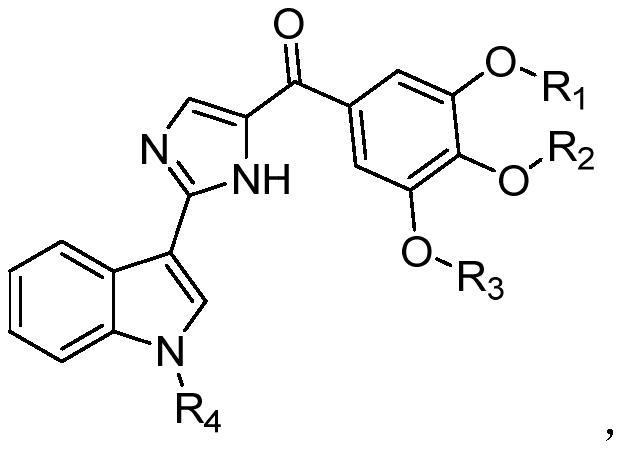
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
54results about How to "Extended dosing interval" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Tripterine nano structure lipid carrier and preparation method and application thereof
InactiveCN102225205AReduce systemic side effectsImprove bioavailabilityOrganic active ingredientsAntipyreticNano structuringTreatment effect
The invention relates to the field of Chinese medicine preparation, in particular to a method for preparing tripterine nano structure lipid carrier containing traditional Chinese medicine monomer and application of the tripterine nano structure lipid carrier in preparation of transdermal drugs used for treating psoriasis, rheumatoid arthritis, skin cancer and breast cancer. The tripterine nano structure lipid carrier is characterized by comprising the following components in parts by weight: 1 part of tripterine, 5-100 parts of mixed lipid, 0.5-10 parts of phospholipid, 0.1-15 parts of poloxamer-188 and 0.5-10 parts of vitamin E and tocopherol polyethylene glycol succinate, wherein the mixed lipid is composed of solid lipid monoglycerine and liquid lipid octylic acid / caprin according to the weight ratio of 1: 0.1-10: 1. Tripterine is prepared into the nano structure lipid carrier, the tripterine nano structure lipid carrier in a semi-solid or liquid preparation form is applied in a transdermal way, bioavailability of the tripterine can be improved, toxic response of tripterine can be reduced, and the nano structure lipid carrier provided by the invention has great clinical application value in the improvement of the treatment effect of tripterine.
Owner:JIANGSU PROVINCE INST OF TRADITIONAL CHINESE MEDICINE
Multiple-effect azole microcapsule suspension formulation with slow-release function and preparation method thereof
InactiveCN101361476AGood slow releaseImprove stabilityBiocidePlant growth regulatorsEngineeringPaclobutrazol
The invention provides a paclobutrazol micro-capsule suspension preparation provided with sustained-release function, which includes the following components according to 100 parts by weight: 10 to 30 parts of film-forming materials, 5 to 40 parts of paclobutrazol, 5 to 10 parts of emulsifying agent and the rest parts of water. The potency of the suspension preparation is more moderate, the potency releases slower and lasts long, thus ensuring the adjustment of steady growth of plants and having the sustained-release function. The invention also provides a preparation method of the paclobutrazol micro-capsule suspension preparation.
Owner:SICHUAN LANYUE SCI & TECH
Drug composition for preventing or treating HIV (human immunodeficiency virus) infection and application thereof
InactiveCN102247590AHigh potencyExtended half-lifeOrganic active ingredientsPeptide/protein ingredientsHuman immunodeficiencyPeptide
The invention relates to a drug composition containing an antiviral peptide, application thereof in prevention or treatment of HIV (human immunodeficiency virus) infection and preparation of the corresponding drugs, and a method for preventing or treating HIV infection by using the drug composition.
Owner:TIANJIN FUSOGEN BIOTECH CO LTD
Compound control-released percutaneous medicine plaster for treating high blood pressure and its preparation method
InactiveCN1633994AImprove treatment efficiencyImprove securityPharmaceutical active ingredientsSheet deliveryBlood drugTreatment hypertension
The invention relates to a compound control-released percutaneous medicine plaster for treating high blood pressure and its preparation method, wherein the paste comprises laminated back lining layer, a pressure-sensitive adhesive framework form medicament-containing layer and a protective film, wherein the compound medicament comprises diuretics, central alpha-acceptor excitant and cirumferential alpha-receptor blocking agent, beta-receptor blocking agent, calcium antagonist and any two of the five categories of medicament for influencing angiotensin II formation and treating high blood pressure. The invention may realize medicine administration once in front of chest or behind the ears, wherein homeostasis blood concentration can be reached.
Owner:王睿 +3
Gatifloxacin gels foreye and its preparing method
InactiveCN1562033AExtended stayImprove bioavailabilityOrganic active ingredientsSenses disorderMedicineGlycerol
Owner:深圳市华晖生物科技有限公司
New erythrocyte-stimulating factor analogues
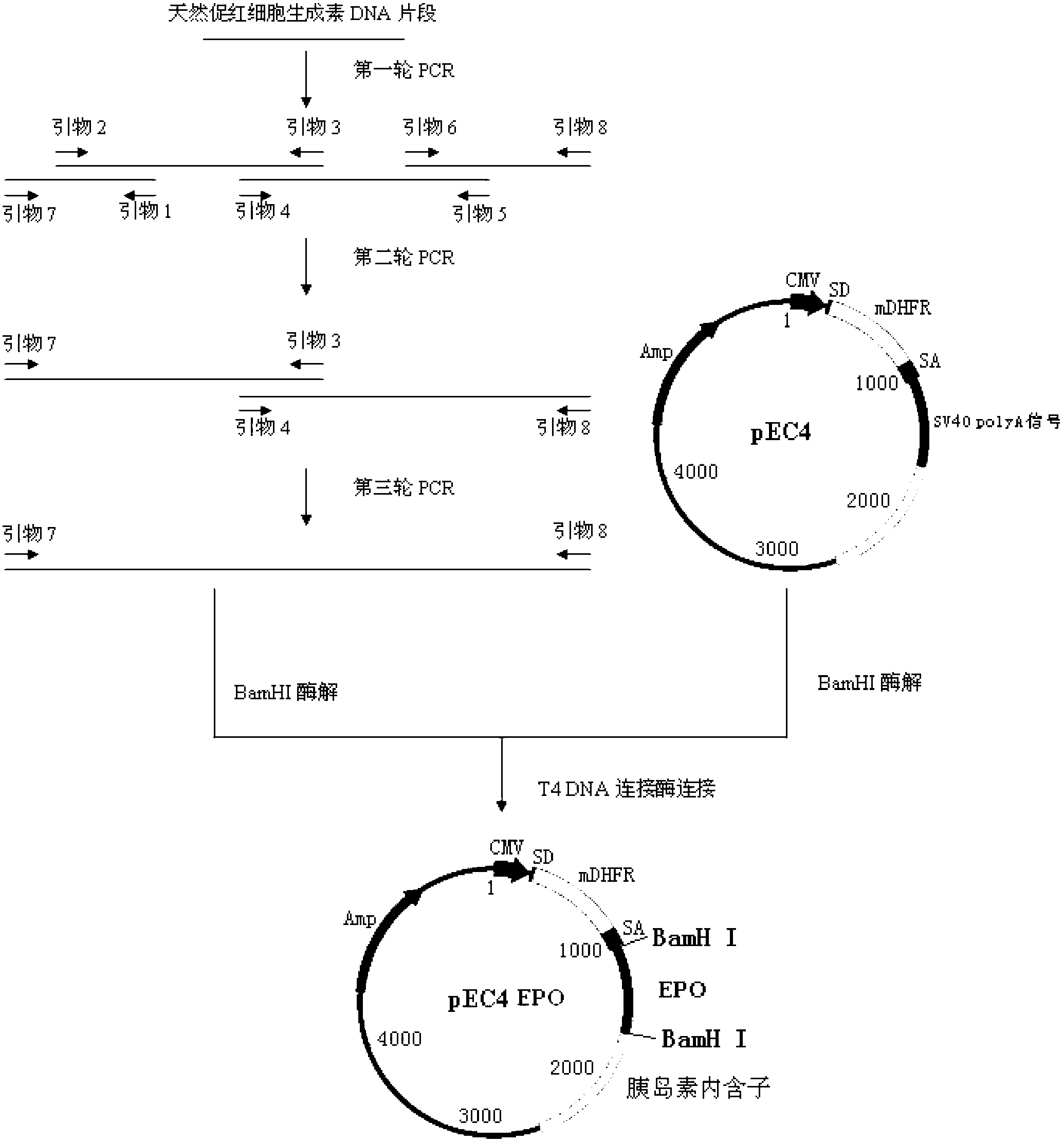
ActiveCN101337988ALong elimination half-lifeExtended dosing intervalPeptide/protein ingredientsErythropoietinEukaryotic plasmidsA-DNA
The invention relates to an erythropoietin analog with at least one extra glycosylation sites or a variant thereof. The invention also relates to a DNA sequence for coding the erythropoietin analog or the variant thereof, and a recombinant plasmid and a host cell for expressing the analog or the variant thereof.
Owner:SHENYANG SUNSHINE PHARMA
Compound injection with probenecid, potassium and beta-lactam antibiotic and its use
InactiveCN1853724AIncrease the total amount of drugReduce dosageAntibacterial agentsOrganic active ingredientsCefotaximePenicillin
A compound powder injection or freeze-dried powder injection is prepared from the sodium (or potassium) salt of probenecid and the beta-lactam kind of antibiotics including penicillin G, ampicillin, ancef, cefuroxime, cefotaxime and cefoxitin. It can elongate the half life of antibiotic, increase the AUC and plasma level and prevent the generation of drug-resistant bacteria.
Owner:吴晓辉
Novel Anti-human ctgf antibody
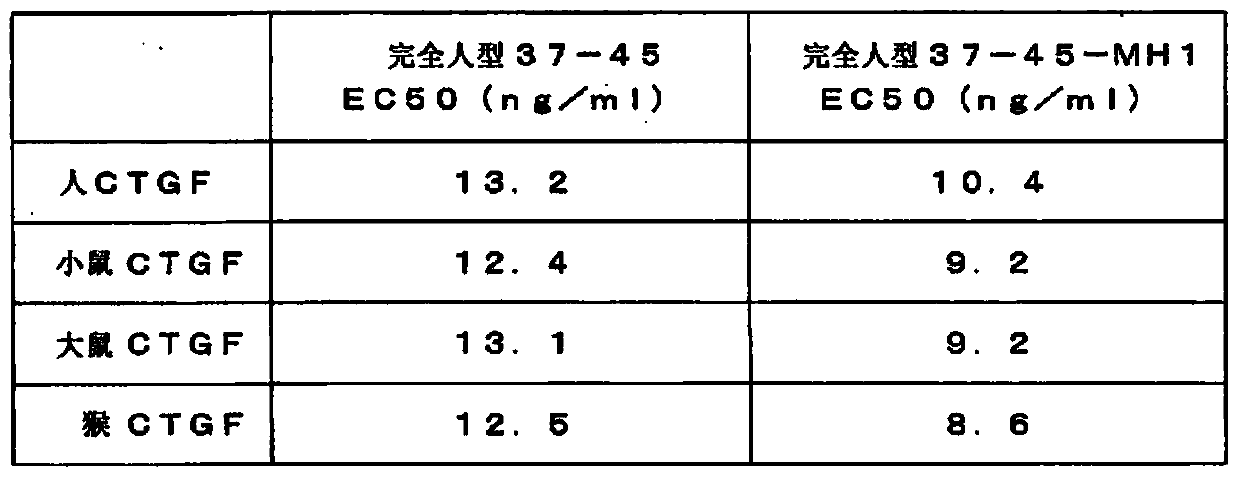
The invention provides an anti-human CTGF antibody having a superior binding activity and / or a superior neutralization activity compared with those of conventional anti-human CTGF antibodies; and a means for preventing or treating various diseases in which human CTGF is involved in the formation of clinical conditions thereof, including renal diseases such as chronic kidney disease and diabetic nephropathy, using the above-mentioned anti-human CTGF antibody. An anti-human CTGF antibody containing a heavy-chain variable region comprising the amino acid sequence represented by SEQ ID NO: 10 and a light-chain variable region comprising the amino acid sequence represented by SEQ ID NO: 4.
Owner:ASTELLAS PHARMA INC
PEG-PLGA sustained release microsphere with encapsulated buprenorphine and preparation method thereof
ActiveCN105596298AGood biocompatibilitySmall particle sizeOrganic active ingredientsPharmaceutical product form changeBuprenorphine HydrochloridePatient compliance
The invention discloses a PEG-PLGA sustained release microsphere with encapsulated buprenorphine and a preparation method thereof. The preparation method is as below: conducting debydrochlorination on buprenorphine to prepare alkali, and conducting a single emulsion method to obtain the PEG-PLGA sustained release microsphere with encapsulated buprenorphine. The weight ratio of buprenorphine (calculated by buprenorphine hydrochloride) to polyethylene glycol-polylactic acid-glycolic acid copolymer is 2-6:10, and the buprenorphine sustained release microsphere has particle size of 10-50 micron. The invention employs for the first time the hydrochloric acid-single emulsion method to prepare the buprenorphine sustained release microspheres with high drug loading, obvious sustained-release effect and good biocompatibility, and can develop a variety of administration approaches, such as intravenous injection, and subcutaneous and intramuscular non-intravenous administration approaches. The invention has good application prospect, and can improve patient compliance.
Owner:SHANDONG UNIV
Gatifloxacin ear drop and its prepn process
InactiveCN1931170AImprove Medication AdherenceAvoid drug resistanceAntibacterial agentsOrganic active ingredientsMedicineWhole body
The gatifloxacin ear drop is prepared with gatifloxacin or its salt as the main component and the supplementary materials including glycerin, ethanol and sterilized water for injection, pure water or distilled water. The present invention also discloses the preparation process of the gatifloxacin ear drop. As one fourth generation oxyquinolone type antibacterial medicine, the gatifloxacin ear drop has high bioavailability, reduced medicine taking times, less systemic untoward reaction, high patient's compliance and less drug resistance.
Owner:王廷春
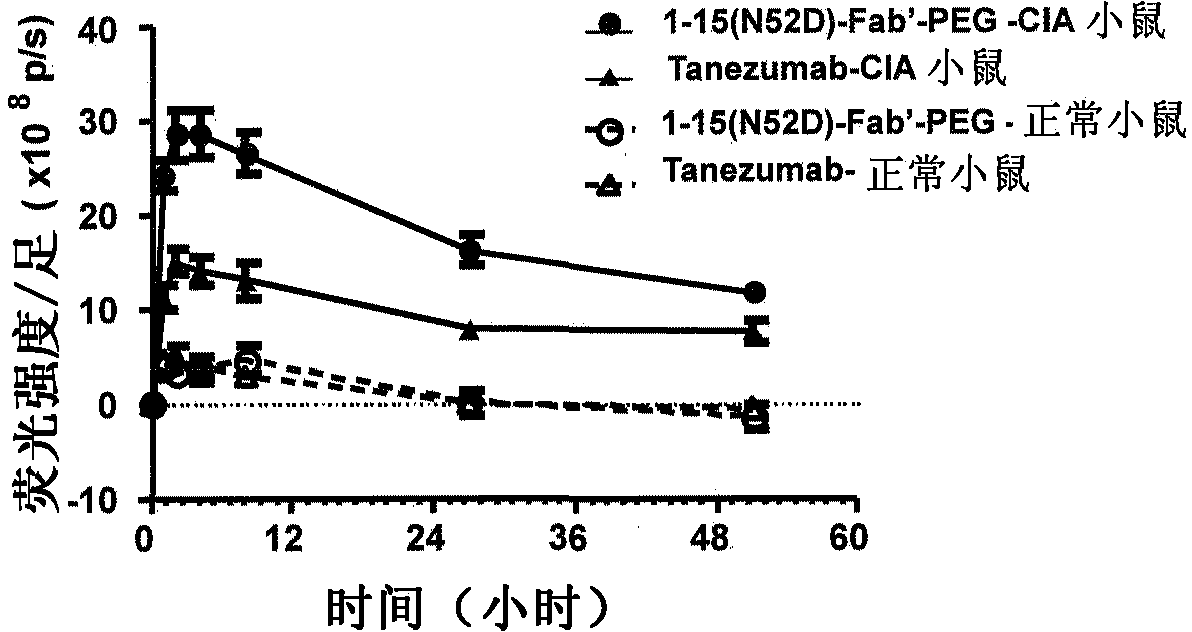
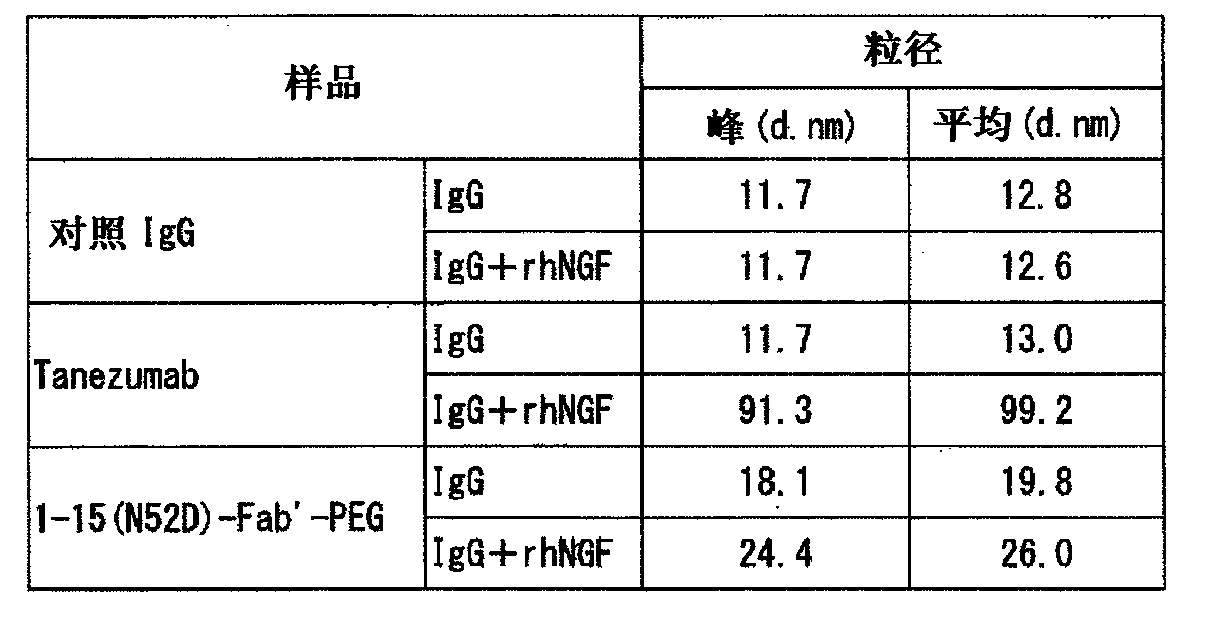
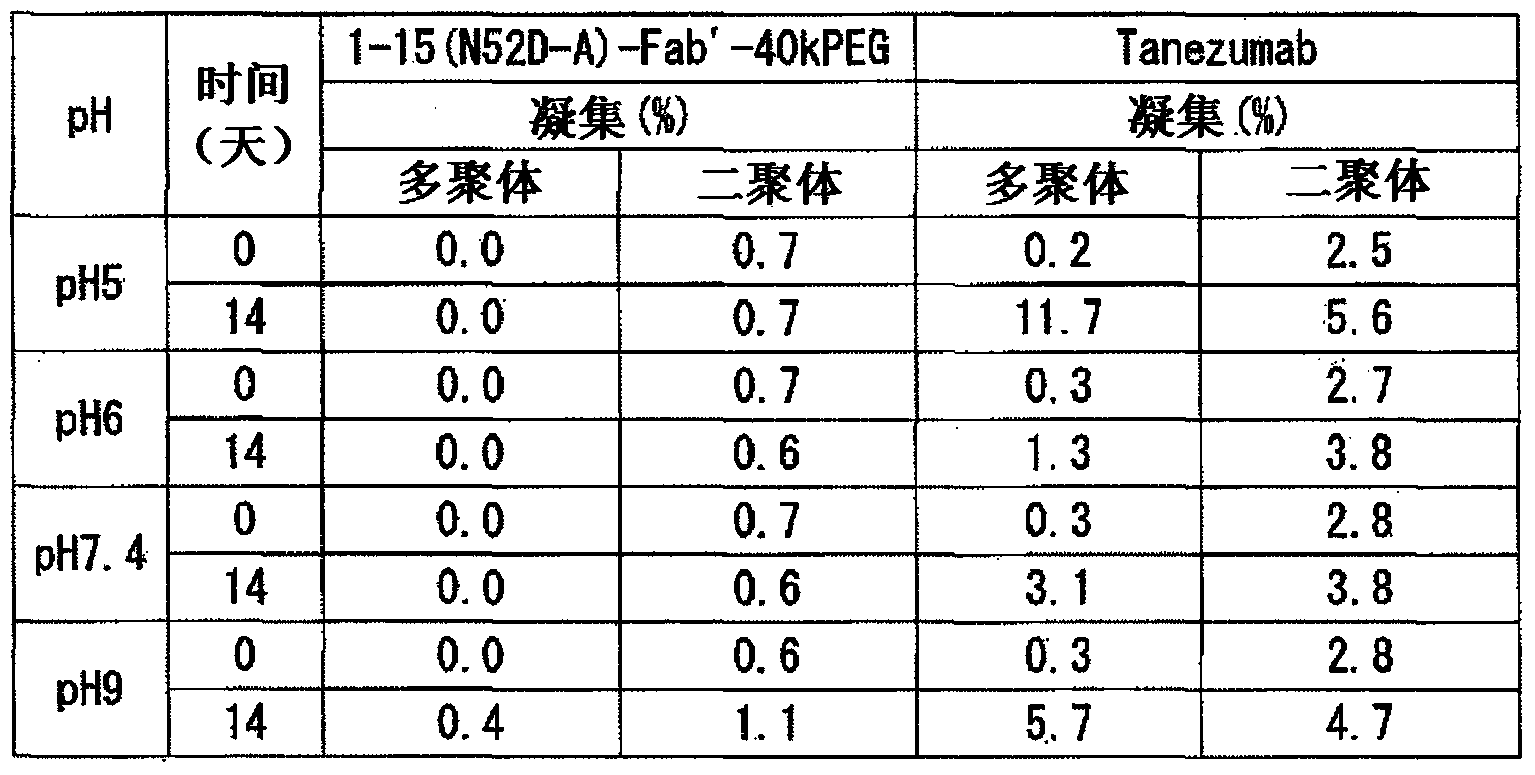
Novel anti-human NGF antibody
Provided are an anti-human NGF antibody which is reduced in the influence on fetuses and the risk of adverse side effects including thrombosis while keeping a high neutralizing activity, and which has excellent safety, or an antigen-binding fragment thereof; and a means, utilizing the antibody or an antigen-binding fragment thereof , for preventing or treating various diseases for which human NGF is involved in the development of a disease state. An Fab' fragment of an anti-human NGF antibody comprises a heavy-chain variable region comprising the amino acid sequence represented by SEQ ID NO: 6 and a light-chain variable region comprising the amino acid sequence represented by SEQ ID NO: 4.
Owner:ASTELLAS PHARMA INC
Preparation method of environmentally-friendly pyrethrin controlled-release microspheres
PendingCN109699687AUniform particlesGood molecular reaction kinetics performanceBiocideAnimal repellantsMicrosphereMagnetization
The invention discloses a preparation method of environmentally-friendly pyrethrin controlled-release microspheres. The method comprises the following steps: (1) performing ultrasonic cleaning on magnetic nanoparticles, and adding ethanol to prepare a magnetic nanoparticle solution; (2) dissolving half of the magnetic nanoparticle solution, a pyrethrin original drug and an environmentally-friendlyhigh-molecular polymer into an oil phase; (3) dissolving the other half of the magnetic nanoparticle solution and an emulsifying agent into a water phase; (4) performing magnetization on the oil phase and the water phase to make the oil phase and the water phase have opposite magnetism; (5) extruding the magnetized oil phase and water phase at a synchronous speed into a supercritical CO2 solvent;(6) adding an antifoaming agent dropwise, and performing a reaction for 12-14 h; (7) performing reduced-pressure drying to obtain controlled-release pyrethrin microspheres; and (8) preparing the pyrethrin sustained-release microsphere wettable powder. According to the preparation method provided by the invention, the pyrethrin controlled-release microspheres prepared by the method have uniform particles and a long lasting period, can reduce frequency of pesticide application and a total amount of the pesticide application, prolong a pesticide application interval, and improve a utilization rate of a pesticide.
Owner:MICROBIOLOGY INST OF SHAANXI
Natural human erythropoietin analogue
ActiveCN103113464ALong elimination half-lifeExtended dosing intervalPeptide/protein ingredientsErythropoietinA-DNAPlasmid
The invention relates to an erythropoietin analogue containing at least one extra glycosylation site or a variant of the erythropoietin analogue. The invention also relates to a DNA (Deoxyribonucleic Acid) sequence for encoding the erythropoietin analogue or the variant thereof as well as a recombinant plasmid and a host cell which are supplied for the analogue or the variant thereof to express.
Owner:SHENYANG SUNSHINE PHARMA
Tripterine nano structure lipid carrier and preparation method and application thereof
InactiveCN102225205BReduce systemic side effectsImprove bioavailabilityOrganic active ingredientsAntipyreticNano structuringPhospholipid
Owner:JIANGSU PROVINCE INST OF TRADITIONAL CHINESE MEDICINE
Hemopoietin analogue
InactiveCN103073631ALong elimination half-lifeExtended dosing intervalPeptide/protein ingredientsErythropoietinEukaryotic plasmidsA-DNA
The invention relates to a hemopoietin analogue with at least one additional glycosylation site or a variant thereof. The invention further relates to a DNA (deoxyribonucleic acid) sequence for coding the hemopoietin analogue or the variant thereof as well as a recombinant plasmid and a host cell for expression of the hemopoietin analogue or the variant thereof.
Owner:SHENYANG SUNSHINE PHARMA
Anti-infection wound-repairing pharmaceutical composition, preparation method and application thereof
InactiveCN112206281APromote growthEnlightenedAntibacterial agentsOrganic active ingredientsBiotechnologyBletilla striata
The invention discloses an anti-infection wound-repairing pharmaceutical composition in the technical field of biomedicines. The anti-infection wound-repairing pharmaceutical composition is prepared from the following raw materials in parts by weight, 30-40 parts of chitosan hydrochloride, 80-120 parts of cunninghamia lanceolata, 30-36 parts of bletilla striata, 2-8 parts of borneol and 450-550 parts of glycerol. The pharmaceutical composition is applied to prepare a film spraying preparation, and the preparation method comprises the following steps, step 1, weighing the raw materials in proportion, processing cunninghamia lanceolata and bletilla striata, and grinding the processed cunninghamia lanceolata and bletilla striata into fine powder; step 2, adding PVA1788 and chitosan hydrochloride to distilled water under water bath condition at 40 DEG C, and conducting magnetic stirring to be completely dissolved; step 3, adding cunninghamia lanceolata powder, bletilla striata powder and borneol, then adding glycerol, continuously conducting magnetic stirring to be completely dissolved, and standing at room temperature until foams are eliminated; step 4, adding absolute ethyl alcohol,conducting uniform stirring, standing for 5 minutes, and conducting bottling; and step 5, conducting sub-packaging and ultraviolet sterilization. The pharmaceutical composition disclosed by the invention has the effects of effusion resistance, adhesion prevention, hemostasis, anti-infection, promotion of wound healing, etc.
Owner:遵义医科大学珠海校区
Desvenlafaxine succinate sustained-release tablets and preparation method thereof
InactiveCN104352469AQuick resultsLittle side effectsOrganic active ingredientsNervous disorderSustained-Release PreparationsDrug release rate
The invention relates to desvenlafaxine succinate sustained-release tablets. The desvenlafaxine succinate sustained-release tablets are prepared from the raw materials in parts by weight: 50 parts of desvenlafaxine succinate, 150-240 parts of sustained-release framework material and 10-100 parts of lubricant. A preparation method of the desvenlafaxine succinate sustained-release tablets comprises the steps of preparing materials, mixing, granulating, mixing, tabletting and carrying out aluminum-plastic packaging. The desvenlafaxine succinate sustained-release tablets disclosed by the invention can be used for effectively curing major depression and are high in effect taking speed, low in side effect, stable in drug release and good in controllability; compared with the tablets currently on the market, the technical advantages are obvious, the bioavailability is improved, novel sustained-release preparations are adopted, and sustained release means delaying the drug release rate of a drug from a dosage form, lowering the organism entering absorption rate of the drug and thus exerting a more stable treatment effect.
Owner:HARBIN SHENGJI PHARMA
Burst release-free irinotecan microsphere and preparation method thereof
InactiveCN104352449AUniform appearanceRelieve painOrganic active ingredientsPharmaceutical non-active ingredientsAcetic acidSide effect
The invention discloses a burst release-free irinotecan microsphere and a preparation method thereof. The irinotecan microsphere comprises irinotecan and a polylactic acid-glycolic acid copolymer (poly(lactic-co-glycolicacid), PLGA), wherein the weight ratio of the irinotecan to the PLGA is 1 to (10-40), and the grain size of the irinotecan microsphere is 1-5 micrometers. A long-acting burst release-free irinotecan microsphere can be prepared from the irinotecan and the PLGA at a specific ratio, and the slow release period of the irinotecan microsphere is longer than 40 days, so the medication frequency is obviously reduced, a peak and valley phenomenon of the medicine is reduced, the bioavailability of the irinotecan is improved and toxic and side effects of the medicine are reduced.
Owner:HEBEI UNIVERSITY OF SCIENCE AND TECHNOLOGY
Pesticide used for preventing and controlling leaf miners and method therefor
InactiveCN101785474APromote absorptionIncreased toxicityBiocideAnimal repellantsPesticide toxicityPESTICIDE ADJUVANTS
The invention relates to a pesticide used for preventing and controlling leaf miners, which is prepared by mixing trichlorphon, pesticide adjuvant and water, wherein the proportion of the trichlorphon (mass), the pesticide adjuvant (volume) and the water (volume) is 1:1:200; and the pesticide adjuvant is of the 75ml of mixture prepared by mixing water and the following raw materials: 14-30g of sodium dodecyl benzene sulfonate, 70-150g of edible white sugar and 14-30g of bone glue. The pesticide has the benefits of improving the pesticide absorption of crops and improving the pesticide toxicity for the larvas of the leaf miners.
Owner:关慧明
Eyedrops for curing eye affection and uses thereof
InactiveCN101239070AExtension of timeImprove utilization efficiencyOrganic active ingredientsSenses disorderSide effectKidney
The invention discloses an eye drop which mainly contains amikacin or amikacin salt and chitosan or the derivative of chitosan. The eye drop comes into being through the dissolving of a medicine compound into the injection water which is then added with leakage pressure adjusting agent, pH value adjusting agent, acid-bases buffer, chemical inhibitor and bacteriostatic agent, then the addition of the full dose of injection water after the dissolving, then the filtering and sterilization of the solution, with the finished product obtained finally after the canning and sealing. The invention, on one hand, reduces the dosage of amikacin through the coordinate antibacterial action of chitosan, the derivative of chitosan and the amikacin, thus reducing the toxic and side effect and particularly reducing the toxicity to ears and kidneys. On the other hand, the invention prolongs the time for which the medicine stays in the eyes through taking advantage of the solution with a certain viscosity obtained after the dissolving of chitosan and the derivative of chitosan into the water, thus improving the use efficiency of the medicine, reducing the medicine using times and prolonging the intervals for medicine using. The invention has a good result in curing the eye infection caused by sensitive bacteria, such as conjunctivitis, keratitis, sclerotitis, etc.
Owner:四川川投医药生物技术有限责任公司
Gatifloxacin gels for eye and its preparing method
InactiveCN1215844CExtended stayImprove bioavailabilityOrganic active ingredientsSenses disorderMedicineGlycerol
The invention discloses a gatifloxacin ophthalmic gel and a preparation method thereof, aiming at overcoming the defects of drug resistance caused by long-term use of the existing ear drops and many times of medication by patients, and providing a gatifloxacin ophthalmic gel eye gel. It is a medicine containing the following percentages by weight: 0.05%-0.4% of gatifloxacin, 0.5%-1.5% of carbomer, and 1%-2% of triethanolamine. The preparation steps are as follows: sprinkle carbomer on the surface of glycerin, grind and infiltrate, then grind with distilled water to make it fully swell; dissolve triethanolamine in distilled water, add supernatant and grind to make gel matrix (III); Add gatifloxacin to distilled water, stir to dissolve; then add propylene glycol and stir well to make a mixed solution (IV); slowly add the mixed solution (IV) to (III), add distilled water to the entire amount, and grind until uniform.
Owner:深圳市华晖生物科技有限公司
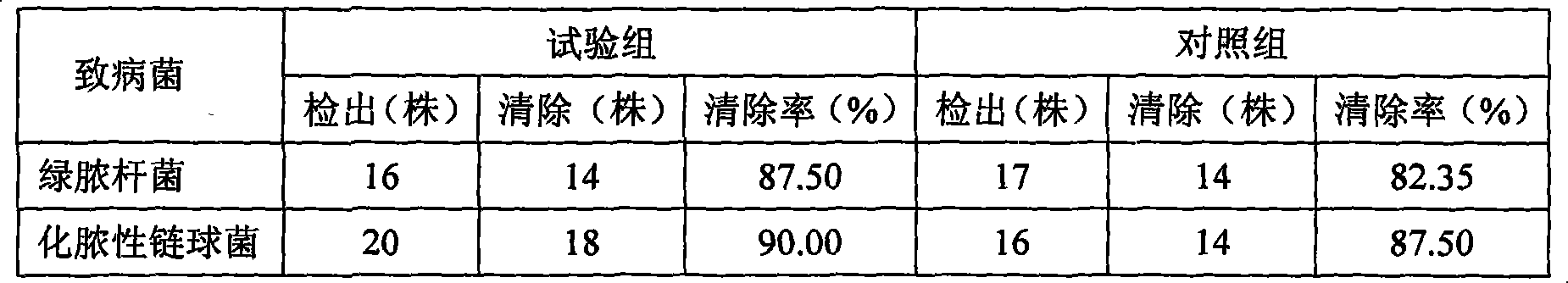
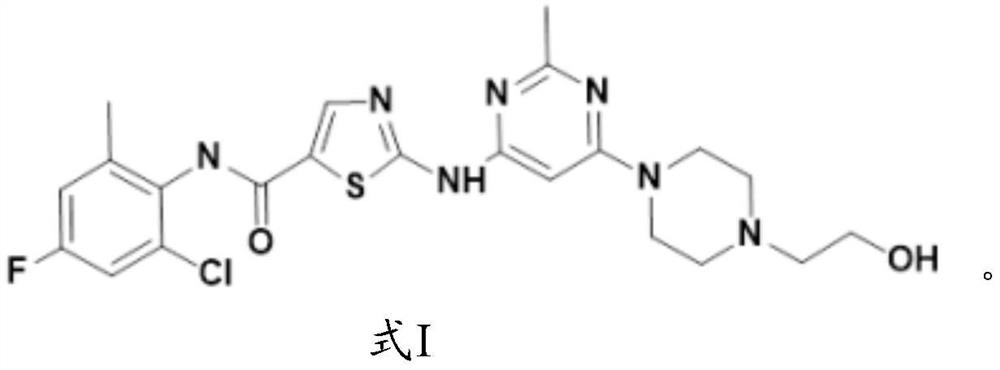
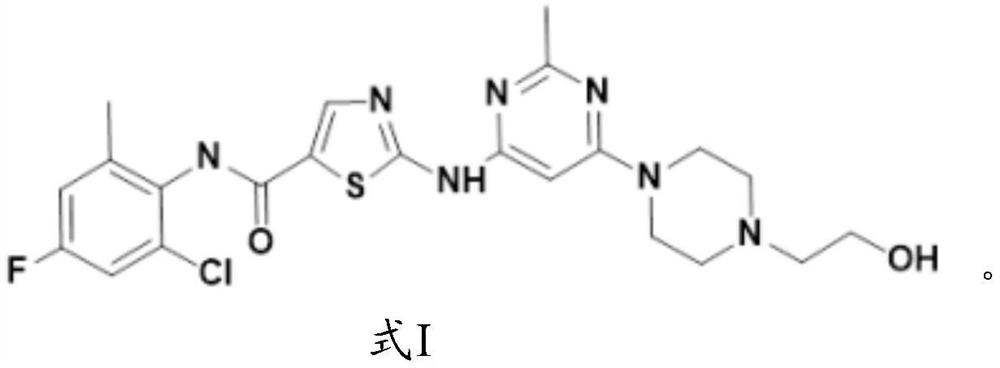
Application of fluorine-substituted 2-aminothiazole-5-aromatic carboxamide
InactiveCN112402422AHigh kinase inhibitory activitySmall doseOrganic active ingredientsNervous disorderDiseaseTyrosine
The application of the invention relates to an application of a compound of a formula I or pharmaceutically acceptable salt, ester, solvate, prodrug, active metabolite, crystal, stereoisomer, tautomeror geometric isomer thereof, or a medical composition comprising the compound of the formula I or the pharmaceutically acceptable salt, the ester, the solvate, the prodrug, the active metabolite, thecrystal, the stereoisomer, the tautomer or the geometrical isomer to preparation of drugs for preventing or treating tyrosine kinase related diseases.
Owner:HUNAN WARRANT PHARMA +2
Indole compound for preparing coronavirus therapeutic drug
PendingCN114805307AEnhanced inhibitory effectImprove stabilityOrganic active ingredientsIsotope introduction to heterocyclic compoundsMetabolic stabilityMedicine
The invention discloses an indole compound for preparing a coronavirus therapeutic drug, and belongs to the field of chemical medicines. The indole compound shown in the general formula (I) or the pharmaceutically acceptable salt thereof has excellent tubulin polymerization inhibition activity, and can inhibit tubulin and destroy cytoskeleton, so that coronavirus cannot be transported, and the purpose of effectively treating virus infection is achieved. Meanwhile, the compound disclosed by the invention is relatively good in metabolic stability and relatively long in metabolic half-life period, so that the medical dosage can be reduced, and the administration time interval can be expanded.
Owner:南京雷正医药科技有限公司
Fusion polypeptide comprising polypeptide region that can be o-glycosylated
PendingCN112654637ALong durationExtended dosing intervalPolypeptide with localisation/targeting motifPeptide/protein ingredientsPharmaceutical drugIntravenous gammaglobulin
Disclosed are: a fusion polypeptide comprising a target polypeptide and a hinge region of an immunoglobulin; a pharmaceutical composition containing the fusion polypeptide; and a method for increasing the in vivo period of a target polypeptide, comprising a step of fusing a hinge region of an immunoglobulin.
Owner:LG CHEM LTD
Mongolian medicine paste for child hyperpyrexia and preparation method and application method thereof
InactiveCN108066681AIncrease irritationPoor oral bioavailabilityAntipyreticAnalgesicsMedicinal herbsHuman body
The invention relates to the technical field of medicine preparations, in particular to a Mongolian medicine paste for child hyperpyrexia and a preparation method and application method thereof. Mongolian medicine eridun-7 soup is used as a powdery preparation for solving the problems that treatment is influenced due to poor adaptability and difficult application during child hyperpyrexia treatment. The oral powdery preparation is changed into an external use paste, extracts of all medicinal materials are used for replacing raw material medicine, and the treatment effect after use is better. The Mongolian medicine paste for child hyperpyrexia has the advantages that 1, the first-pass effect of the liver and degradation of the medicine in the gastrointestinal tract can be avoided, medicineabsorption is not influenced by gastrointestinal factors, and the individual difference of medicine application is reduced; 2, the medicine can enter the human body at the constant speed for a long time after one-time medicine application, the frequency of medicine application is reduced, and the interval of medicine application is prolonged; 3, the medicine application area is changed, the medicine application dosage is adjusted, and a patient takes the medicine by himself / herself; 4, the Mongolian medicine paste has the sustained or controlled release effect, the medicine can be slowly released at a certain speed, the constant plasma concentration is formed in the skin accordingly, and convenience is provided for prolonging the treatment time of the medicine.
Owner:内蒙古民族大学附属医院
Norethisterone acetate-containing compound estradiol transdermal sustained release preparation and preparation method thereof
ActiveCN103054879AAvoid degradationAvoid first pass effectOrganic active ingredientsSkeletal disorderControlled releaseTransdermal patch
The invention discloses a norethisterone acetate-containing compound estradiol transdermal sustained release preparation and a preparation method thereof. The preparation comprises a backing layer, a protective film and a frame layer between the backing layer and the protective film, wherein the frame layer comprises an upper frame layer, a lower frame layer and a controlled release film between the upper frame layer and the lower frame layer; and the backing layer covers the surface of the lower frame layer. The upper and lower frame layers are respectively wrapped by estradiol and norethisterone acetate in a certain ratio, an intermediate frame layer is wrapped by a special transdermal patch of the controlled release film, estrogen estradiol effects in relieving or eliminating climacteric syndromes and preventing osteoporosis are fully played, and the preparation also has the characteristics of small stimulus to human bodies, small side effects, quality stability and long effective time.
Owner:ZHEJIANG YATAI PHARMA
Paliperidone sustained-release microspheres, injection thereof and preparation method of the sustained-release microspheres
ActiveCN103893129BUniform appearanceNo adhesionOrganic active ingredientsPowder deliveryMicrosphereGlycolic acid
The invention provides a paliperidone sustained release microsphere and an injection thereof, and a preparation method of the sustained release microsphere. The paliperidone sustained release microsphere disclosed by the invention comprises paliperidone and a polylactic acid-glycolic acid copolymer, wherein the weight ratio of the paliperidone to the polylactic acid-glycolic acid copolymer is (0.160-0.190):(0.5-0.9); the grain size of the paliperidone sustained release microsphere is 20-50mum. The paliperidone sustained release microsphere is prepared for the first time, and is high in medicament loading capacity, long in slow release period and good in biocompatibility.
Owner:NEW FOUNDER HLDG DEV LLC +2
A nitric oxide releasing ointment and its preparation method and application
ActiveCN110433134BReduce the burden onObvious curative effectAntibacterial agentsAntimycoticsSodium methoxidePolyvinyl alcohol
The invention discloses a nitric oxide releasing ointment, a preparation method and application thereof. The method comprises the following steps: (1) dissolving polyethyleneimine in an organic solvent, adding sodium methoxide, mixing uniformly, carrying out NO loading reaction, and obtaining polyethyleneimine NO donor material; (2) dissolving polyethyleneimine NO donor material The body material is dissolved in polyvinyl alcohol aqueous solution to obtain polyethyleneimine NO donor material solution; (3) liquid paraffin, glyceryl monostearate, white vaseline and lanolin are mixed uniformly to obtain an oil phase; glycerin, Add Span 80 and Tween 80 into water and mix evenly to obtain a water phase; add the water phase to the oil phase to obtain a mixed solution; (4) add polyethyleneimine NO donor material solution to the mixed solution to obtain a released Nitric oxide ointment. The prepared ointment has good antibacterial and slow release effects on various bacteria, and is of great significance for the treatment of various microbial diseases and the improvement of people's health.
Owner:JINAN UNIVERSITY
Novel anti-human IL-23 receptor antibody
The present invention provides anti-human IL-23R antibodies with superior activity and / or species cross-reactivity compared with existing anti-human IL-23R antibodies, and various diseases that use the antibodies to prevent or treat human IL-23R involved in pathogenesis. way of disease. An anti-human IL-23R antibody, which respectively comprises the heavy chain variable region composed of the amino acid sequence shown in SEQ ID NO: 5, 9, 13, 17, 21, 25 or 29 and the amino acid sequence represented by SEQ ID NO: 7, 11, 15, 19, 23, 27 or 31 of the amino acid sequence shown in the light chain variable region.
Owner:ASTELLAS PHARMA INC
Norethisterone acetate-containing compound estradiol transdermal sustained release preparation and preparation method thereof
ActiveCN103054879BAvoid degradationAvoid first pass effectOrganic active ingredientsSkeletal disorderTransdermal patchSide effect
The invention discloses a norethisterone acetate-containing compound estradiol transdermal sustained release preparation and a preparation method thereof. The preparation comprises a backing layer, a protective film and a frame layer between the backing layer and the protective film, wherein the frame layer comprises an upper frame layer, a lower frame layer and a controlled release film between the upper frame layer and the lower frame layer; and the backing layer covers the surface of the lower frame layer. The upper and lower frame layers are respectively wrapped by estradiol and norethisterone acetate in a certain ratio, an intermediate frame layer is wrapped by a special transdermal patch of the controlled release film, estrogen estradiol effects in relieving or eliminating climacteric syndromes and preventing osteoporosis are fully played, and the preparation also has the characteristics of small stimulus to human bodies, small side effects, quality stability and long effective time.
Owner:ZHEJIANG YATAI PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com