Novel Anti-human ctgf antibody
An antibody and sequence technology, applied in the direction of antibodies, anti-animal/human immunoglobulins, animal/human proteins, etc., can solve the problems of insufficient binding activity and insufficient CTGF neutralization activity, etc., to achieve the goal of giving Improvement of drug method, improvement of treatment effectiveness and patient compliance, effect of strong anti-fibrotic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
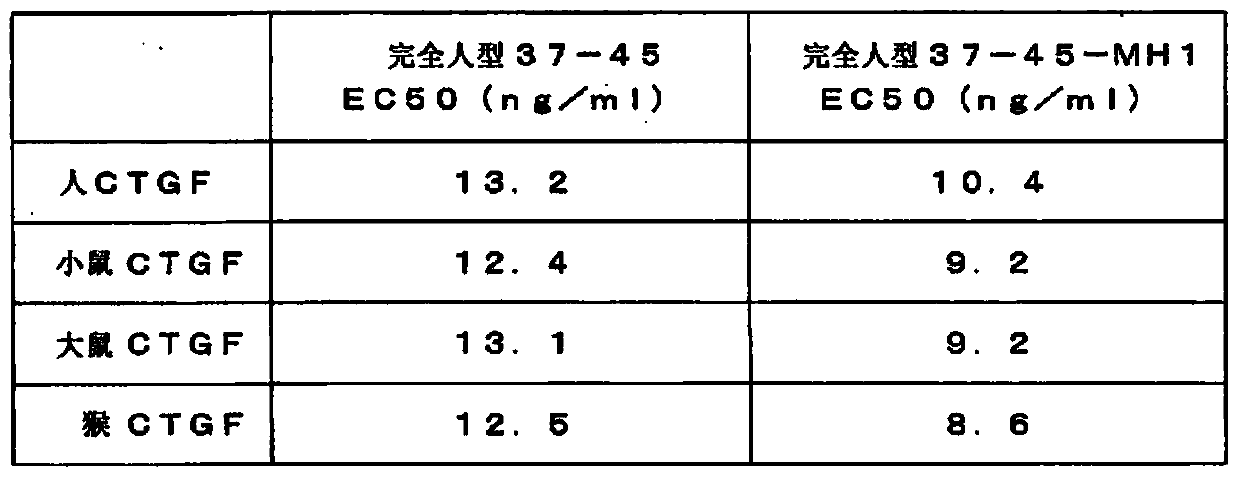
Image
Examples
Embodiment 1
[0092] (Example 1: the acquisition of CTGF protein from various sources)
[0093]The present inventors obtained human CTGF protein as an antigen for making anti-CTGF antibodies. The full-length gene (sequence number 13) of human CTGF is integrated in the expression vector (pcDNA3.1; Invitrogen Company), the vector that will be made uses FreeStyle MAX Reagent (Invitrogen Company) as gene transfer reagent to FreeStyle293 cell (Invitrogen Company) ) for gene transfer. The cells were cultured in a serum-free culture system using FreeStyle293 expression medium (Invitrogen) to obtain a culture supernatant containing human CTGF protein. Proteins were purified from the obtained culture supernatants using HiTrap heparin columns and CM columns (GE Healthcare Japan), and used for the following experiments. For mouse, rat and monkey CTGF proteins, the same method was used to obtain them.
Embodiment 2
[0094] (Example 2: Immunization to VelocImmune mice)
[0095] Anti-human CTGF antibody was obtained by immunizing VelocImmune mice. In order to increase the diversity of the antibodies obtained, the present inventors conducted research on various immunization methods, administration routes, adjuvants, immunization periods, and the like. As an immunogen, purified human CTGF was used and mixed with an adjuvant for immunization. As the route of administration, plantar administration and intraperitoneal administration were investigated. As an adjuvant, TiterMax Gold (CytRx), complete Freund's adjuvant (Sigma), and incomplete Freund's adjuvant (Sigma) were investigated. As further added immune stimulators, CpG oligonucleotides and aluminum phosphate gel (BRENNTAG) were investigated. As the immunization period, 3 weeks to 14 weeks were studied. After several immunizations, blood was collected from the tail vein of the mice, and the titer was monitored to select VelocImmune mice ...
Embodiment 3
[0097] (Example 3: Production of hybridomas producing anti-human CTGF antibodies)
[0098] Final immunization (intravenous or intraperitoneal administration of the antigen) was performed on mice selected after confirming the increase in antibody titer. According to a conventional method, the spleen, lymph nodes, etc. of the immunized mice were removed, lymphocytes were collected, and they were fused with mouse myeloma cells SP2 / 0 to prepare hybridomas. The hybridomas were subjected to limiting dilution, and on the basis of obtaining single clones, antibodies were purified from the supernatant using a protein A or protein G column (GE Healthcare Japan).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com