Freeze-dried hemagglutination inhibition test antigen for detecting mycoplasma synoviae, antigen combination and preparation method
A technique for mycoplasma synovial bursae and hemagglutination inhibition test, applied in the field of freeze-dried hemagglutination inhibition test antigen and antigen combination for detection of mycoplasma synovial bursa Less difficult to operate, stable antigen properties, and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1: Isolation and screening of MS JS2018-4 strain
[0056] 1. Experimental materials
[0057] Mycoplasma broth basal medium (product number: CM0403B) and mycoplasma agar basal medium (product number: CM0401B) were purchased from OXOID; arginine, cysteine and coenzyme I were purchased from Sangon Bioengineering (Shanghai) Co., Ltd.; pig serum was purchased from Jinyuankang Bioengineering Co., Ltd.
[0058] Preparation method of improved Frey's liquid medium: mycoplasma broth basal medium (OXOID, product number: CM0403B) 25.5g / L, glucose 3.3g / L, coenzyme Ⅰ 100mg / L, arginine 400mg / L, cysteine 100mg / L, 0.02% phenol red indicator, each component was added into ultrapure water, and the pH was adjusted to 7.8. Use a 0.22 μm bacterial filter to filter and sterilize, and add 15% to 20% pig serum.
[0059] The preparation method of improved Frey's solid medium: mycoplasma broth basal medium (OXOID, product number: CM0403B) 35.5g / L, autoclave at 121°C for 15min, coo...
Embodiment 2
[0067] Example 2: Preparation and application of freeze-dried MS hemagglutination inhibition test antigen
[0068] 1 Experimental materials
[0069] 1.1 Strains
[0070] The MS JS2018-4 strain was isolated, identified and preserved by the Key Open Laboratory of Livestock and Poultry Infectious Diseases of the Ministry of Agriculture of Yangzhou University, and was preserved in the China Center for Type Culture Collection (China, Wuhan, Wuhan University) with the preservation number: CCTCC M20191100.
[0071] 2 Experimental methods
[0072] 2.1 Strain cultivation
[0073] 2.1.1 Obtaining of seed bacterial liquid
[0074] Take the MS JS2018-4 isolate strain, inoculate it into fresh modified Frey's liquid medium, place it in an incubator at 37°C, and cultivate it at 220r / min for 24h, until the color of the medium changes from purple red to orange yellow. Then absorb the bacterial liquid to inoculate the freshly improved Frey's liquid medium at a ratio of 1:10, culture for 24 ...
Embodiment 3
[0121] Example 3: Preparation and application of freeze-dried MG hemagglutination inhibition test antigen
[0122] 3.1 Mass preparation of MG hemagglutination inhibition test antigen
[0123] A batch of MG hemagglutination inhibition test antigens were prepared in large quantities according to the freeze-dried MS hemagglutination inhibition test antigen preparation conditions determined in 2.1-2.4 of Example 2.
[0124] 3.2 Detection of MG hemagglutination inhibition test antigen
[0125] 3.2.1 HA titer determination
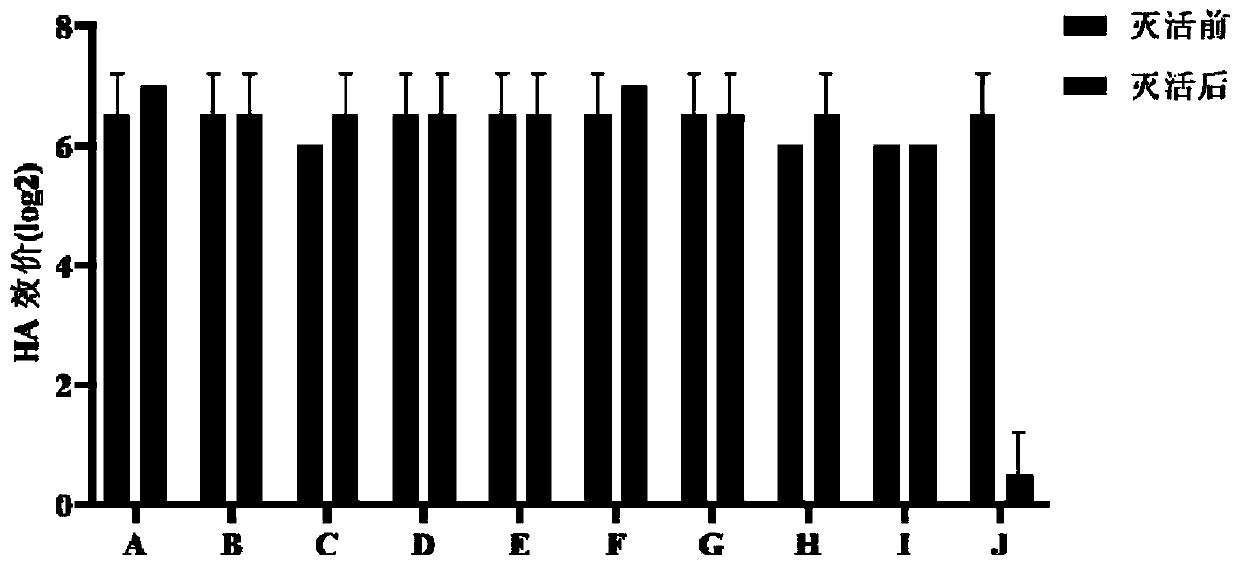
[0126] Use 2ml of PBS solution to dissolve the hemagglutination inhibition test antigen, and measure the HA titer. The results showed that the HA titers of 10 randomly selected bottles of freeze-dried antigens were all 2 8 .
[0127] 3.2.2 Specificity test
[0128] Using the method of HI test, the MG, MS, NDV, AIV, IBV positive serum and SPF chicken serum were tested for HI by using the prepared MG hemagglutination inhibition test antigen. The results are ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com