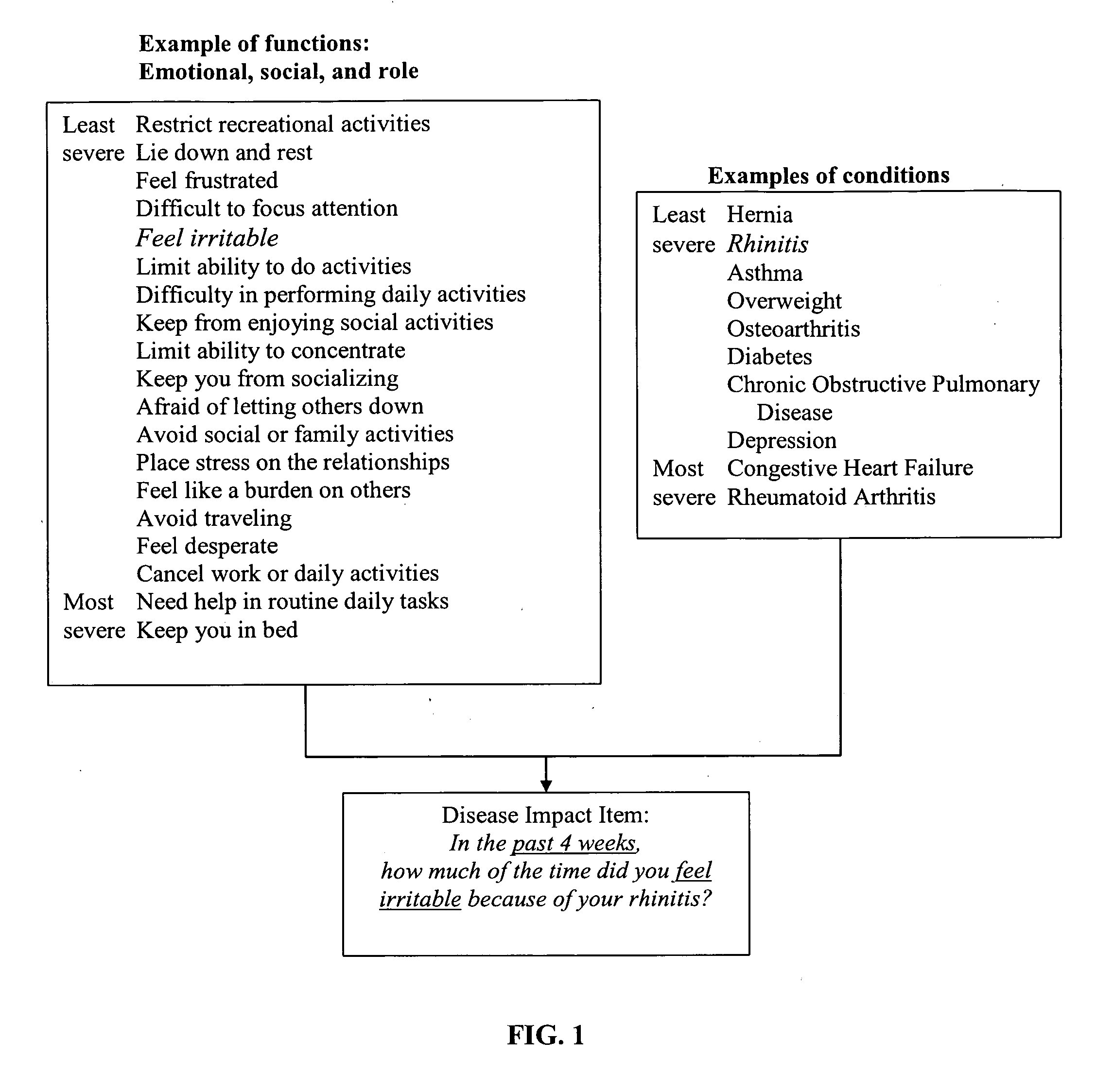
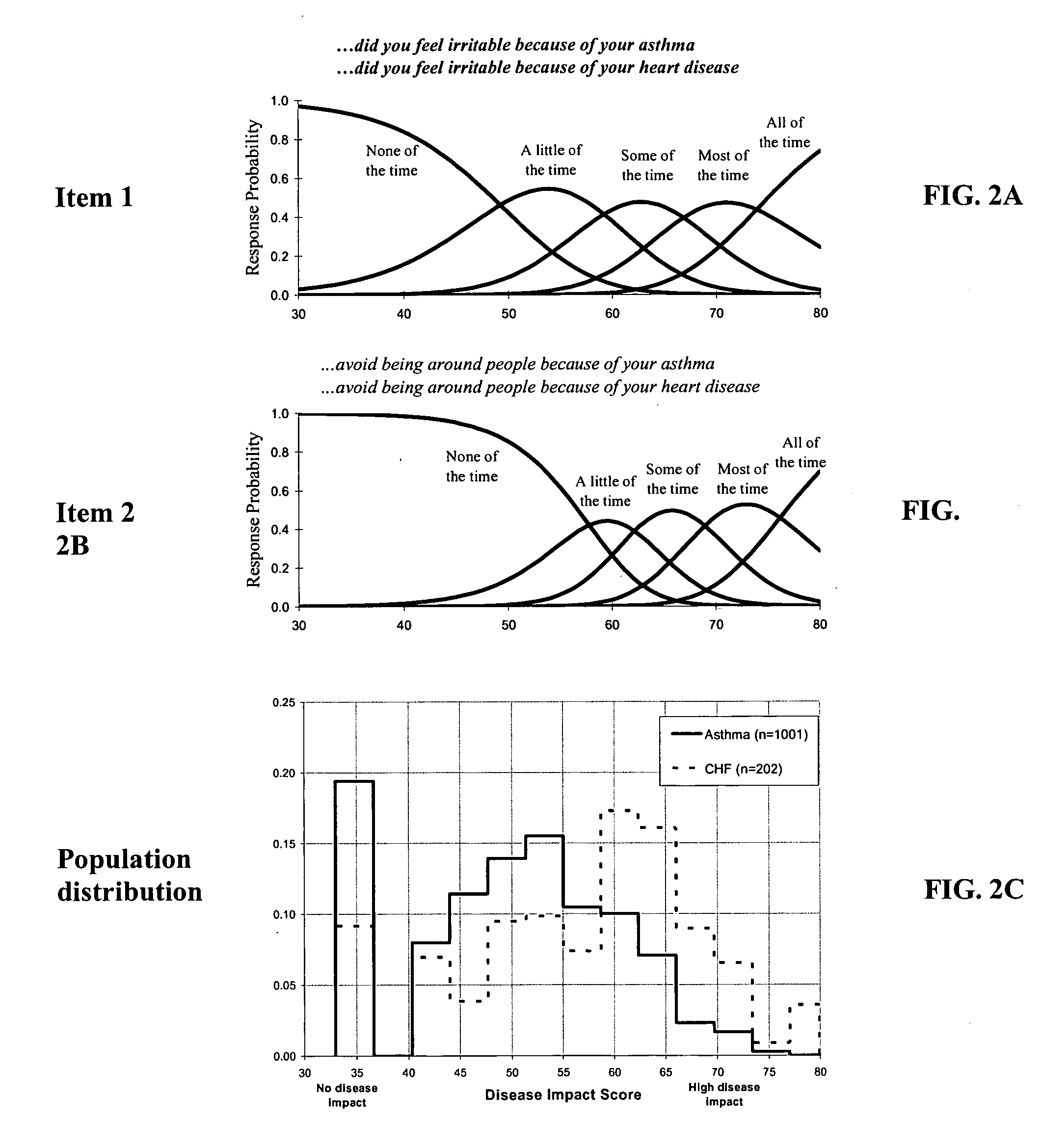
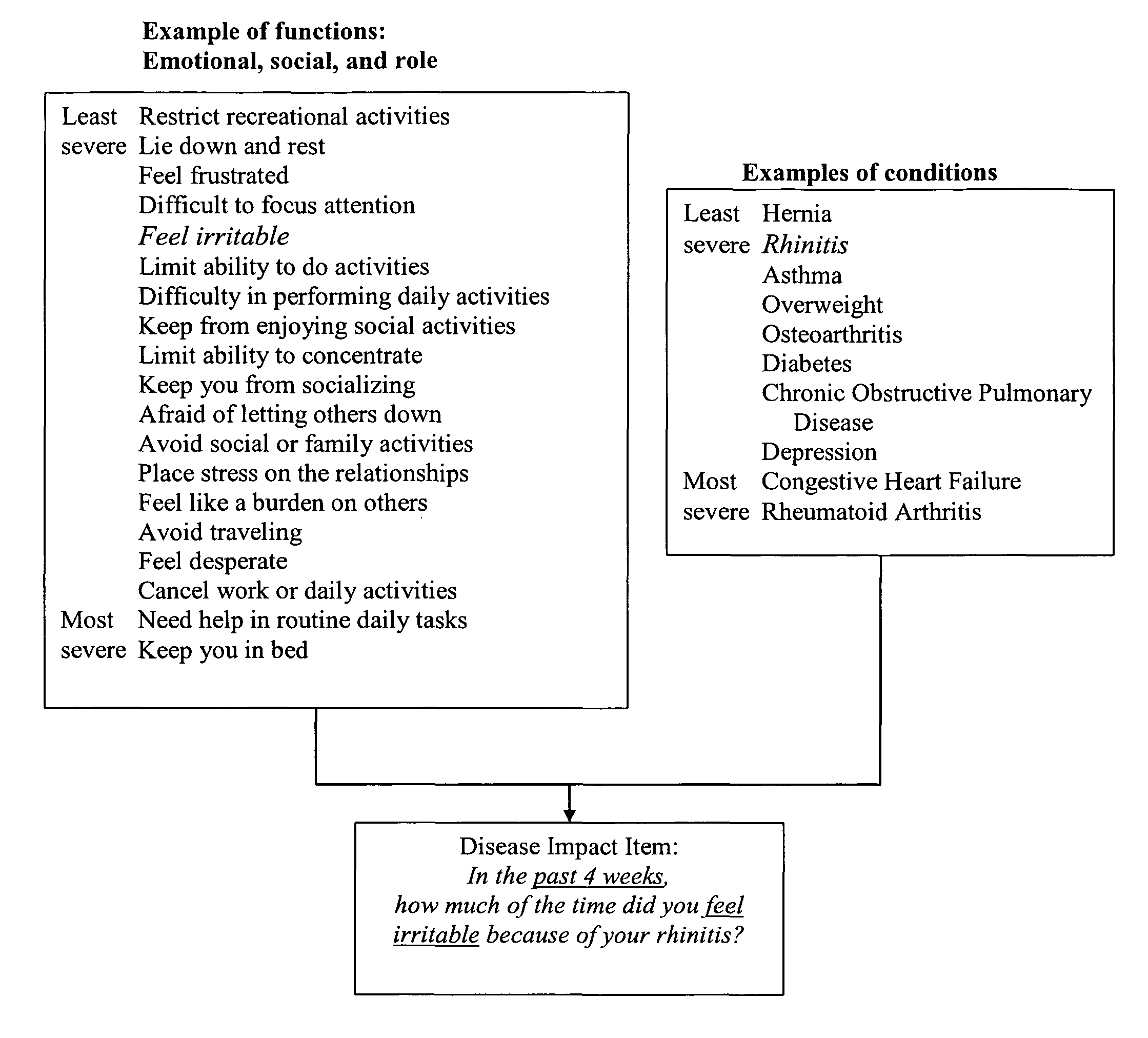
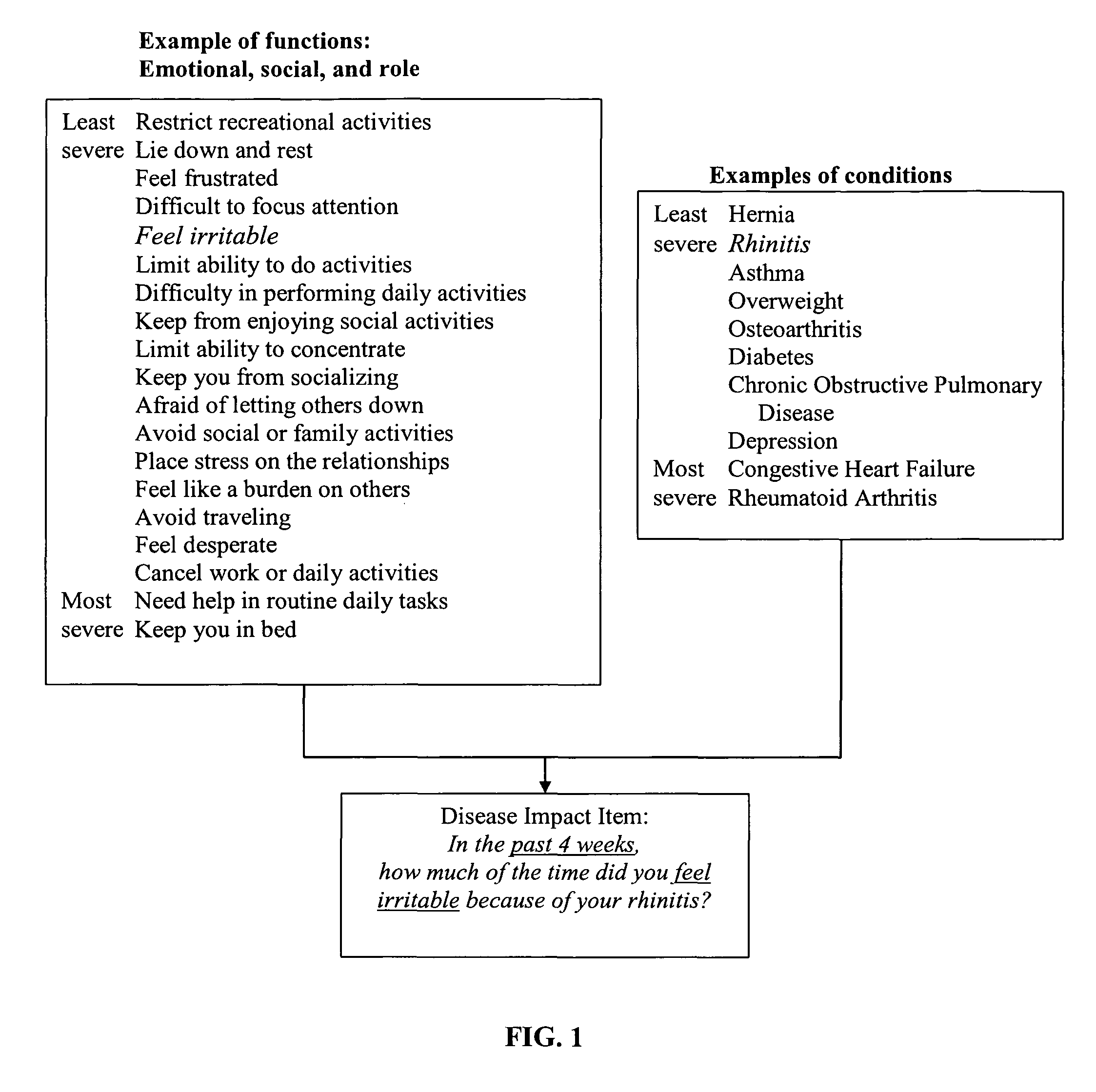
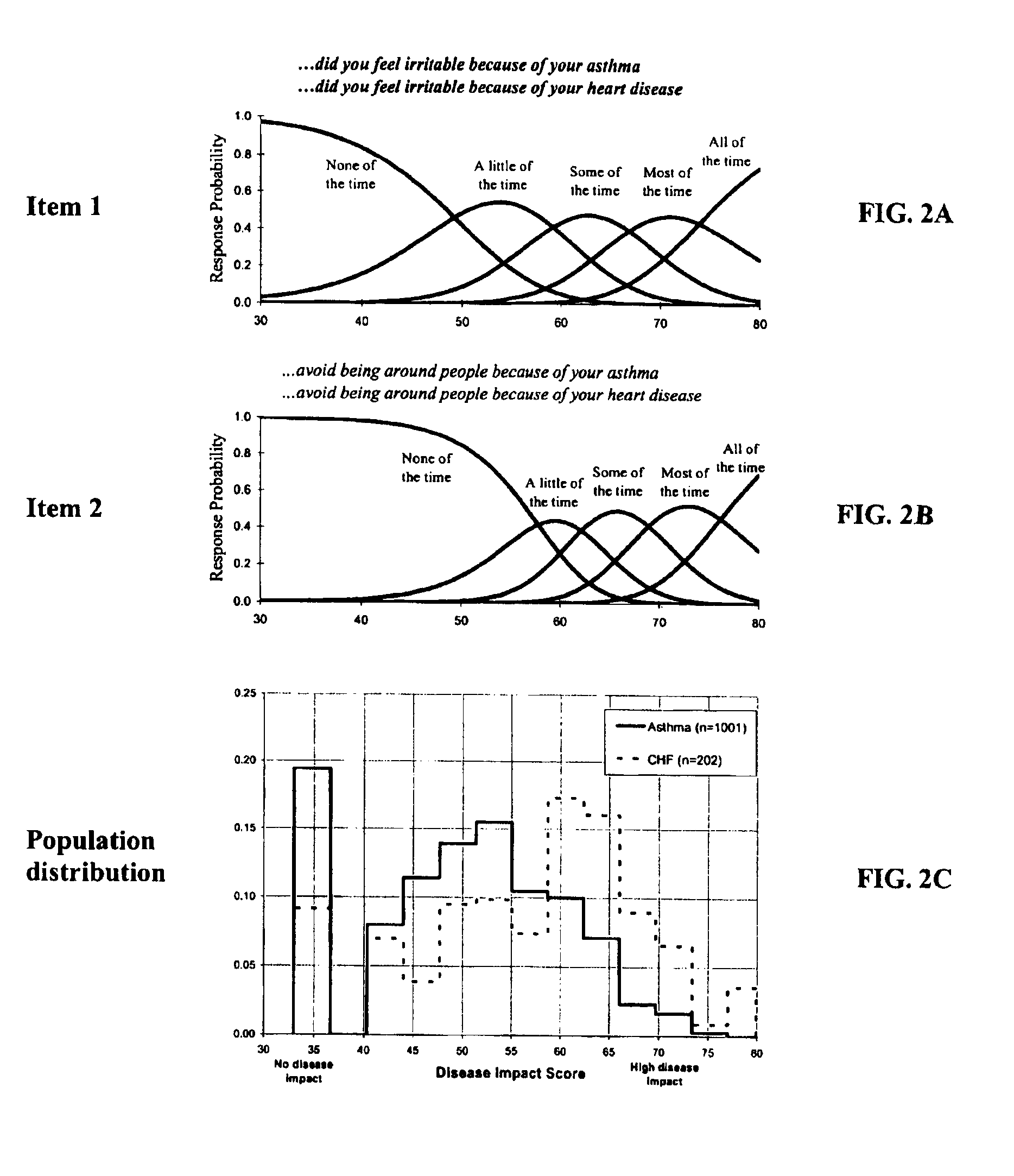
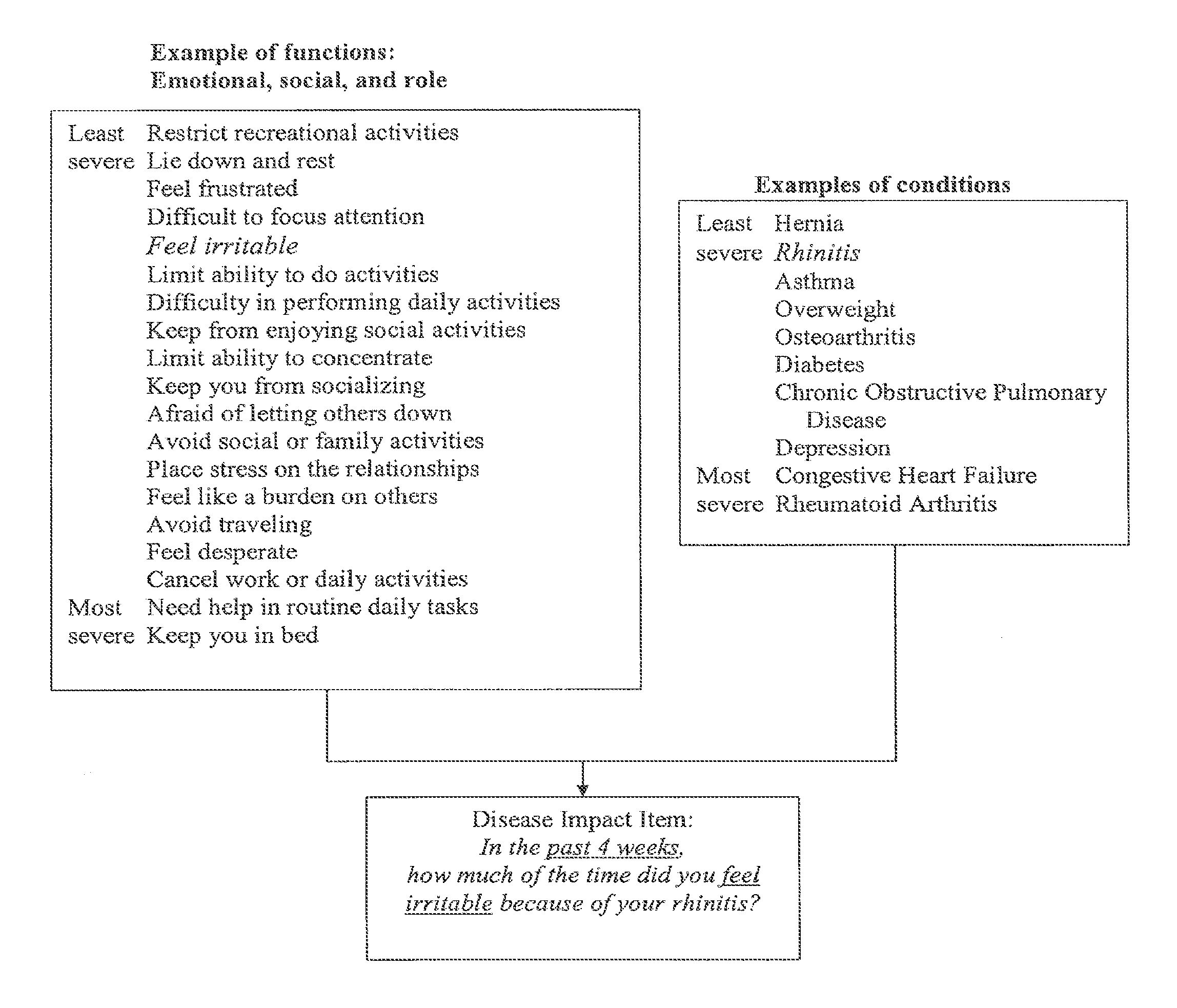
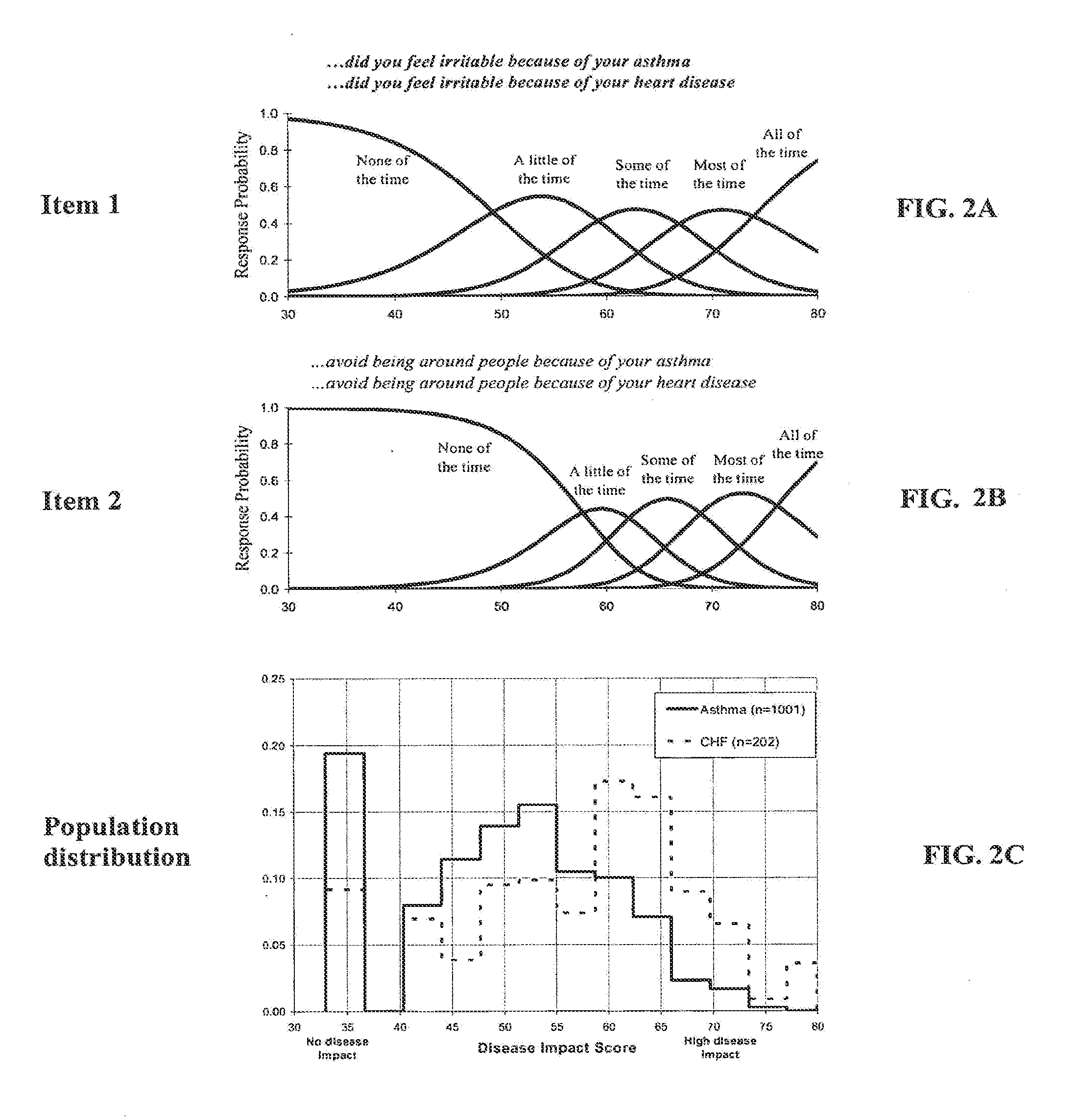
Method, system and medium for assessing the impact of various ailments on health related quality of life
InactiveUS20060218007A1Low costSpeed up the processComputer-assisted medical data acquisitionOffice automationDiseaseHealth related quality of life
The present invention relates to a system and method for assessing the impact of an ailment on a health related quality of life domain of a patient using a standardized common metric. The standardized common metric of the present invention enables the impact of various ailments to be compared.
Owner:QUARTERMASTER NEWCO LLC
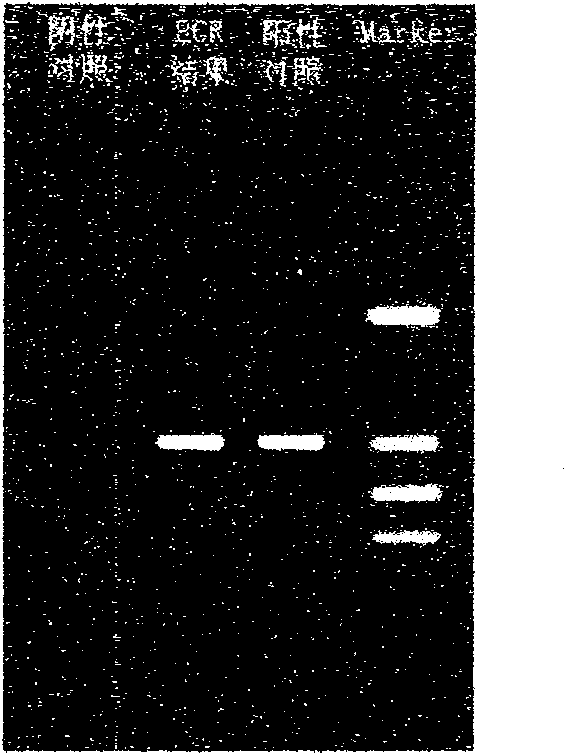
Fluorescent PCR (Polymerase Chain Reaction) detection kit for African swine fever virus, preparation method and application method
ActiveCN108300808AGood sensitivity and specificityReduce missed detectionMicrobiological testing/measurementEnzymeNegative control
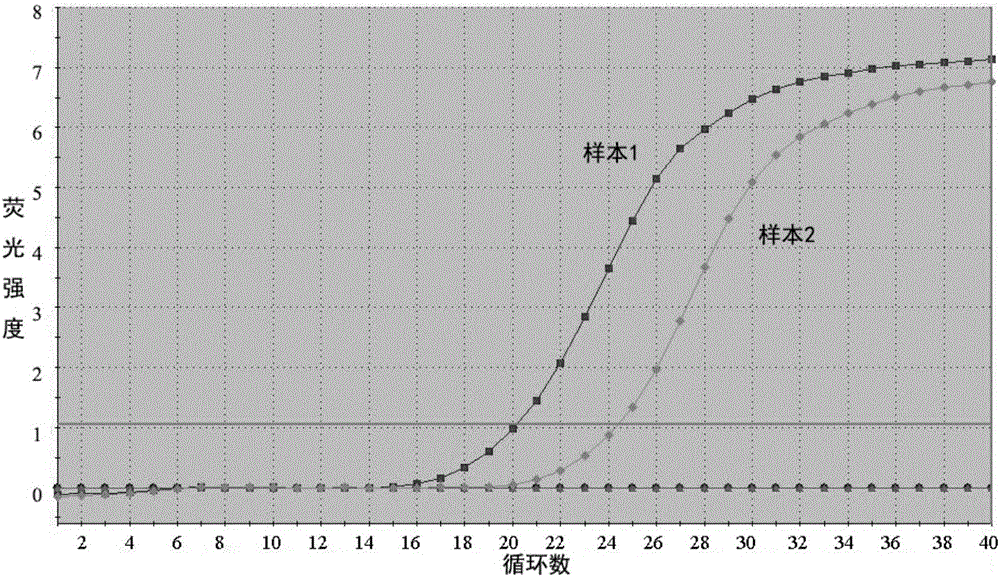
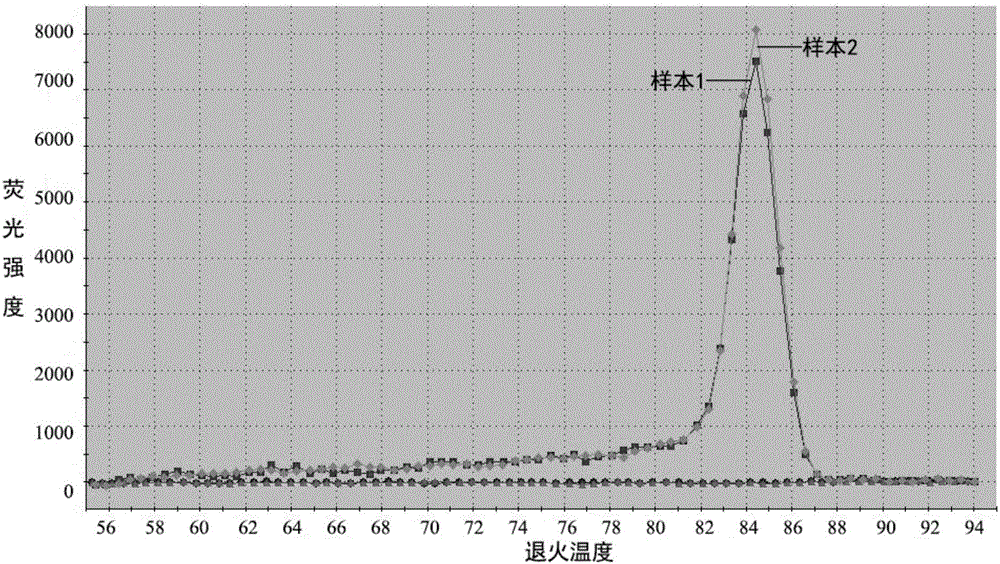
The invention discloses a fluorescent PCR (Polymerase Chain Reaction) detection kit for African swine fever virus (ASFV). The fluorescent PCR detection kit comprises an ASFV-reaction solution, a DNA (Deoxyribonucleic Acid) enzyme mixed solution, an ASFV-internal standard, an ASFV-positive control, an ASFV-negative control, a nucleic acid releasing reagent and an ASFV-quantitative reference material, wherein the ASFV-reaction solution is prepared from primers: ASF03F03 and ASF03R03; internal standard primers: IPC05F01 and IPC05R01; a probe ASF03P and an internal standard probe IPC05P; the primers and the probes correspond to highly conserved fragments of an African swine fever virus p72 gene. The invention further discloses a method for preparing the fluorescent PCR detection kit for the African swine fever virus and a detection method utilizing the fluorescent PCR detection kit.
Owner:湖南国测生物科技有限公司 +1
Method, system and medium for assessing the impact of various ailments on health related quality of life
InactiveUS7818185B2Convenient treatmentSmall administration feeComputer-assisted medical data acquisitionDiagnostic recording/measuringDiseaseHealth related quality of life
The present invention relates to a system and method for assessing the impact of an ailment on a health related quality of life domain of a patient using a standardized common metric. The standardized common metric of the present invention enables the impact of various ailments to be compared.
Owner:QUARTERMASTER NEWCO LLC
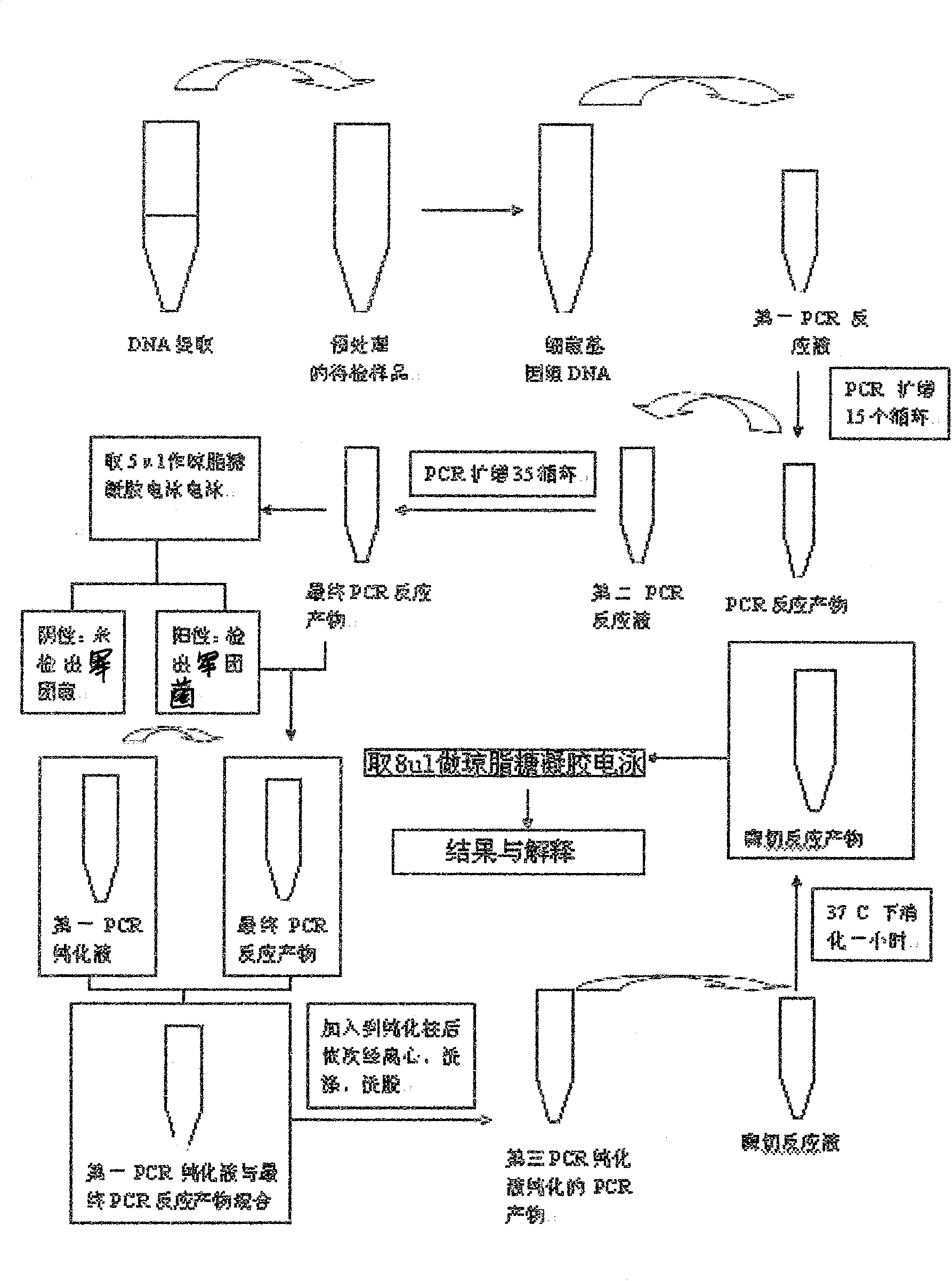
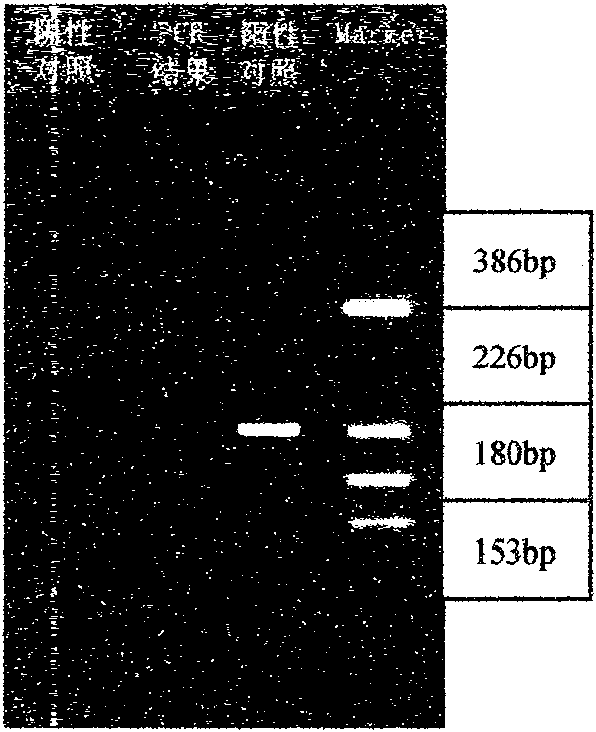
Rapid detection kit of legionella bacteria and detection method thereof
ActiveCN101597647AEasy to useGood sensitivity and specificityMicrobiological testing/measurementMicroorganism based processesGenomic DNABiology
The invention discloses a rapid detection kit of legionella bacteria, comprising bacteria genomic DNA extracting solution, PCR reaction solution, PCR product purification solution and restriction endonuclease solution; wherein the solutions are respectively placed in a container; the PCR reaction solution comprises a first PCR reaction solution and a second PCR reaction solution; and the first PCR reaction solution and the second PCR reaction solution are respectively placed in containers. The invention also discloses a detection method of the kit. The invention can be used for detecting not only clinical samples such as the legionella bacteria in sputum and bronchial lavage fluid, but also the legionella bacteria in water sample, and has the advantages of high speed, simple and convenient and easy usage.
Owner:GUANGZHOU KINGMED DIAGNOSTICS CENT
Method, system and medium for assessing the impact of various ailments on health related quality of life
InactiveUS20130144645A1Convenient treatmentSmall administration feeFinanceHealth-index calculationDiseaseHealth related quality of life
The present invention relates to a system and method for assessing the impact of an ailment on a health related quality of life domain of a patient using a standardized common metric. The standardized common metric of the present invention enables the impact of various ailments to be compared.
Owner:OPTUMINSIGHT LIFE SCI
Gastric cancer molecular marker hsa_circ_0006633 and its application
ActiveCN107177669AGood sensitivity and specificityGastric cancer tissue specificity is goodMicrobiological testing/measurementDNA/RNA fragmentationFluorescenceMolecular biology
The invention relates to a gastric cancer molecular marker hsa_circ_0006633 and its application. The nucleotide sequence of the molecular marker is shown as SEQ ID NO.1, and the molecular marker is a closed loop RNA molecular marker. The molecular marker has significant expression in a gastric cancer tissue and extremely good gastric cancer correlation. Through applying CircRNA chip, real-time fluorescence quantification PCR detection and other technologies and combining with characteristics of simplicity, economy and practicability, the feasibility of using circRNA as a gastric cancer screening marker is explored; the gastric cancer molecular marker is of significance in clinical diagnosis and application.
Owner:叶国良
Peste des petitis ruminants virus N protein monoclonal antibody, test strip comprising same and preparation method thereof
InactiveCN105017416AGood sensitivity and specificityQuick checkMicroorganism based processesImmunoglobulins against virusesPolyclonal antibodiesImmunochromatographic test
The invention belongs to the technical field of animal virus detection and particularly relates to a peste des petitis ruminants virus N protein monoclonal antibody, a test strip comprising the same and a preparation method thereof. The test strip comprises backing, a sample pad, a fluorescent microsphere-marked peste des petitis ruminants virus monoclonal antibody composite pad, a nitrocellulose membrane with spotted detection line and control line and a water absorption pad, wherein the detection line is coated with a goat anti-peste des petitis ruminants virus polyclonal antibody, the control line is coated with staphylococcus A protein, the peste des petitis ruminants virus monoclonal antibody is the peste des petitis ruminants virus N protein monoclonal antibody which is generated by a peste des petitis ruminants virus monoclonal antibody hybridoma cell strain 117-6H6, and the preservation number of the peste des petitis ruminants virus monoclonal antibody hybridoma cell strain 117-6H6 is CCTCC NO. C2014233. The established des petitis ruminants virus fluorescent immunochromatographic test strip detection test strip can be used for rapid detection and field detection of PPRV, so that the situation that PPRV rapid detection and field detection methods and detection tools are lacking at home is solved.
Owner:杨俊兴
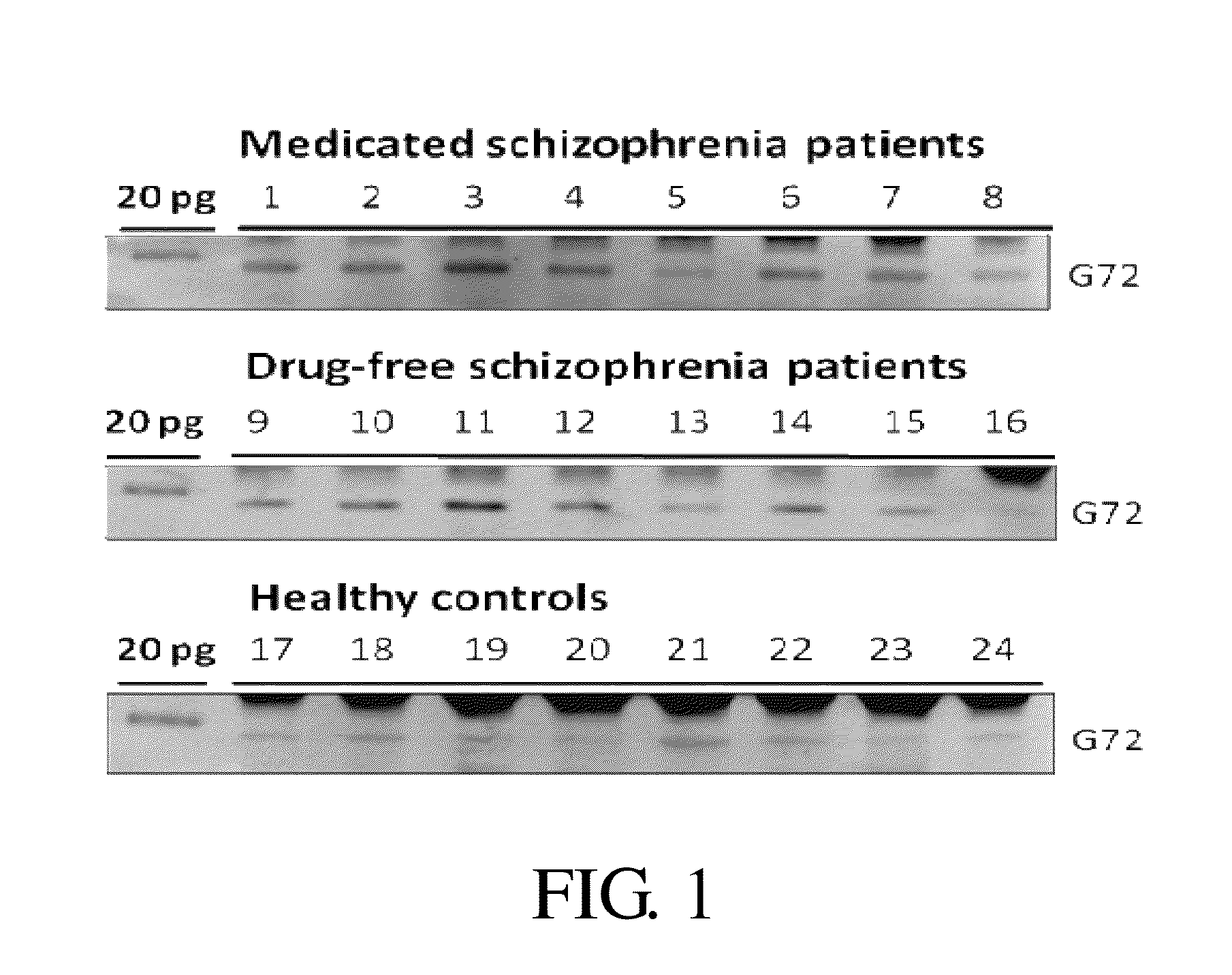
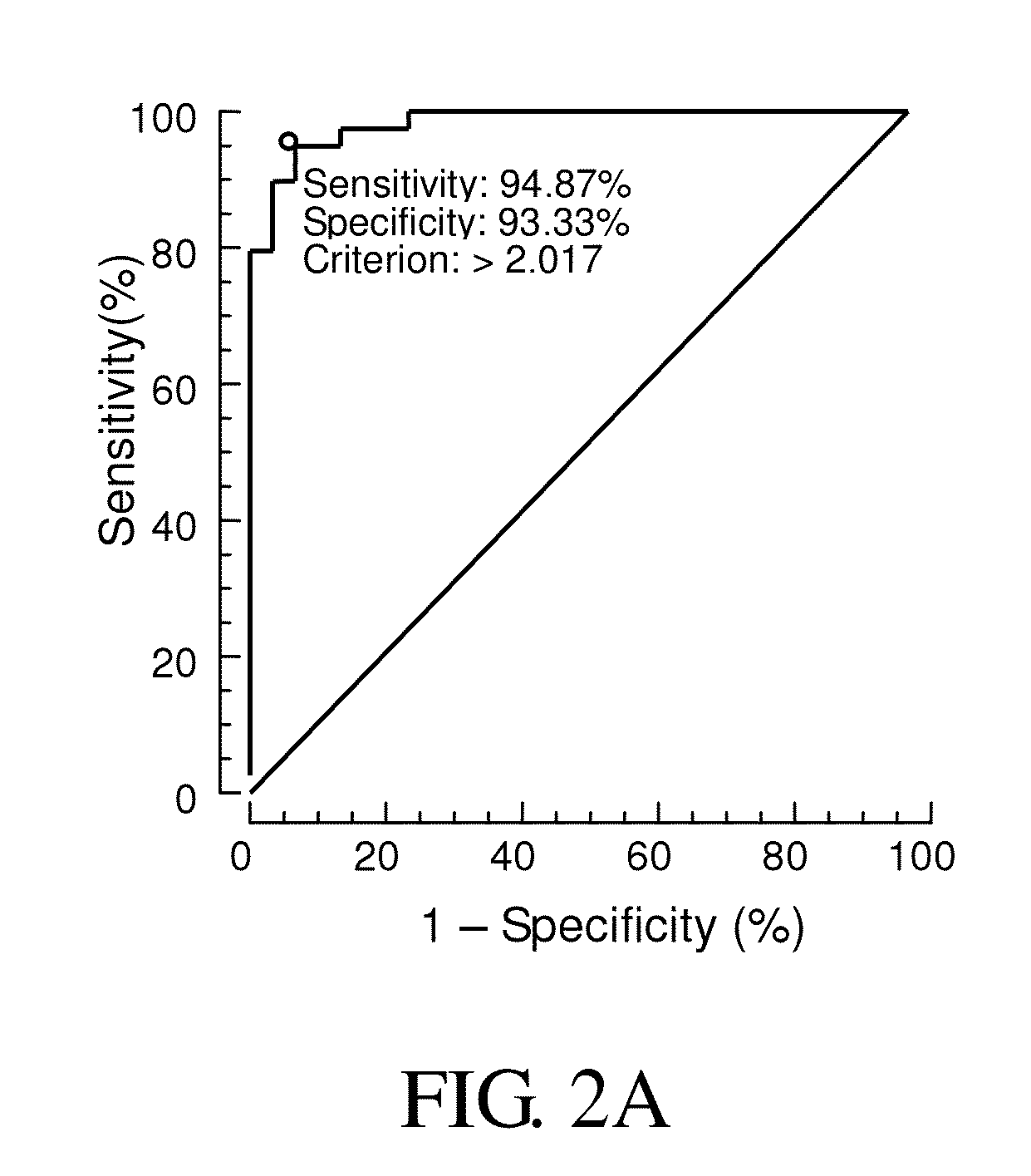
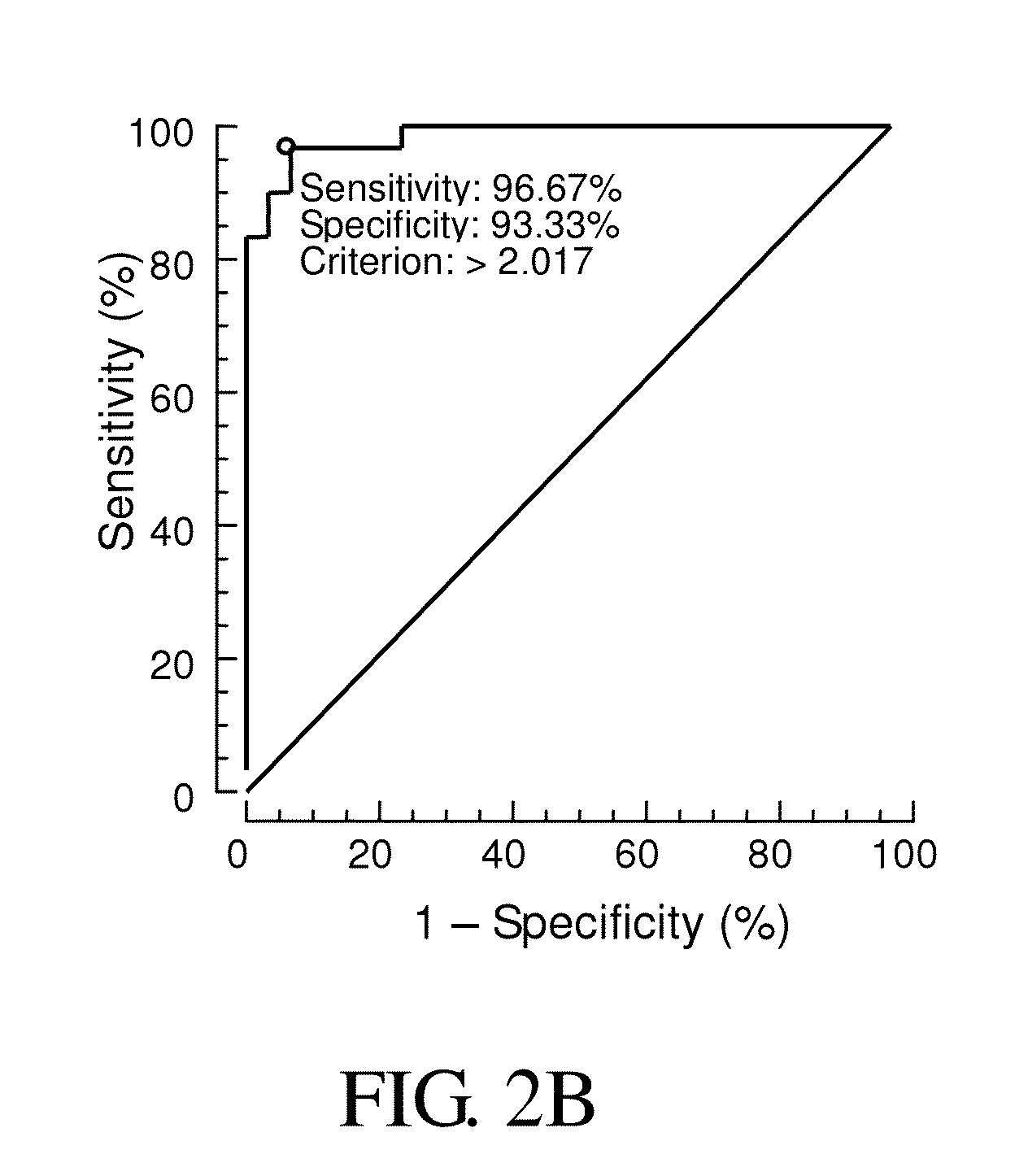
Compositions and methods for diagnosis of schizophrenia
InactiveUS20140171413A1Good sensitivity and specificityElevationOrganic active ingredientsBiocideGene productIncreased risk
Provided is a method for diagnosis of schizophrenia, which comprises: detecting G72 gene product in a body fluid sample from a subject by an assay to determine G72 expression level; comparing said G72 expression level to a baseline G72 expression; and relating the G72 expression level to the patient's risk of schizophrenia by assigning an increased risk of schizophrenia when said G72 expression level is greater than said baseline G72 expression. Through detecting G72 expression level in a peripheral sample, the method can be simply performed by an in vitro assay and accurately predict or diagnose a subject with schizophrenia.
Owner:CHINA MEDICAL UNIVERSITY(TW)
An oligonucleotide gene chip and its application in the detection of various germs
InactiveCN102260738AGood repeatabilityStrong specificityMicrobiological testing/measurementNucleotideOligonucleotide
The invention discloses an oligonucleotide gene chip and application of the oligonucleotide gene chip to the detection of various bacteria. Probes of the oligonucleotide gene chip can be hybridized with polymerase chain reaction (PCR) amplification products of M. tuberculosis, M. bovis, M. avium, M. paratuberculosis and Brucella respectively; and the probes have nucleotide sequences shown as SEQ ID No:25, SEQ ID No:31, SEQ ID No:35, SEQ ID No:37 and SEQ ID No:39 respectively. The gene chip is applied to the detection of the bacteria and has high repeatability, and interassay and intraassay coefficients of variation (CV) are less than 15 percent. The gene chip shows high accuracy on the detection of a pathogenic bacterium sample to be detected, which indicates that the method has high specificity and sensitivity; the high-throughput and parallel detection is realized; detection results are quick and accurate; the whole process from the preparation of nucleic acid to the finishing of the detection only needs 6 to 8h; and the gene chip has broad prospect in preparing reagents and products for pathogen detection, import and export quarantine and epidemiological analysis.
Owner:SOUTH CHINA AGRI UNIV
Colon cancer diagnostic marker and application thereof
ActiveCN109884300AGood sensitivity and specificityGood clinical valueComponent separationPyridoxic AcidPantothenic acid
The invention discloses a colon cancer diagnostic marker and application thereof. The colon cancer diagnostic marker is urine metabolites and includes at least one of pseudouridine, N2, N2-dimethylguanosine, kynurenine, valine, N-acetylL-valine, N-acetyl-L-glutamic acid, 5-hydroxytryptophan, alpha, beta-didehydrotryptophan, hippuric acid, aspartyl-phenylalanine, taurine, pantothenic acid, 2,3-pyridinedicarboxylic acid, 4-pyridoxic acid, L-carnitine and xanthine. Based on the marker, the invention further provides a colon cancer detection kit which can effectively distinguish colon cancer patients from healthy people and has high accuracy, sensitivity and specificity. A sample selected by the colon cancer diagnostic marker and the colon cancer detection kit is a urine sample, preferably themiddle part of fasting morning urine, invasive samples such as tissues and blood are avoided, and the colon cancer diagnostic marker and the colon cancer detection kit have higher patient complianceand clinical application value.
Owner:CHINA PHARM UNIV
Detection method for AMACR auto-antibody and its application in prostatic cancer diagnosis
InactiveCN101344525AGood sensitivity and specificityHigh purityMaterial analysisOptical absorbanceAntigen
The invention discloses an AMACR auto-antibody test method and the application of the method in prostatic cancer diagnosis, which relates to the field of extracorporeal immune detection, and provides an ELISA detection method of an AMACR auto-antibody, that comprises the following steps: a specific antigen AMACR is used for enveloping a millipore reaction plate, blood serum to be measured is added into the millipore reaction plate, and simultaneously blank contrast, negative contrast and positive contrast are set; enzyme tag secondary antibody is added, and color reaction, termination reaction and reading are implemented; an optical absorbance is read at the 450nm position of an ELISA reader, normal blood serum is used as feminine to contrast optical absorbance Bo with the optical absorbance B of a sample to be measured, and if B is more than or equal to 2.1 times of the Bo value, the prostatic cancer diagnosis result is determined to be positive. The AMACR auto-antibody test method is applied to the prostatic cancer diagnosis and used for detecting the AMACR auto-antibody in human blood that is used as a marker in the prostatic cancer diagnosis. Compared with existing prostatic cancer diagnosis methods in the current domestic market that detect PSA concentration in human blood, the AMACR auto-antibody test method has the advantages of greatly-raised sensitivity and specificity.
Owner:无锡纳生生物科技有限公司
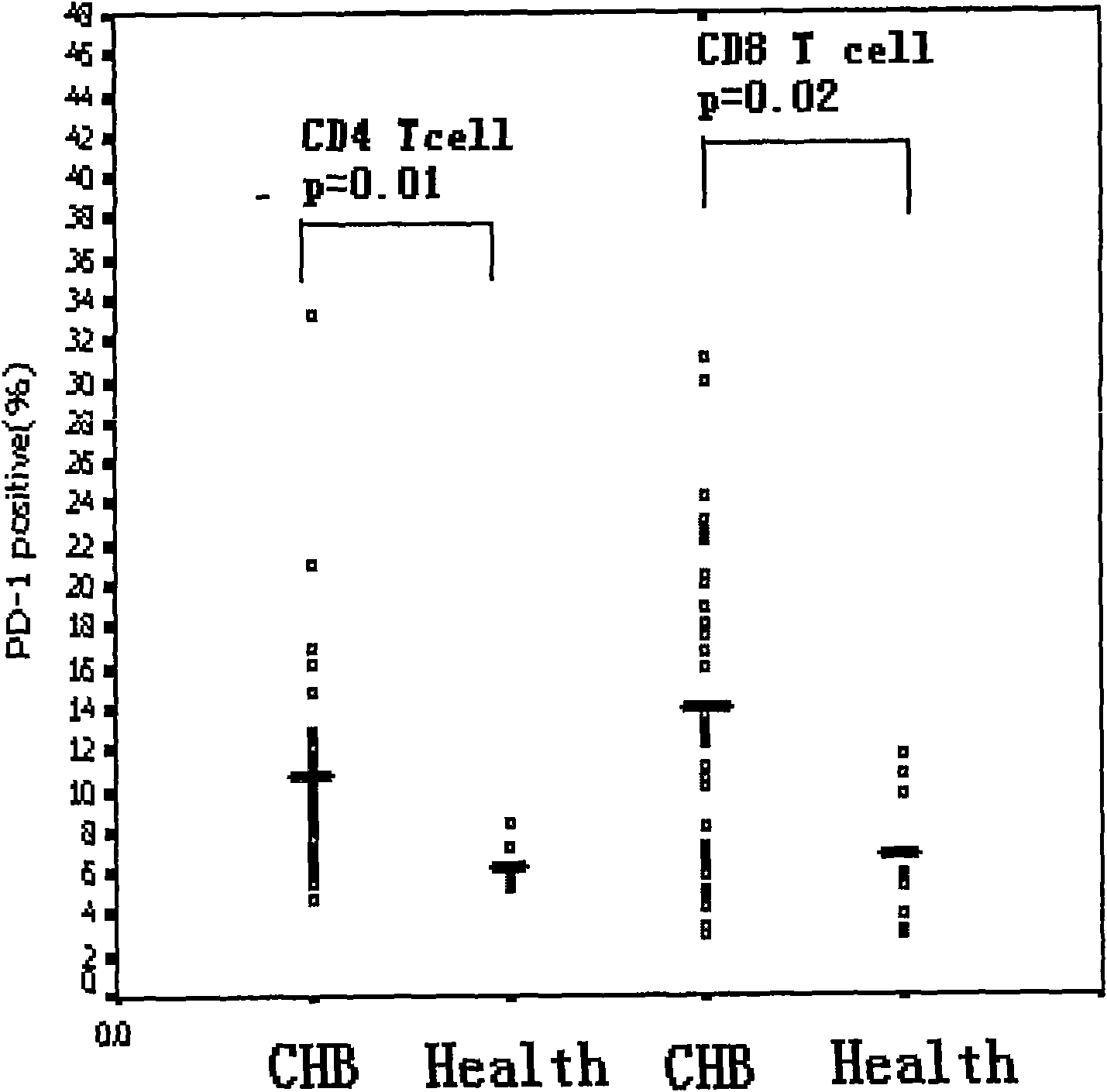
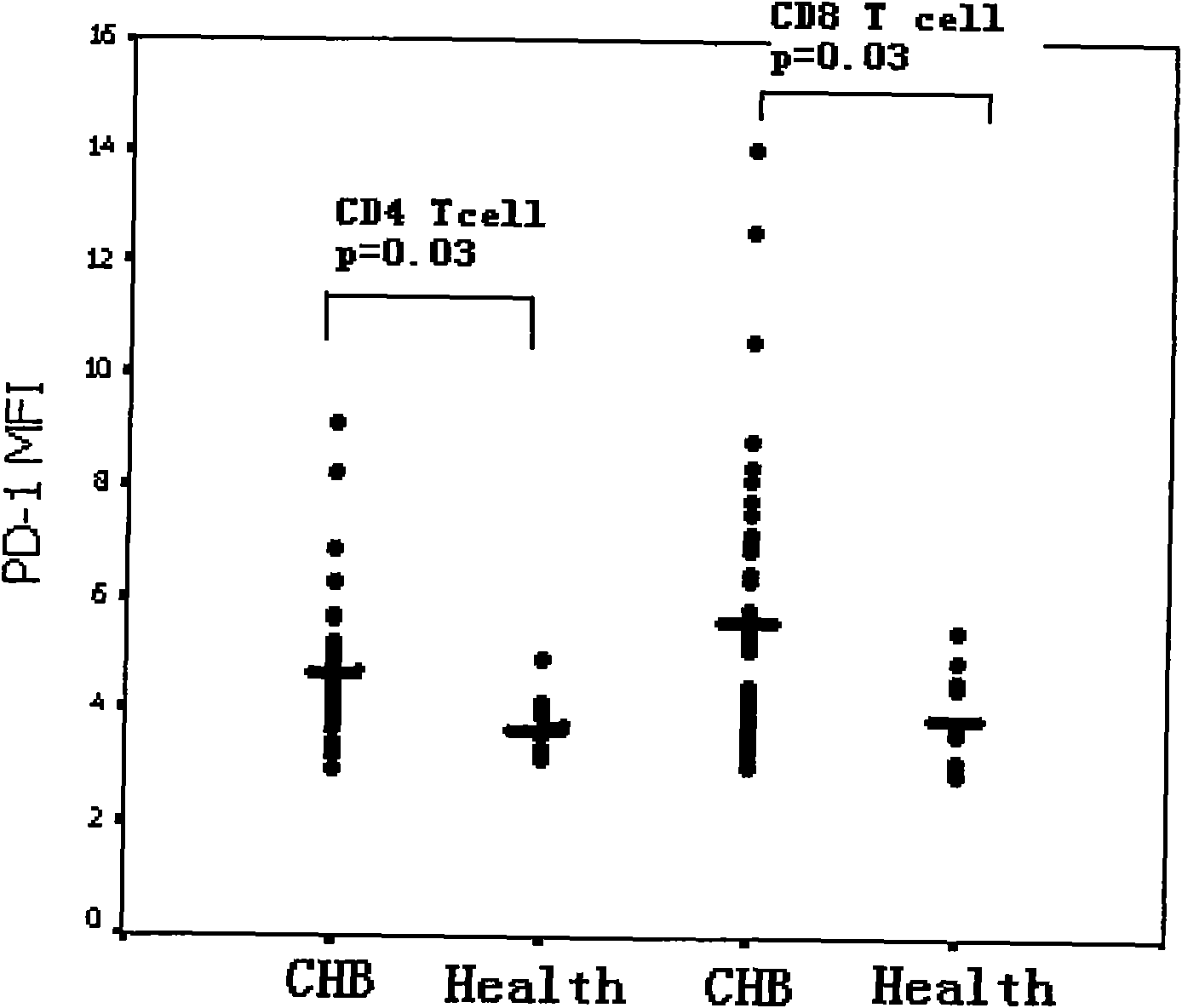
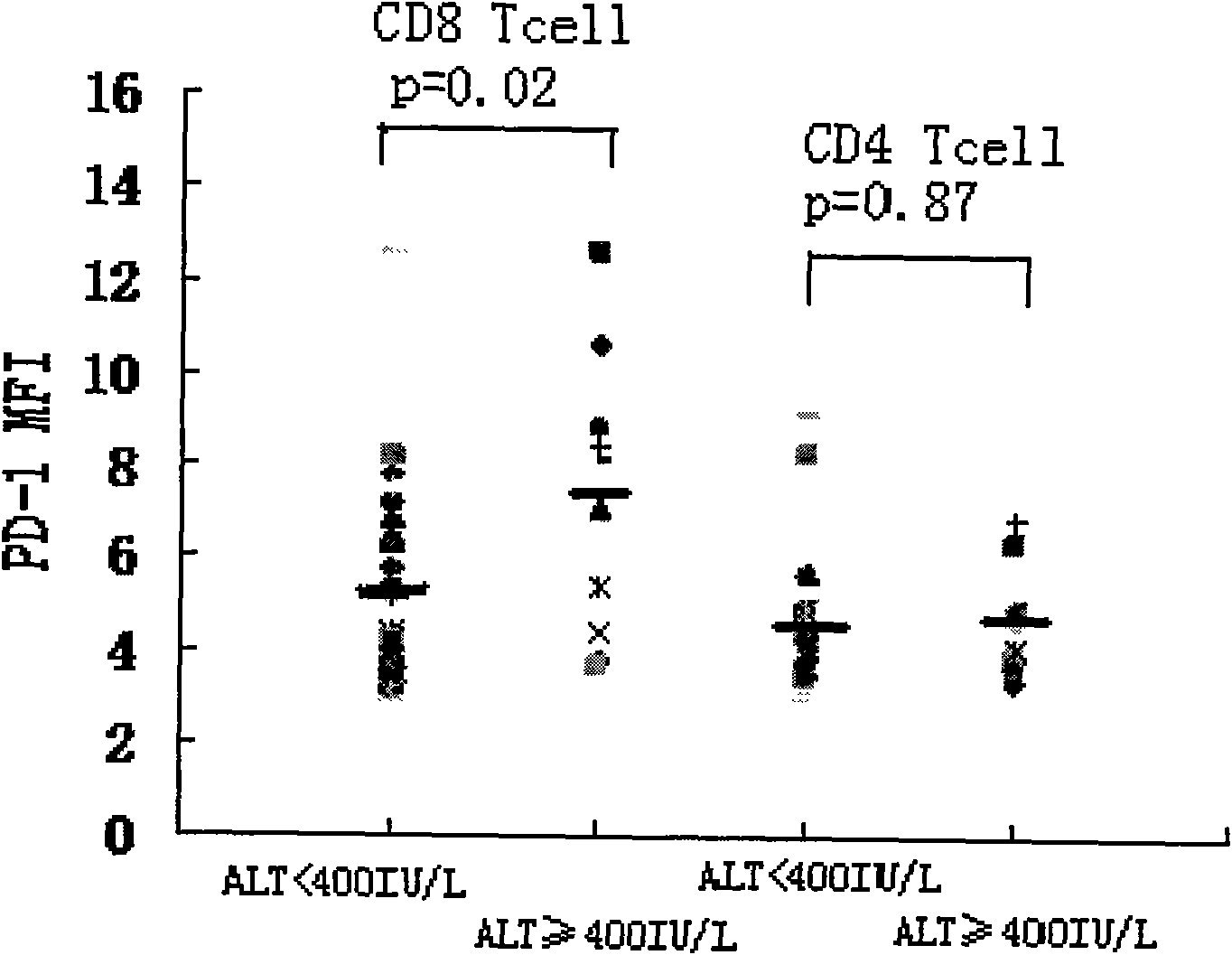
Diagnostic method for immune state of chronic hepatitis B patient
InactiveCN101625361AGood sensitivity and specificityEasy to operateMaterial analysisTrue positive rateMedical diagnosis
The invention relates to the medical diagnosis technical field, in particular to a diagnosis method for immune state of chronic hepatitis B patient. The diagnosis method for immune state of a chronic hepatitis B (CHB) patient includes the following steps: collecting peripheral blood sample of a chronic hepatitis B patient and anti-freezing processing; detecting PD-1 of peripheral blood total CD8<+> T cell in the peripheral blood sample; and analyzing PD-1 single cell expression of the peripheral blood total CD8<+> T cell. The diagnosis method of the invention has good sensitivity and specificity, simple operation and less time consumption, and meanwhile the invention can provide important information for knowing immune state of patient in clinical and further can provide effective information for deciding and adjusting a treatment scheme in clinical CHB antivirus treatment and has far-reaching significance for improving and predicting CHB healing efficacy.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Method for detecting pectobacterium carotovorum based on loop-mediated isothermal amplification technology, primer combination of method and detection kit
ActiveCN105018631AGood sensitivity and specificityAccurate detectionMicrobiological testing/measurementDNA/RNA fragmentationPectobacterium carotovorumLoop-mediated isothermal amplification
The invention relates to a method for detecting pectobacterium carotovorum based on a loop-mediated isothermal amplification technology, a primer combination of the method and a detection kit. An LAMP primer set used for detecting the pectobacterium carotovorum is composed of a positive inner primer FIP, a negative inner primer BIP, a positive outer primer F3, a negative primer B3 and a loop primer LOOP. The primer combination and the detection kit are high in specificity and good in flexibility and are obviously superior to other detection means in the prior art. Compared with a traditional PCR, the detection method and the kit are visual in result, easy to operate and low in cost; detection of pectobacterium carotovorum in the total DNA in various plant morbidity tissues can be achieved, a pectobacterium carotovorum bacterial suspension can directly detected, and a foundation is laid for direct field diagnosis of diseases.
Owner:INST OF VEGETABLE & FLOWERS CHINESE ACAD OF AGRI SCI
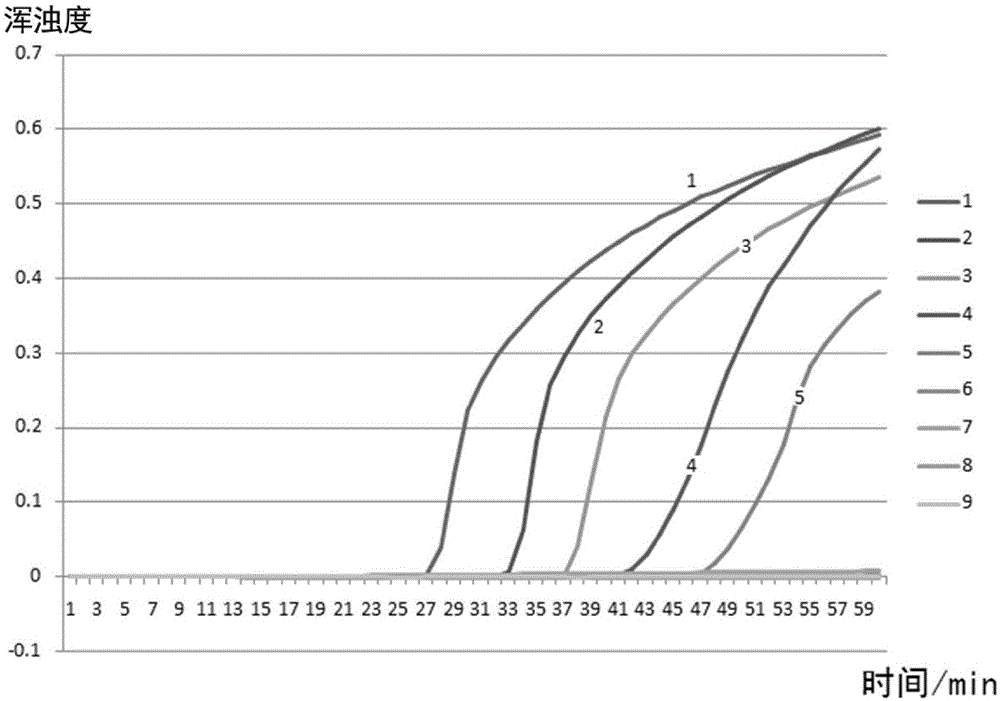
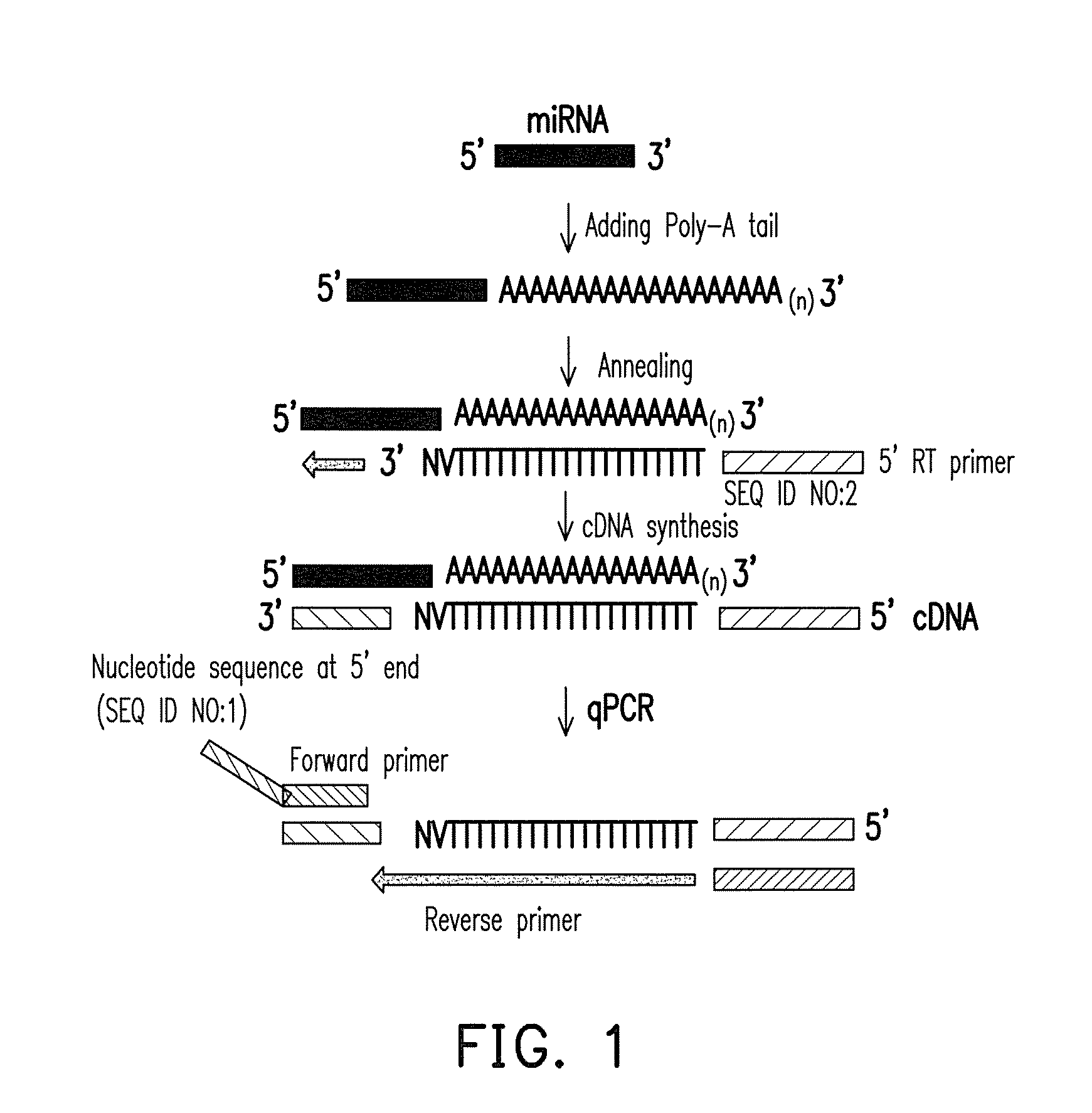
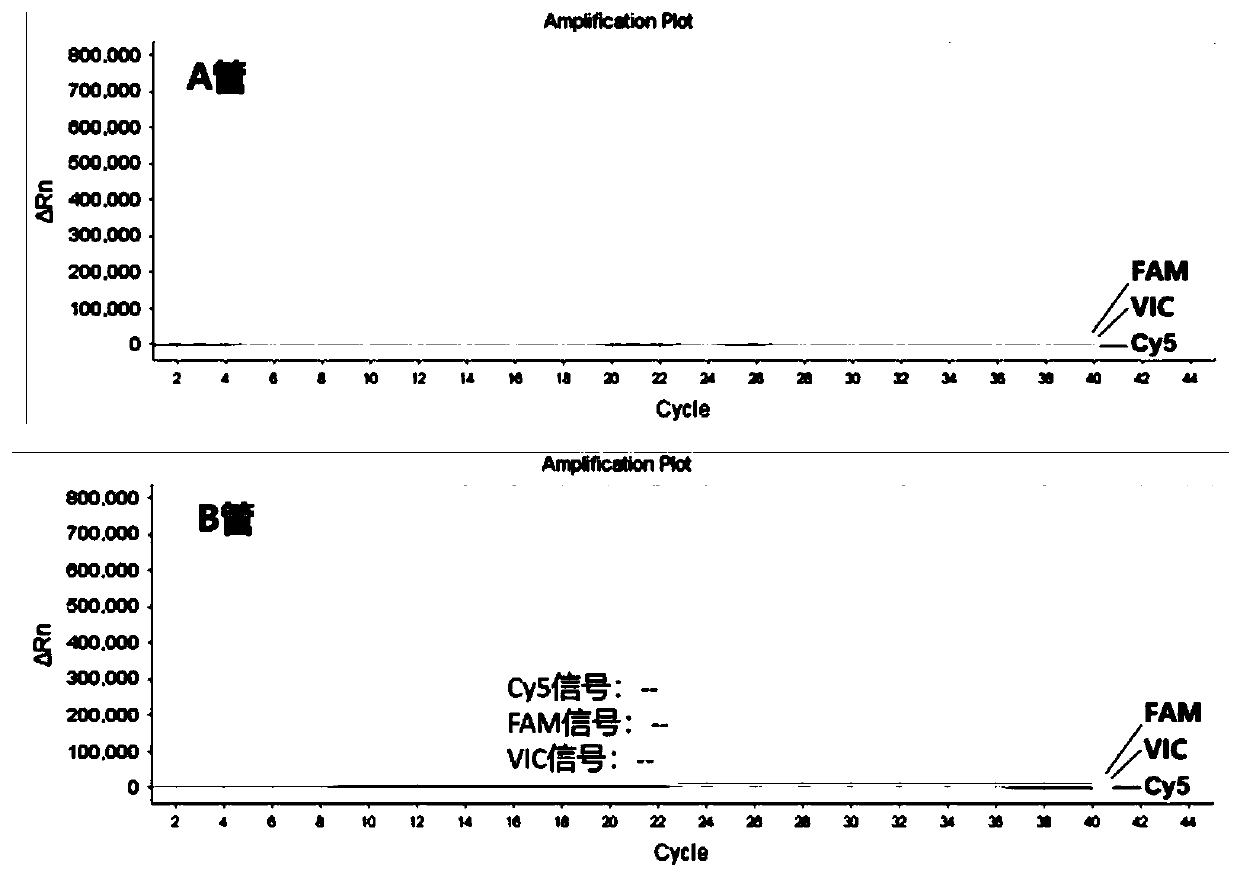
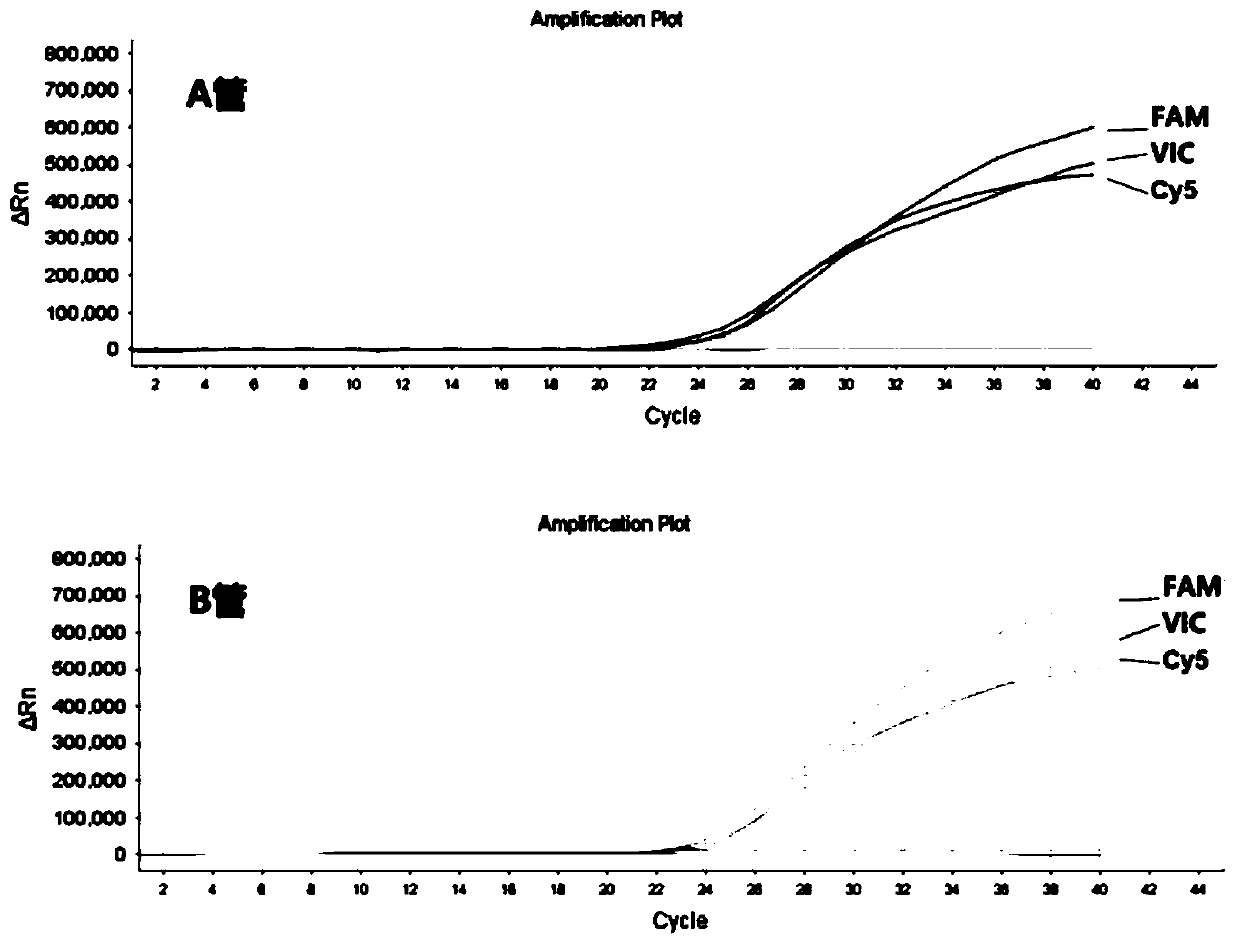
NUCLEOTIDE SEQUENCE, UNIVERSAL REVERSE PRIMER, UNIVERSAL RT PRIMER, METHOD FOR DESIGNING PRIMER AND miRNA DETECTION METHOD
ActiveUS20170022544A1Good sensitivity and specificityReduced cost and ease of useMicrobiological testing/measurementNucleotide sequencNucleotide
A nucleotide sequence having SEQ ID NO:1, a universal reverse primer having SEQ ID NO:2, a universal RT primer, a method for designing primer, and a miRNA detection method are provided. In the miRNA detection method, the universal RT primer is used for the cDNA synthesis of a miRNA sample and the universal reverse primer is used for cDNA molecule amplification in qPCR quantitative detection. The universal reverse primer sequence, the nucleotide sequence, and the design rules are used in the method for designing primer, so as to design a primer for qPCR quantitative detection.
Owner:CRACKERBIO INC
Kit and method for polymorphism detection of metabolism ability genes MTHFR and MTRR of folic acid
InactiveCN111118138ASystem stabilityGood sensitivity and specificityMicrobiological testing/measurementDNA/RNA fragmentationWild typeGenotype
The invention provides a kit and method for genotyping detection of polymorphism sites of metabolism ability genes of folic acid. The kit includes a mutation-type reaction system and a wild-type reaction system, wherein the reaction systems include rs1801133 groups, rs1801131 groups and rs1801394 groups, and the structures of primers, probes and inhibitors in each group are disclosed. The problemsof high requirements for template quality and insufficient timeliness can be solved during detection of polymorphism typing of the metabolism genes MTHFR and MTRR of folic acid, only the two tubes ofreaction systems are needed, one-time sampling and one-time closed detection can be realized, and polymorphism of three sites of 677C>T, 1298A>C and 66A>G of the gene MTHFR are detected in real time;and the kit and method have stable systems and good sensitivity and specificity.
Owner:重庆浦洛通基因医学研究院有限公司
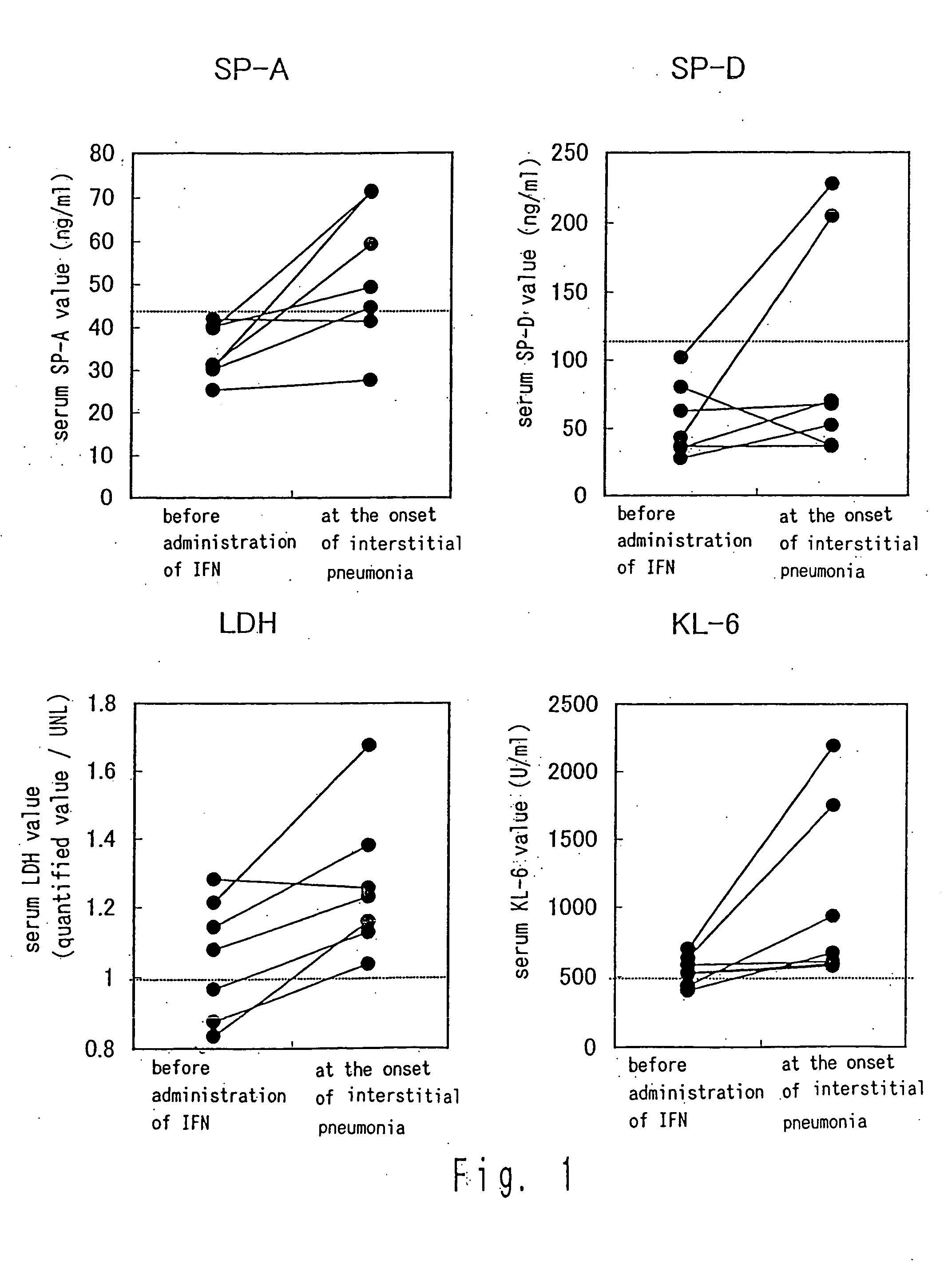
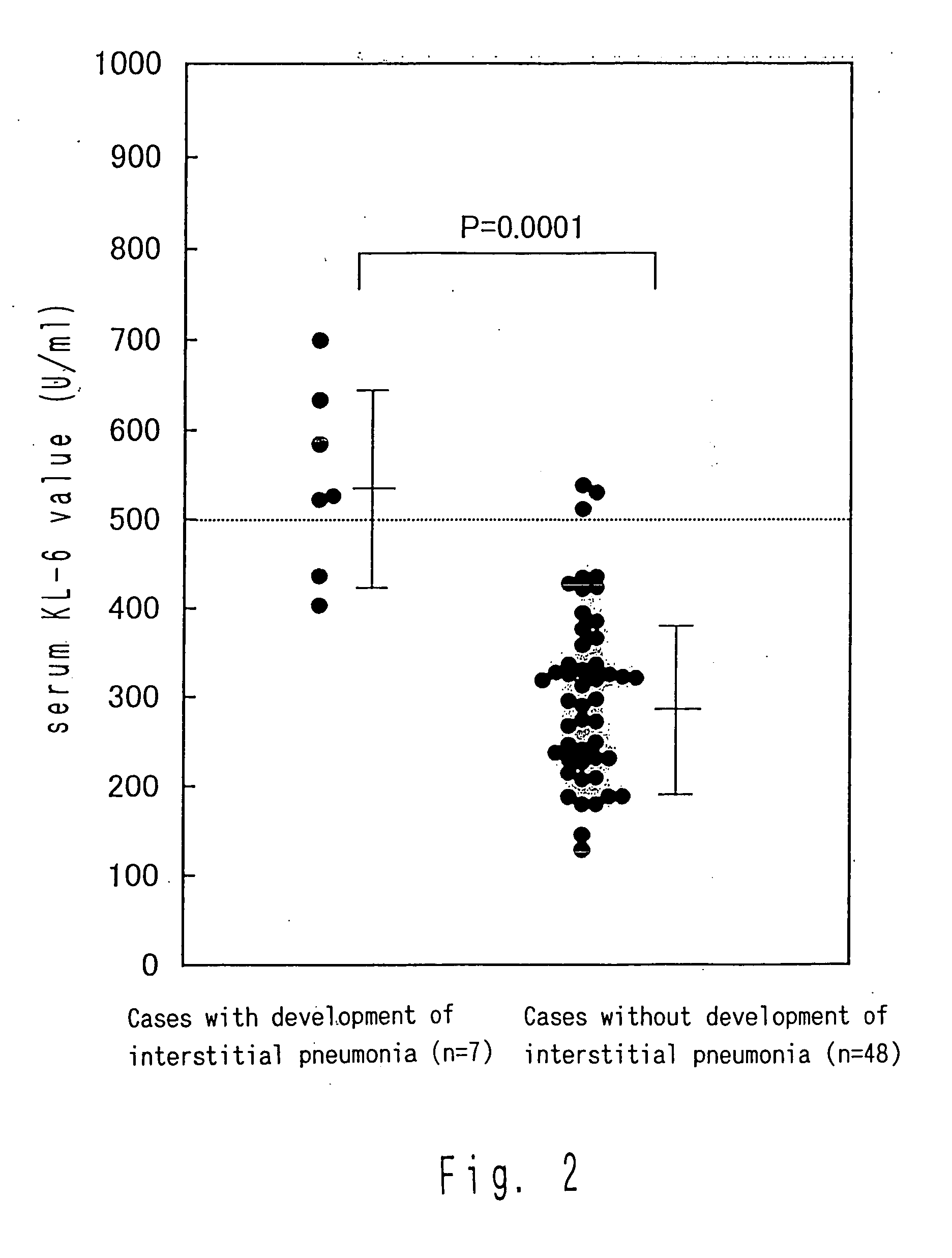
Method for predicting development of interstitial pneumonia by quantifying MUC-1
InactiveUS20050084846A1Good sensitivity and specificityHigh sensitivityMicrobiological testing/measurementImmunoglobulins against cytokines/lymphokines/interferonsDrugNormal values
Development of interstitial pneumonia due to administration of a drug can be predicted by quantifying MUC-1 in a body fluid; and determining that a possibility for the development of interstitial pneumonia is high when the amount of MUC-1 exhibits a value higher than a normal value.
Owner:EISIA R&D MANAGEMENT CO LTD
Porcine pseudorabies virus and porcine circovirus 3 double fluorescent quantitative PCR detection primer, probe, kit and method
PendingCN112553372AGood sensitivity and specificityGood repeatabilityMicrobiological testing/measurementDNA/RNA fragmentationNucleotidePorcine circovirus
The invention provides a porcine pseudorabies virus and porcine circovirus 3 double fluorescent quantitative PCR detection primer, a probe, an amplification reagent and a method, and belongs to the technical field of virus detection. The primer probe comprises primers PRV gD real-F1, PRV gD real-R2, PCV3-real-F2 and PCV3-real-R2, and the nucleotide sequences of the fluorescent probes PRV gD and PCV3, and the PRV gD real-F1, PRV gD real-R2, PRV gD, PCV3-real-F2, PCV3-real-R2 and PCV3 are sequentially shown as SEQ ID No.1-6. The primer probe, the amplification reagent and the method have excellent sensitivity, specificity, repeatability and stability on detection of the porcine pseudorabies virus and the porcine circovirus 3.
Owner:浙江省动物疫病预防控制中心
Porcine reproductive and respiratory syndrome and porcine Japanese B encephalitis dual one-step RT-PCR (Reverse Transcription-Polymerase Chain Reaction) diagnosis kit
ActiveCN103215389AGood sensitivity and specificityEasy to operateMicrobiological testing/measurementMicroorganism based processesMolecular biologyJapanese encephalitis viruses
The invention discloses a porcine reproductive and respiratory syndrome and porcine Japanese B encephalitis dual one-step RT-PCR (Reverse Transcription-Polymerase Chain Reaction) diagnosis kit which comprises 100 micro-milliliters of DL2000, 20 micro-milliliters of RT=PCR one-step enzyme, 250 micro-milliliters of enzyme buffer solutions, 300 micro-milliliters of RNase Free dH2O, 80 micro-milliliters of mixed primers ( respectively with the concentration of 10 micromoles / liter), 20 micro-milliliters of positive controls and 20 micro-milliliters of negative controls. According to the invention, two pairs of primers are designed according to the sequences of a PRRSV (Porcine Reproductive and Respiratory Syndrome Virus) OFR7 N gene and a JEV (Japanese Encephalitis Virus) NS1 gene which are contained in a GenBank; and the dual one-step RT-PCR diagnosis kit for detecting a porcine reproductive and respiratory syndrome virus and a porcine Japanese B encephalitis virus is successfully developed through the optimization of reaction conditions, and the dual one-step RT-PCR diagnosis kit has sensibility and specificity on the two viruses. The dual one-step RT-PCR diagnosis kit disclosed by the invention can be used for clinically detecting the porcine reproductive and respiratory syndrome and the porcine Japanese B encephalitis.
Owner:GUIZHOU INST OF ANIMAL HUSBANDRY & VETERINARY
Specific antibody against pesticide phosmet
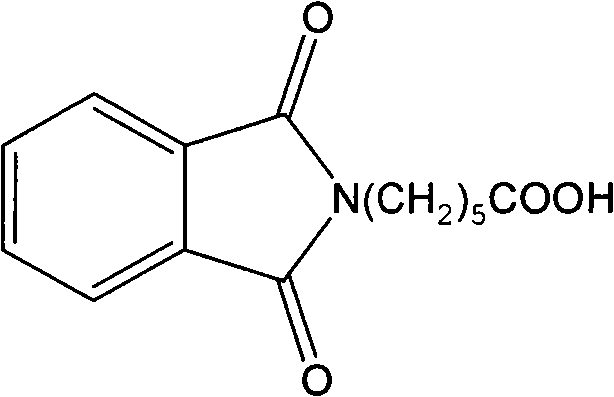
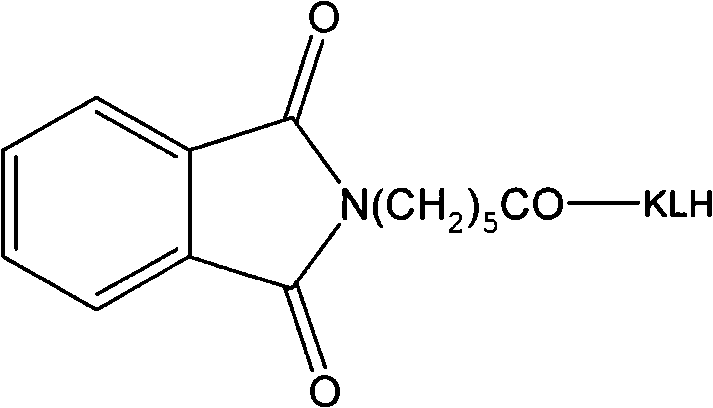
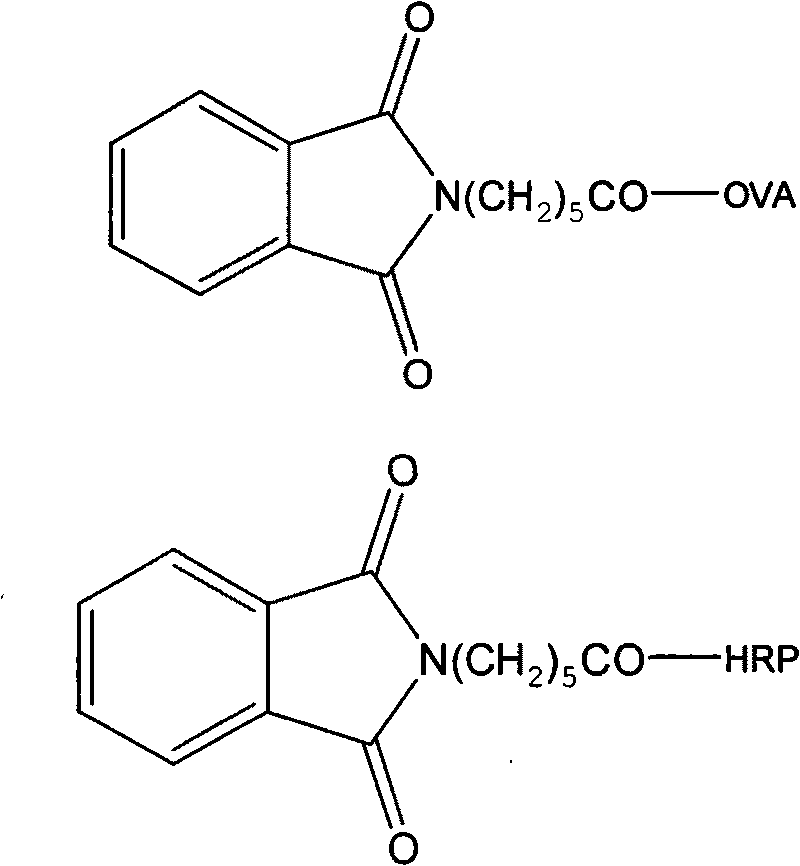
InactiveCN101747436AHigh similarityGood sensitivity and specificitySerum immunoglobulinsDepsipeptidesChemistryOrganic synthesis
The invention relates to a synthetic method of an artificial antigen of phosmet and an antibody preparation process. The invention belongs to the technical field of immunochemistry of small molecule compounds of pesticides and residue analysis and relates to organic synthesis, immunochemistry, biochemistry and the like. The invention solves the problem that the traditional physical and chemical analysis methods are complicated, high in cost and slow in analysis, and provides simple, quick, sensitive and accurate immunoassay. The designed and synthesized hapten N-phthaloyl-6-aminocaproic acid is linked with the carrier protein such as hemocyanin and horseradish peroxidase respectively to synthesize the artificial antigen and an enzyme-labelled antigen. The antibody is prepared through animal immunization of the artificial antigen, blood collection and antiserum extraction and purification. With good stability, high specificity and sensitivity, the antibody can be used for quick immunoassay of the pesticide organophosphorus and has good application prospect.
Owner:TIANJIN UNIVERSITY OF SCIENCE AND TECHNOLOGY
Freeze-dried hemagglutination inhibition test antigen for detecting mycoplasma synoviae, antigen combination and preparation method
PendingCN111537714AAntigen is stableGood sensitivity and specificityBacteriaMicroorganism based processesHemagglutinationFreeze dry
The invention belongs to the technical field of veterinary diagnosis, and particularly relates to a freeze-dried hemagglutination inhibition test antigen for detecting mycoplasma synoviae (MS), an antigen combination and a preparation method. The freeze-dried hemagglutination inhibition test antigen for MS is prepared by the steps of selecting a mycoplasma synoviae CCTCC No.M 20191100 strain, andcarrying out seed liquid culture, enlarged culture, inactivation, concentration and freeze drying. The invention further discloses the combination of the freeze-dried hemagglutination inhibition testantigen with a Mycoplasma gallisepticum (MG) hemagglutination inhibition test antigen. The hemagglutination inhibition test antigen and the combination thereof provided by the invention are stable inproperty and easy to store. The HI detection method established by utilizing the antigen is simple and rapid to operate and low in cost, and has good sensitivity and specificity.
Owner:YANGZHOU UNIV
Method for detecting drug-resistant mutation sites of campylobacter jejuni carbostyril antibiotics
ActiveCN103695559ASimplify complex proceduresGood sensitivity and specificityMicrobiological testing/measurementMicroorganism based processesDrugCampylobacter jejuni
The invention discloses a method for detecting drug-resistant mutation sites of campylobacter jejuni carbostyril antibiotics. The method comprises the following steps: by taking campylobacter jejuni of which the 257th site of a gyrA gene coding sequence is C as a wild type standard strain, taking campylobacter jejuni of which the 257th site of the gyrA gene coding sequence is T as a mutant type standard strain, respectively and simultaneously performing high-resolution melting curve analysis with campylobacter jejuni to be detected; determining whether the 257th site of the gyrA gene coding sequence of the campylobacter jejuni to be detected is C or T according to the comparison result of the high-resolution melting curve; if the high-resolution melting curve of the campylobacter jejuni to be detected is consistent with that of the wild type standard strain, selecting the campylobacter jejuni to be detected as a strain of which the 257th site of the gyrA gene coding sequence is C, and if the high-resolution melting curve of the campylobacter jejuni to be detected is consistent with that of the mutant type standard strain, selecting the campylobacter jejuni to be detected as a strain of which the 257th site of the gyrA gene coding sequence is T.
Owner:CHINA AGRI UNIV
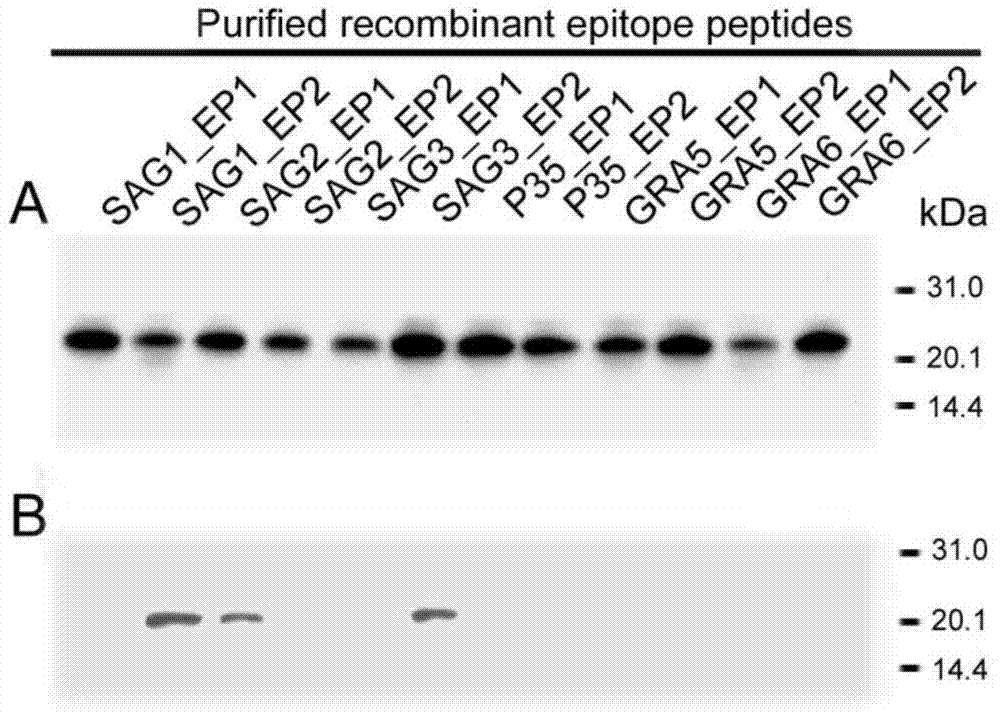
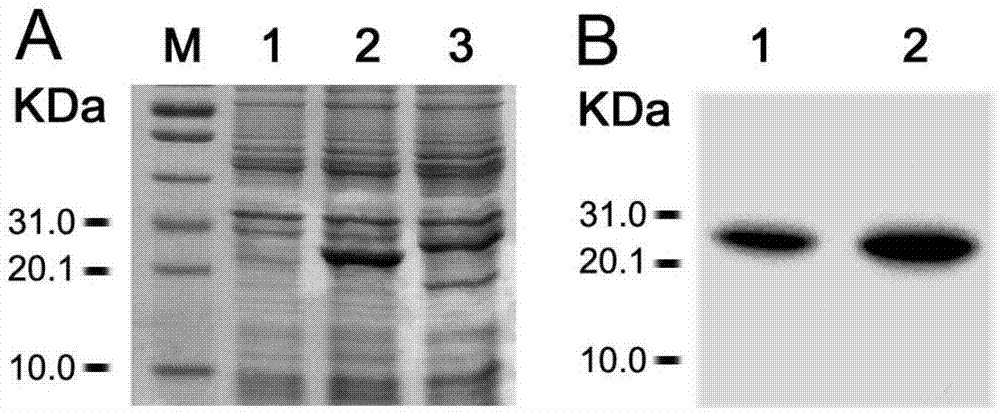
Fusion protein TgMEP as well as preparation method and application thereof
InactiveCN102827292AGood sensitivity and specificityEasy to prepareBiological testingHybrid peptidesPolymerase chain reactionDisease
The invention relates to a fusion protein TgMEP as well as a preparation method and application of the fusion protein TgMEP. An amino acid sequence of the fusion protein TgMEP is shown in a SEQ ID NO.2. The construction and preparation method comprises the following steps of: optimizing a gene sequence of the TgMEP to obtain a sequence shown in a SEQ ID NO.1; synthetizing the optimized gene sequence in vitro by using a method for overlapping polymerase chain reaction (PCR); directionally cloning to Pet-32c expression type carrier by two enzyme cutting sites of Nco1 and Xho1; converting the built multi-epitope peptide recombination expression vector TgMEP / Pet-32c into BL21 host bacteria; expressing soluble fusion protein with the size of 23kDa by virtue of induction; ultrasonically pulverizing the expressed bacteria and separating to obtain supernatant; and filtering the supernatant by a filtering film to perform affinity purification by using a nickel column to obtain the fusion protein TgMEP. The fusion protein TgMEP can be used for detecting bow worm disease serum IgG and IgM antibodies.
Owner:THE SECOND AFFILIATED HOSPITAL OF NANJING MEDICAL UNIV
Mammary gland non-malignant tumor and malignant tumor blood serum special protein and uses thereof
InactiveCN101354393AGood sensitivity and specificityMaterial analysis by electric/magnetic meansBiological testingPremalignant NeoplasmSpecific protein
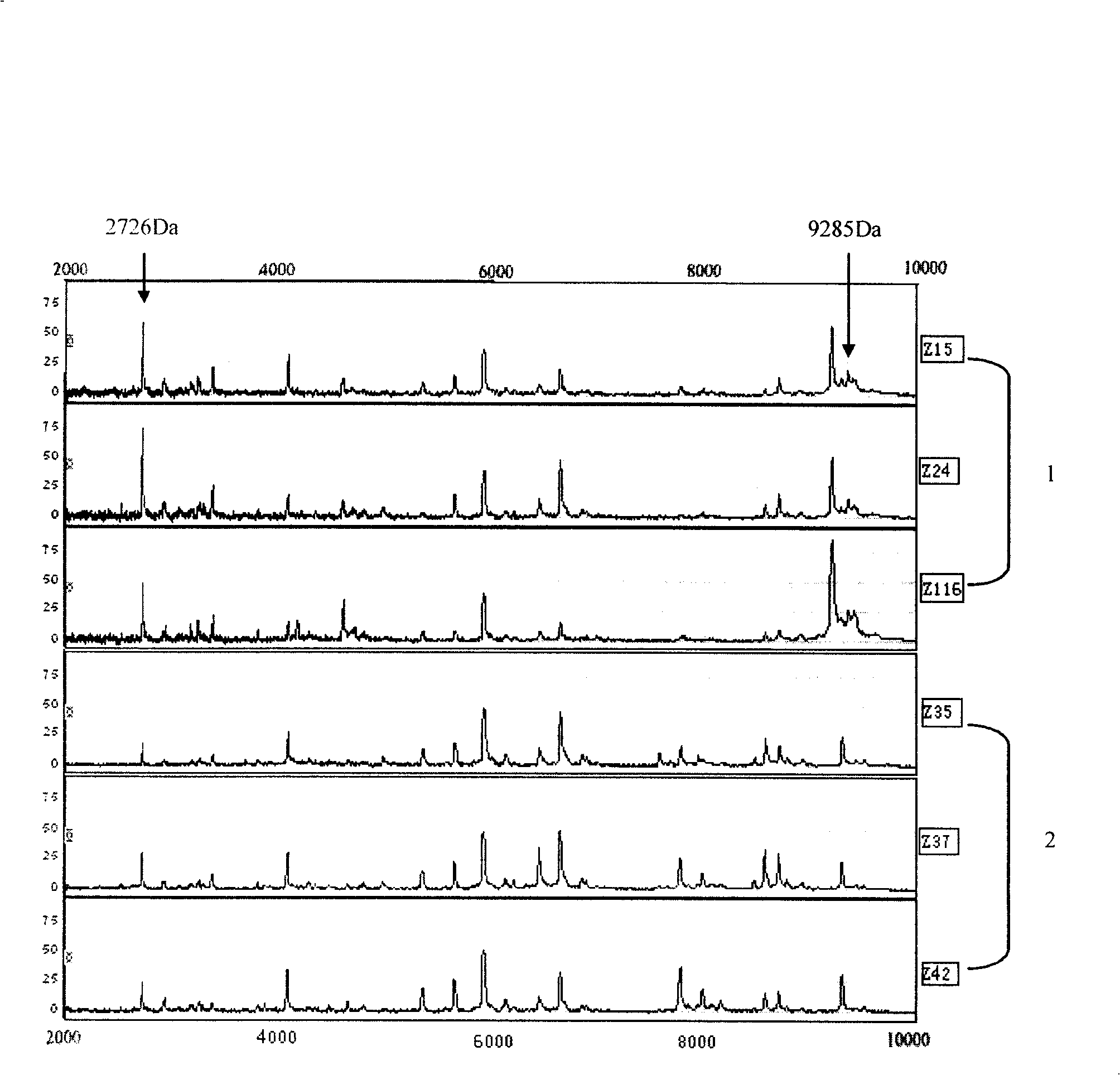
The invention discloses a benign and malignant mammary tumour serum specific protein and application thereof, belonging to the field of biological detection technique. The invention uses an SELDI-TOF-MS technique, establishes the fingerprint of serum protein between the benign mammary tumour patient and breast carcinoma patient, carries out the variation analysis and searches new protein markers, thus dividing the benign and malignant mammary tumour. As the SELDI-TOF-MS technique is used by the invention, mass spectrometric detection, comparison and analysis are carried out on the serum of the peripheral blood of benign and malignant mammary tumour patients from the point of view of proteomics, thus establishing effective mammary tumour serum protein fingerprint which is used for the risk evaluation method used when the nasopharyngeal carcinoma is detected and distinguished outside the body. The method is a detection method with the sensitiveness and specificity better than that of the traditional method.
Owner:福建省肿瘤医院
Composite assay for detecting a clinical condition
InactiveUS20130203058A1Improve abilitiesHigh sensitivityMicrobiological testing/measurementChromosomal AbnormalityBioinformatics
The invention generally relates to methods for screening patients for one or more clinical conditions using a composite assay. According to certain aspects, methods of the invention involve isolating at least one nucleic acid from a biological sample obtained from the subject, detecting at least one sequence mutation and a chromosomal abnormality in the at least one nucleic acid in a single assay format, and identifying a clinical condition in said subject when both the sequence mutation and the chromosomal abnormality are present.
Owner:PHYSICIANS CHOICE LAB SERVICES
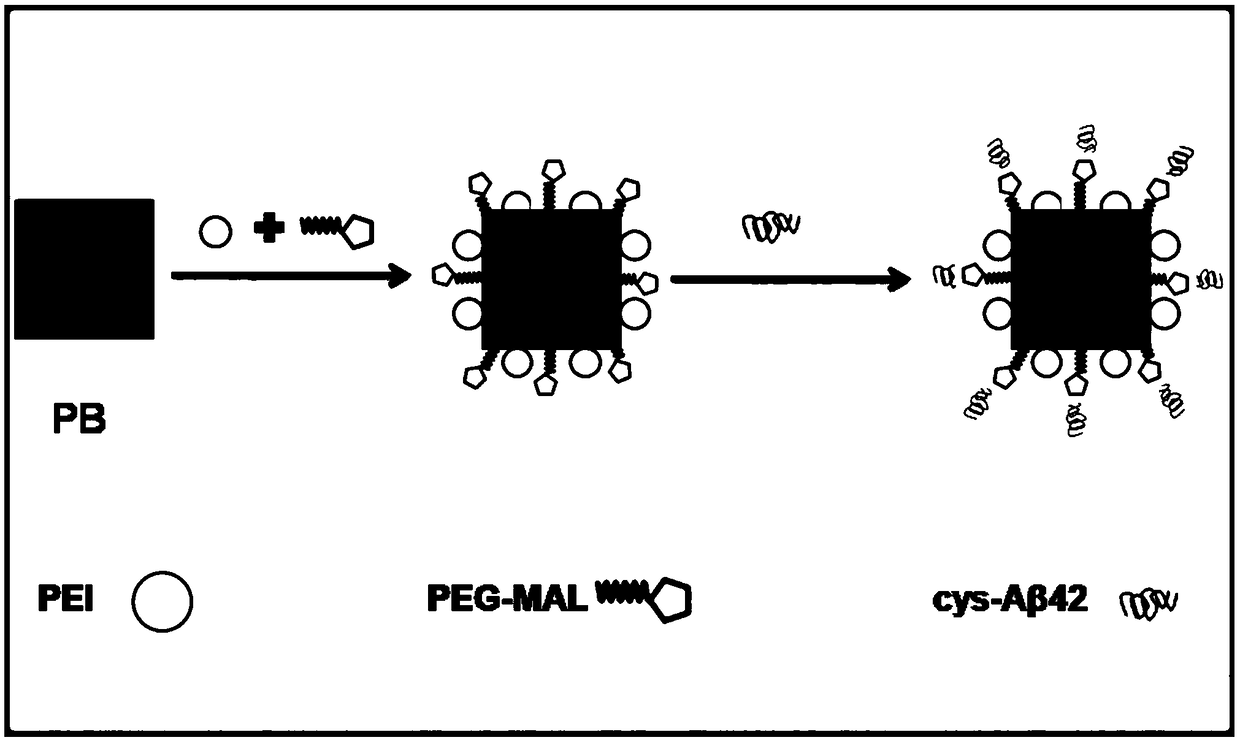
Prussian blue nano MRI tracer agent as well as preparation method and application thereof
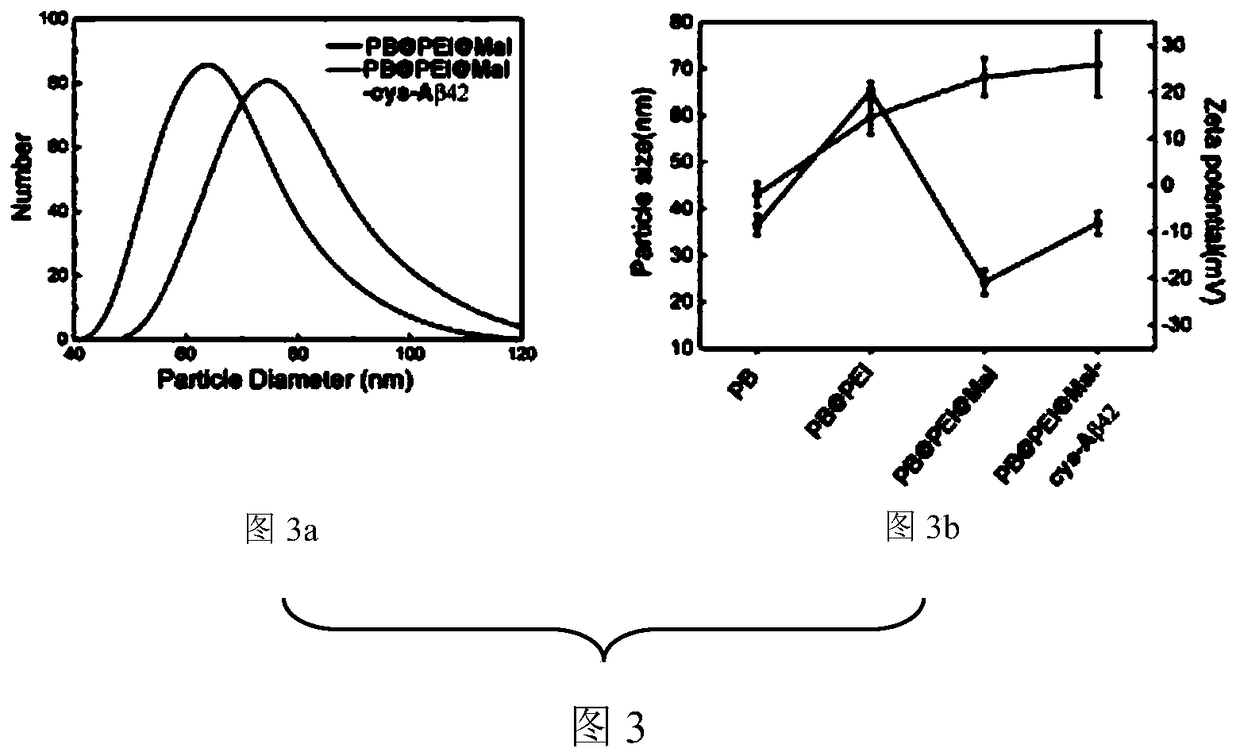
ActiveCN109045311AGood sensitivity and specificityGood stabilityNanomedicineNanosensorsNuclear medicineAmyloid
The invention discloses a Prussian blue nano MRI tracer agent as well as preparation method and application thereof, and provides nontoxic Prussian blue nanoparticles-based MRI tracer agent with highspecificity and sensitivity. The maleinimidized Prussian blue nanoparticles combined with cysteine-modified beta-amyloid protein can detect the Abeta deposited in the brain of an Alzheimers patient and clearly image the Abeta on MRI. In the invention, an MRI tracer agent capable of passing through the blood brain barrier and being specifically combined with the Abeta is prepared by adopting Abetahomologous polypeptide as a target of beta amyloid protein in brain and the Prussian blue as an imaging agent. As a detection agent of molecular images, the MRI tracer agent disclosed by the inventioncan provide a new means of AD early diagnosis and curative effect evaluation in the future.
Owner:王金环
Method for examining liver cancer
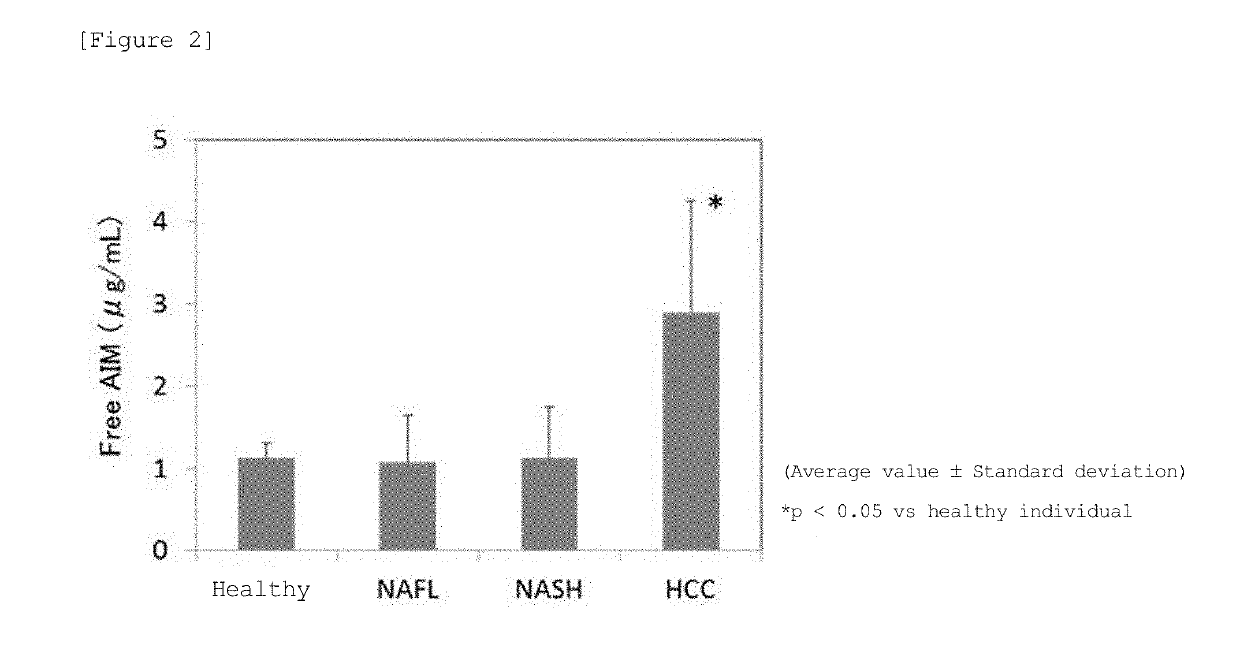
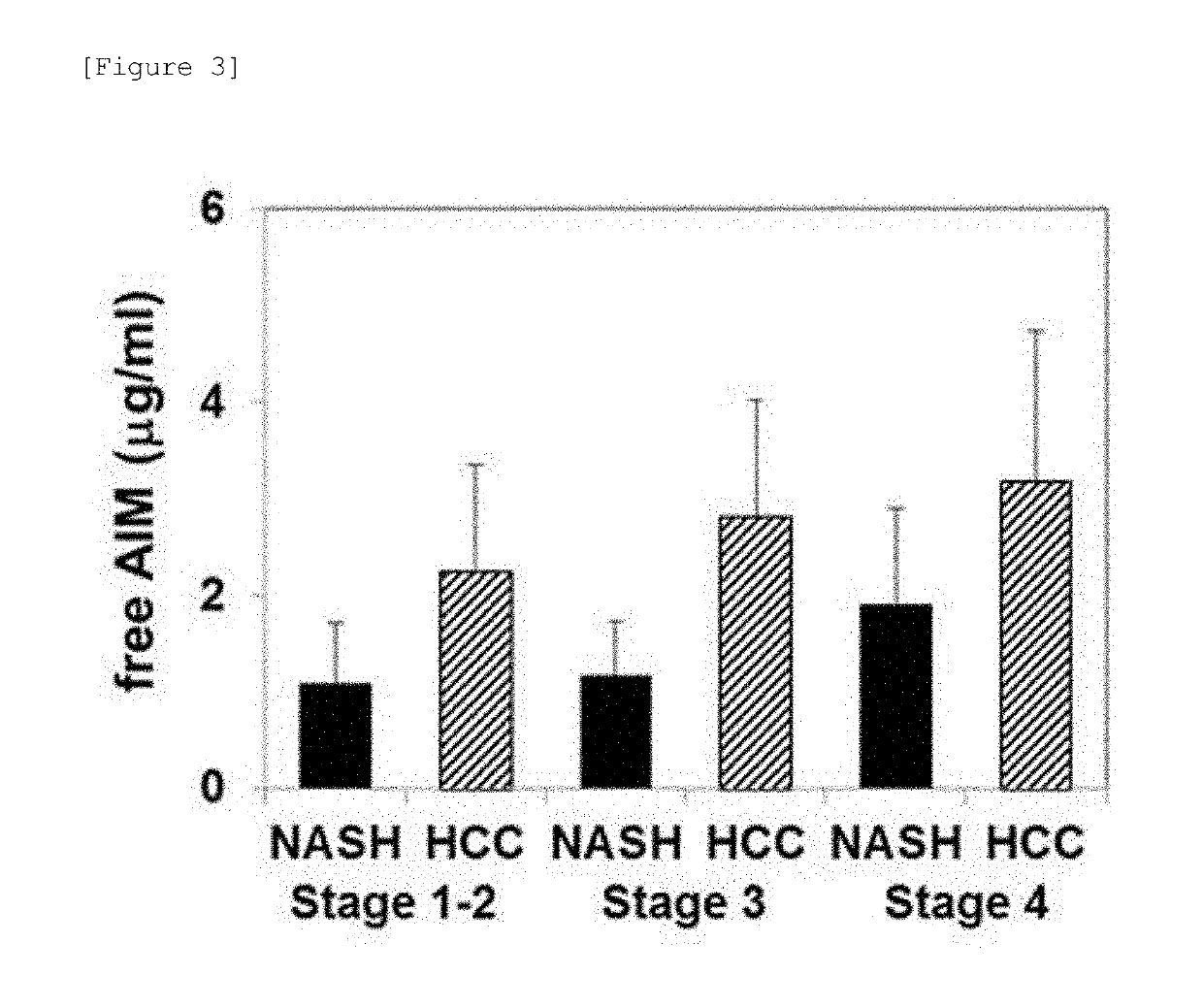
InactiveUS20190317096A1High sensitivityStrong specificityMicrobiological testing/measurementMaterial analysisTrue positive rateHepatica
A method for examining liver cancer or a method for assisting diagnosis of liver cancer that is superior in sensitivity and specificity, as well as a kit that can be used therefor is provided. The present invention provides a method for detecting liver cancer or a method for assisting diagnosis of liver cancer comprising a step of detecting or quantifying free AIM in a biological sample derived from a test subject, as well as a kit for examining or assisting diagnosis of liver cancer comprising an antibody that binds to free AIM.
Owner:SEKISUI MEDICAL CO LTD +2
Beamforming techniques for ultrasound microcalcification detection
ActiveUS20160296202A1High sensitivityStrong specificityHealth-index calculationOrgan movement/changes detectionUltrasonographyChannel data
A medical ultrasound acquisition-data analysis device acquires channel data (144) via ultrasound received on the channels, uses the acquired channel data to estimate data coherence and derive dominance of an eigen-value of a channel covariance matrix and, based on the estimate and dominance, distinguishes microcalcifications (142) from background. Microcalcifications may then be made distinguishable visually on screen via highlighting, coloring, annotation, etc. The channel data operable upon by the estimating may have been subject to beamforming delays and may be summed in a beamforming procedure executed in the estimating. In the estimating and deriving, both field point-by-field point, multiple serial transmits (116, 118) may be used for each field point. In one embodiment results of the estimating and deriving are multiplied point-by-point and submitted to thresholding.
Owner:KONINKLJIJKE PHILIPS NV
Testing for Blood Group Immunological Reaction Without the Use of Anti-Human Globulin
InactiveUS20080280310A1Accelerate binding processShorten reaction timeBiological testingGroup A - bloodBlood donor
A method testing for blood antigens (known as forward blood grouping) is presented wherein known antibodies are attached to a solid surface and the red blood cell sample for immunological reaction is centrifuged to physically overcome the natural repellent force between two red cells (know in the industry as the zeta potential) and to allow for the antigen antibody reaction to occur more rapidly. A reverse blood grouping procedure utilizes synthetic or purified antigens, which are attached directly attached directly to a solid surface. The surface is then contacted with the patient's serum and then centrifuged to allow the antigen antibody reaction to occur. The cells are then washed and a second labeled antibody of known concentration is added as an indicator. This is a method of performing a major crossmatch that does not utilize anti-human immunoglobin. In an alternative major crossmatch procedure, a binder is used to attach red blood cell membranes from a blood donor and the serum from a recipient is allowed to undergo an immune reaction with these membranes on the solid surface. Antibody screening and antibody identification are carried out by attaching known antigen carrying cells to a solid surface. The solid surface is contacted with the unknown solution which will undergo an immune reaction to the extent that antibodies specific to the previously adhered antigens are present and will bind. Red blood cells or synthetic labeled particles are used as the indicator mechanism.
Owner:PANAGOPOULOS LOUIS
Analysis method for application of circRNAs in lung adenocarcinoma
PendingCN114058703AGood sensitivity and specificityEarly diagnosis improvesMicrobiological testing/measurementPulmonary adenocarcinomaCancer research
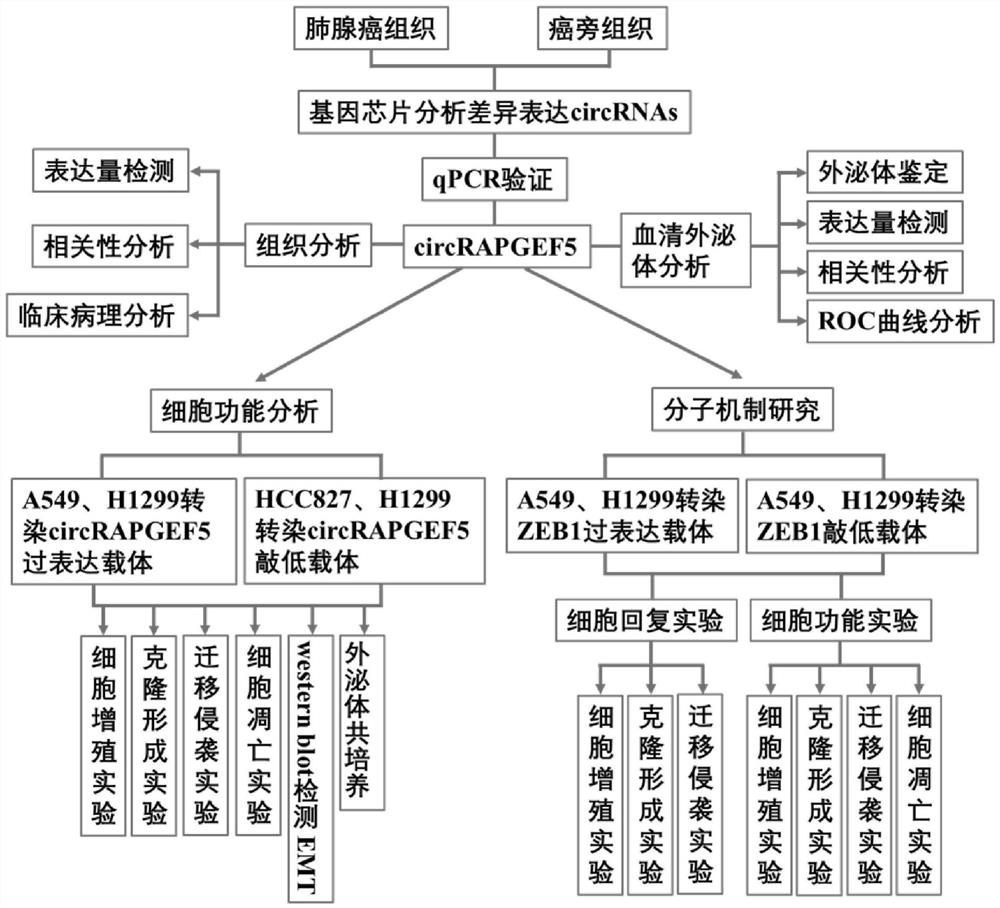
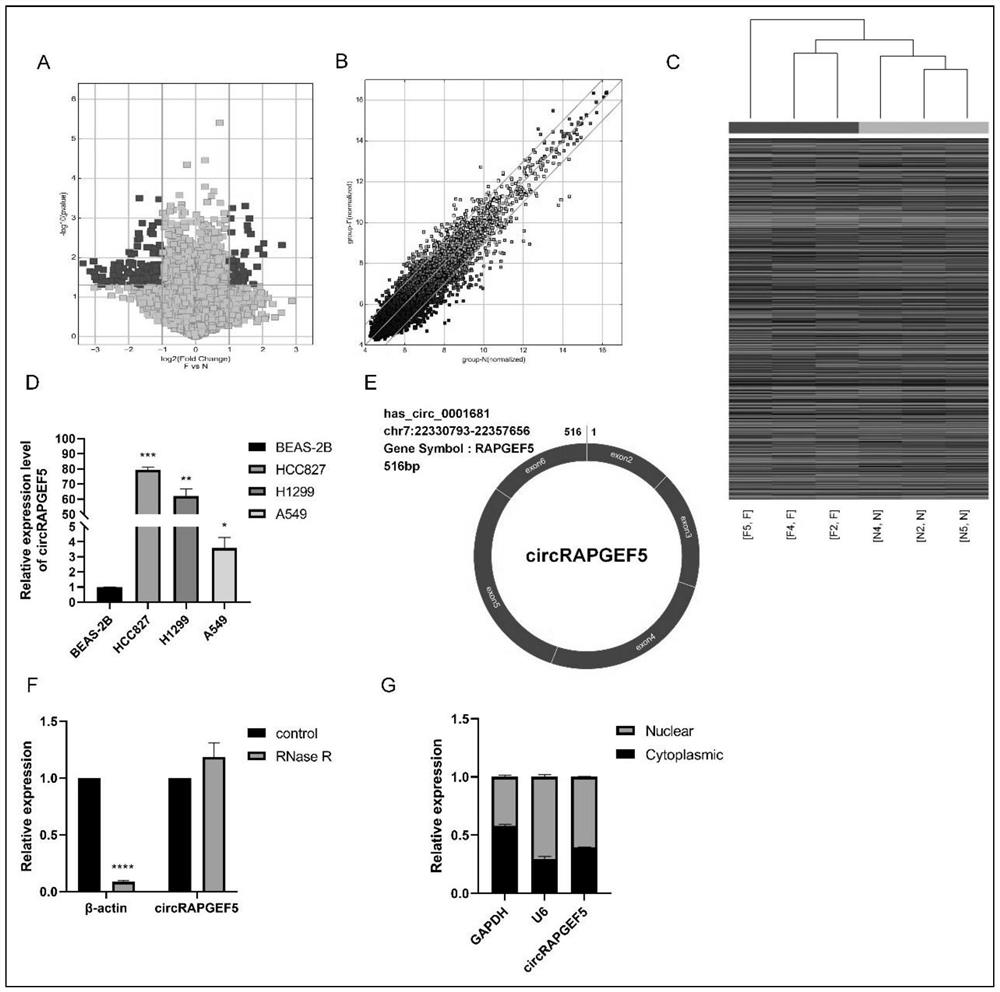
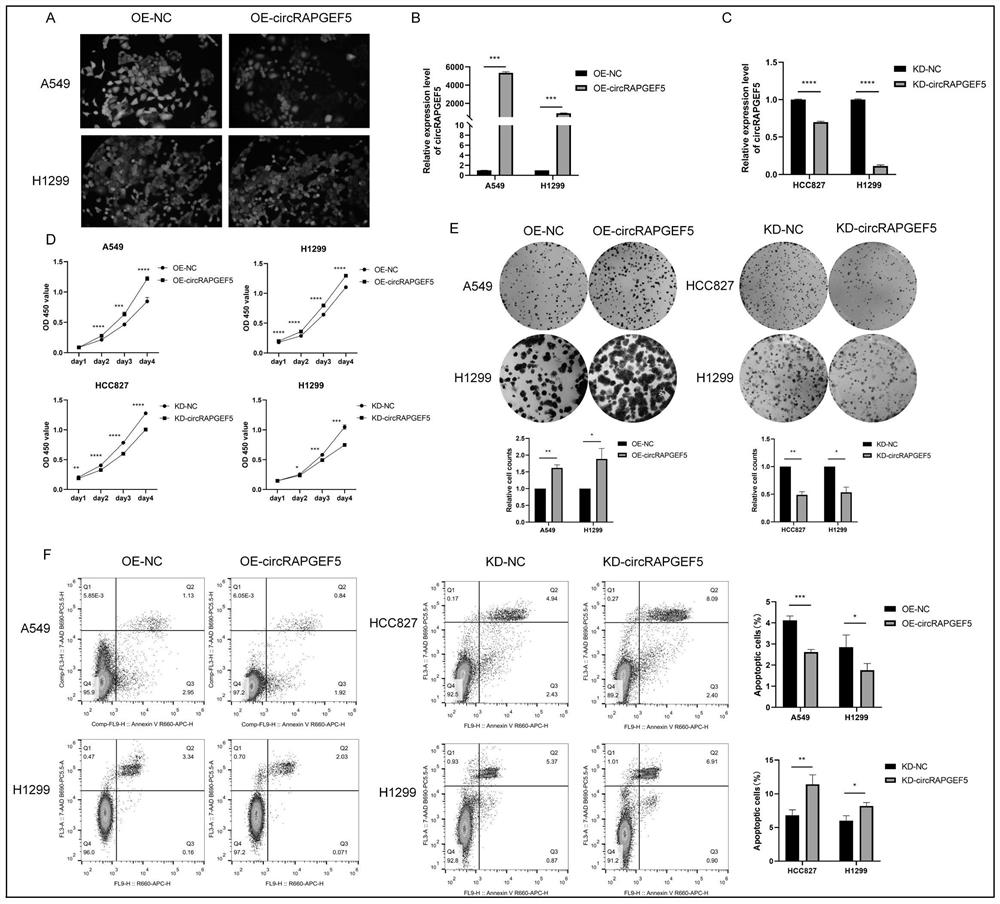
The invention discloses an analysis method for application of circRNAs in lung adenocarcinoma, and the method comprises the following steps of: acquiring differentially expressed circRNAs in lung adenocarcinoma, and acquiring high-expression circRAPGEF5; and analyzing the influence of circRAPGEF5 on proliferation, apoptosis, migration and invasion of lung adenocarcinoma cells. The invention lays a foundation for early diagnosis of lung adenocarcinoma, preparation of lung adenocarcinoma targeted drugs and preparation of products for judging the disease risk of lung adenocarcinoma patients.
Owner:THE FIRST AFFILIATED HOSPITAL OF WENZHOU MEDICAL UNIV
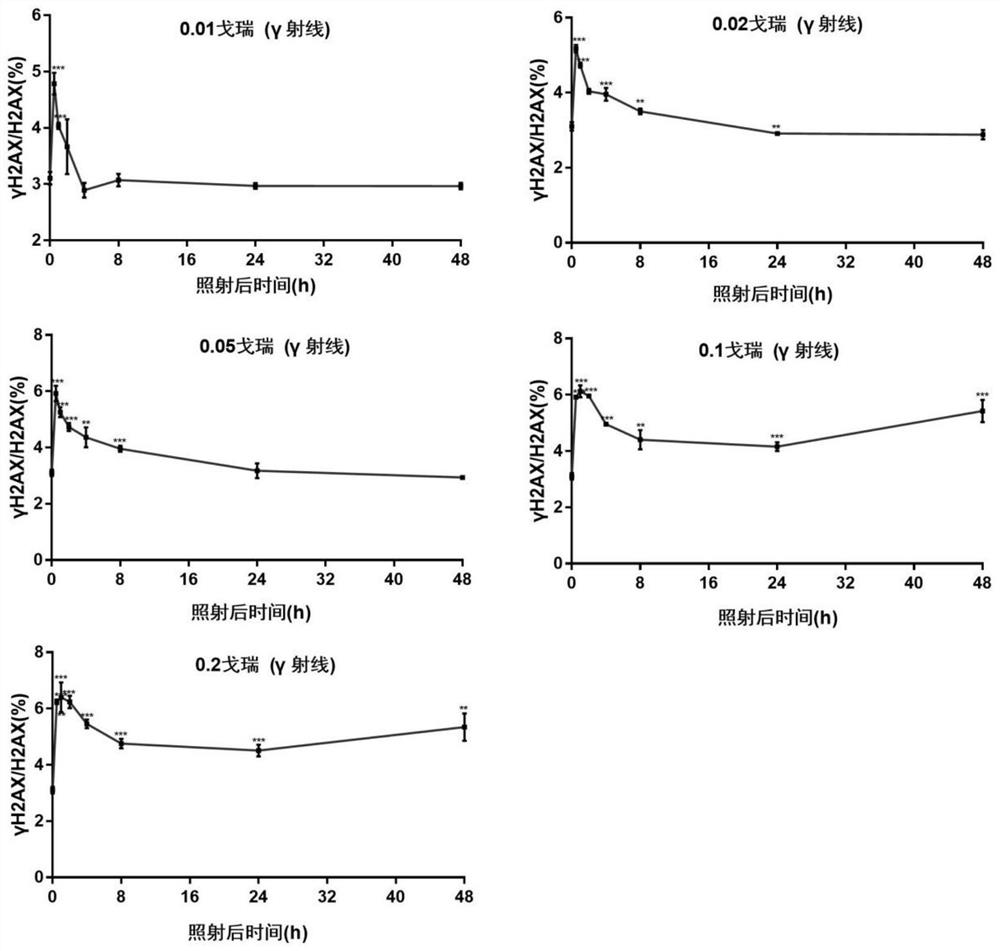
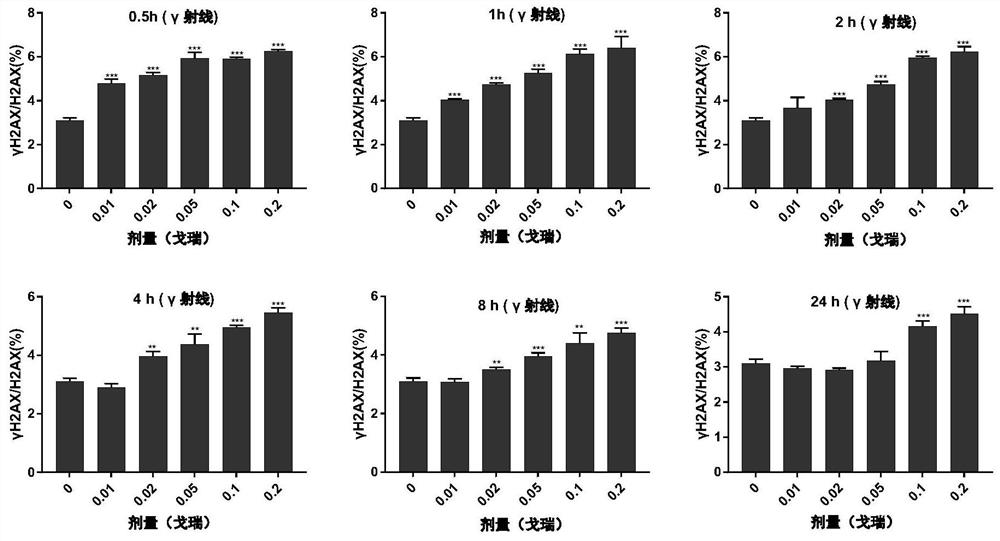
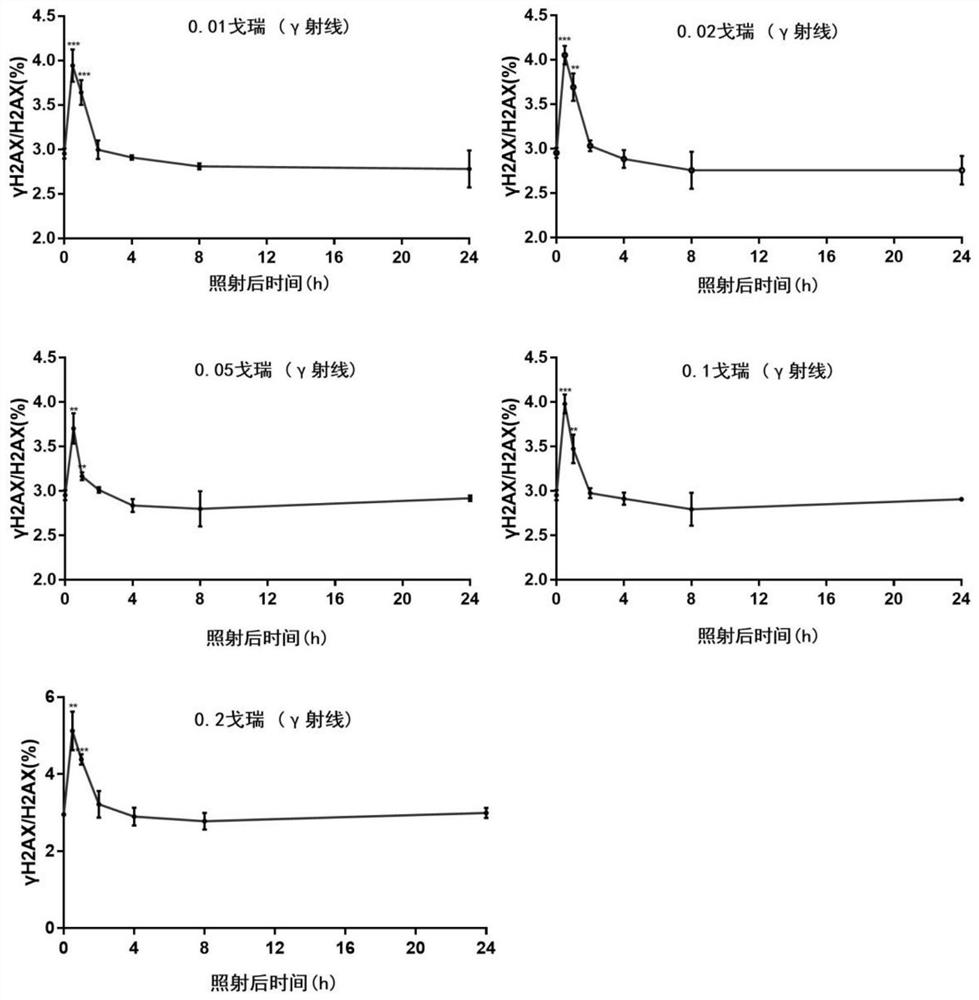
Method for detecting environmental low-dose ionizing radiation
ActiveCN113625325AGood sensitivity and specificityAccurate detectionComponent separationDosimetersNuclear medicineRadiochemistry
The invention relates to a method for detecting environmental low-dose ionizing radiation, and belongs to the technical field of radiation detection. The method comprises the following steps: 1) taking cells to be detected and cells of a negative control group, and respectively extracting cell nucleuses to obtain the cell nucleuses; 2) extracting histone from the cell nucleus obtained in the step 1) to obtain histone; (3) detecting the molar content of gamma H2AX and H2AX in the histone; 4) when the molar content ratio of gamma H2AX to H2AX of the cells to be detected is higher than that of the negative control group under the same treatment condition, judging that the cells are subjected to low-dose ionizing radiation; according to the fitted curve, obtaining the specific dose of the ionizing radiation on the cells to be detected by combining the molar content ratio of the gamma H2AX to the H2AX of the cells to be detected. According to the method, low-dose ionizing radiation in the environment can be detected, whether the ionizing radiation exists in the environment or not can be identified, and the radiation reference dose can be provided.
Owner:ACADEMY OF MILITARY MEDICAL SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com