Fusion protein TgMEP as well as preparation method and application thereof
A fusion protein and sequence technology, applied in the field of genetic engineering, can solve the problems of high preparation cost, difficult standardization, and low diagnostic efficiency of toxoplasmosis diagnostic antigen, and achieve the effect of simple preparation, good sensitivity and specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
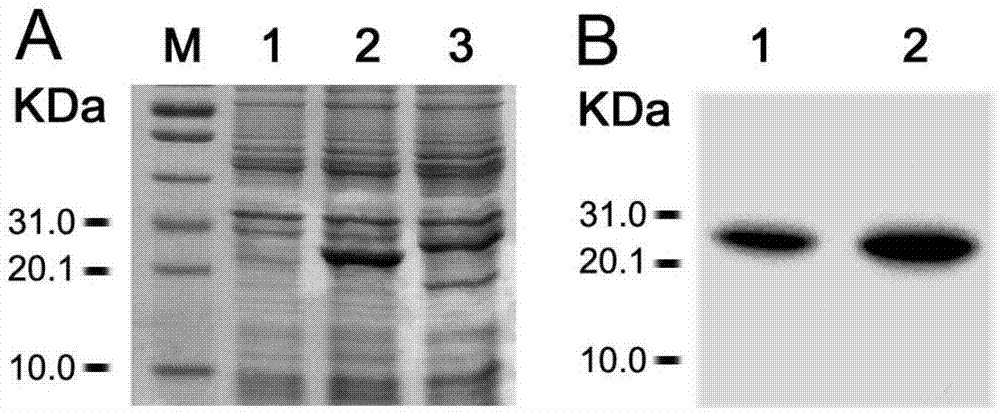
[0020] Example 1 Expression, purification and immunoreactivity detection of Toxoplasma gondii multi-epitope peptide recombinant fusion protein TgMEP
[0021] [1] Screening and identification of B cell epitopes of the main diagnostic antigen of Toxoplasma gondii
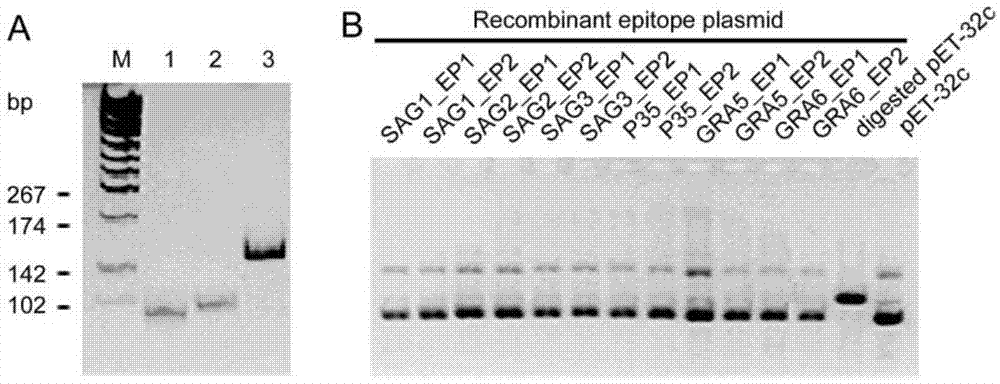
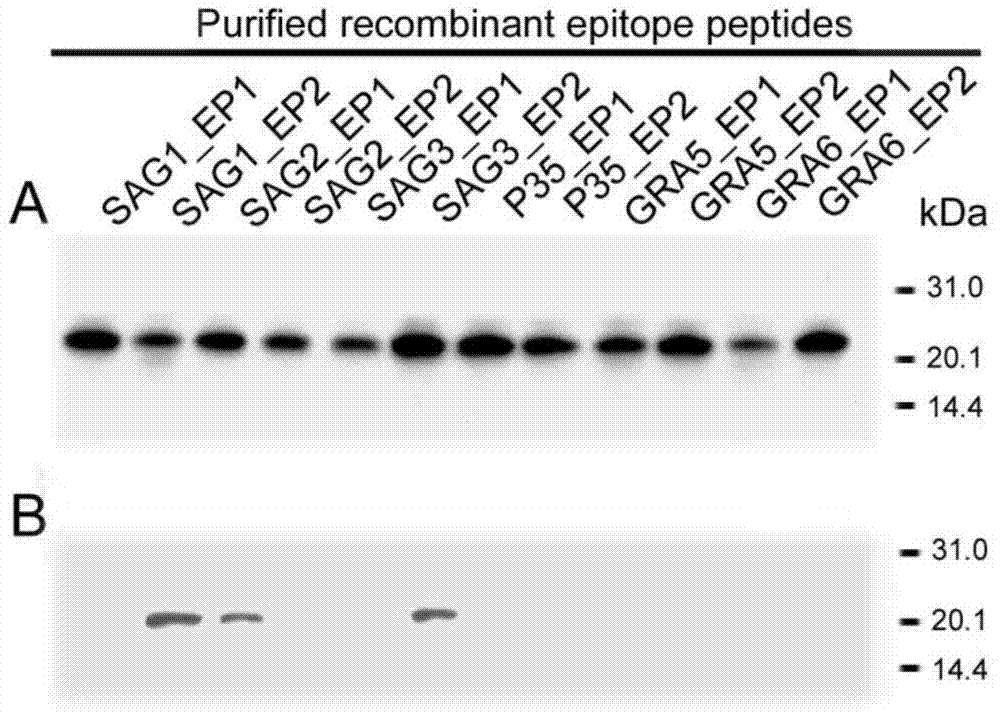
[0022] Using molecular biology software BioSun, DNAstar combined with Hopp&woods hydrophilicity parameters, accessibility parameters, polarity parameters, flexibility parameters and secondary structure parameters to analyze the major surface antigen 1 (SAG1) and major The B cell epitopes of surface antigen 2 (SAG2), major surface antigen 3 (SAG3), tachyzoite surface antigen P35, compact granule protein 1 (GRA1), and compact granule protein 6 (GRA6) were analyzed; each antigen molecule was selected Two predicted B cell epitopes, two complementary oligonucleotide single strands were designed according to the sequence of each epitope, annealed to form double strands and then cloned into the prokaryotic expression vector ...
Embodiment 2
[0032] Example 2 Application of recombinant fusion protein TgMEP in toxoplasmosis diagnostic kit
[0033] [1] Establishment of recombinant fusion protein TgMEP ELISA
[0034] Orthogonal experiments were used to determine the best detection conditions for the enzyme-linked immunosorbent assay for the detection of toxoplasmosis IgG and IgM antibodies, and the coating concentrations of the recombinant fusion protein TgMEP for the detection of IgG and IgM antibodies were respectively: 1 μg / ml and 2 μg / ml. The diagnostic cut-off value was the mean of 15 negative sera plus 2 standard deviations, and the diagnostic cut-off values of ELISA for IgG and IgM antibody detection were 0.14 and 0.16, respectively. The specific detection steps are as follows: Coat polystyrene enzyme-labeled strips with 100 μl / well of carbonic acid buffer containing recombinant fusion protein, overnight at 4°C, block with PBST containing 5% skimmed milk powder at 37°C for 1 hour, and wash the plate three ti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com