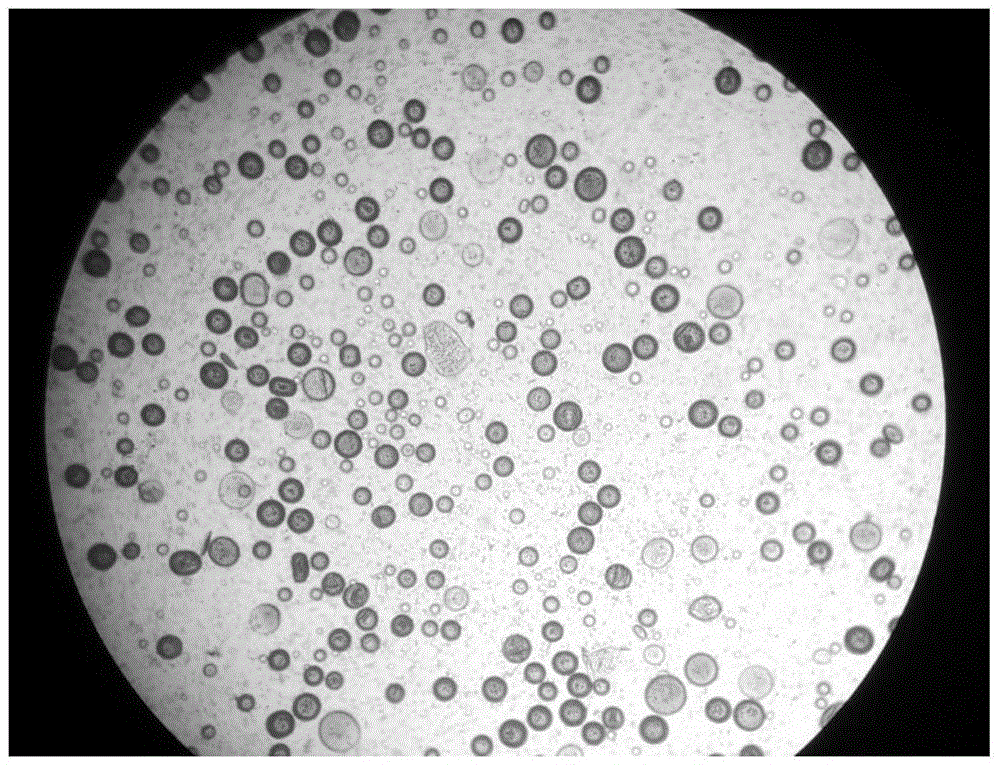
PEG-PLGA sustained release microsphere with encapsulated buprenorphine and preparation method thereof
A technology of PEG-PLGA and slow-release microspheres, applied in the field of pharmaceutical preparations, to achieve the effects of alleviating pain, good biocompatibility, and increasing patient compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1: Preparation of buprenorphine sustained-release microspheres
[0044] (1) Weigh 10 mg of buprenorphine hydrochloride, dissolve it in 0.5 ml of methanol, and add N,N,N',N'-tetramethyl ethylenediamine and stirred overnight. Obtain 20mg / ml (according to buprenorphine hydrochloride) buprenorphine base, standby.
[0045] (2) Weigh 20 mg of polyethylene glycol-polylactic acid-glycolic acid copolymer carrier material and dissolve it in 900 μl of dichloromethane, and dissolve completely. Measure 100 μl of the above buprenorphine base solution and add it into the carrier material solution, so that the final volume of the organic phase is 1 ml. Ultrasonic dissolved and mixed, and then stirred at 800r / min for 4h to mix thoroughly.
[0046] (3) Under stirring conditions, use a needle to draw the organic phase deep into the bottom of the liquid surface and slowly add it to an aqueous solution of polyvinyl alcohol with a mass fraction of 1%, emulsify for 30 minutes at...
Embodiment 2
[0049] Embodiment 2: Preparation of buprenorphine sustained-release microspheres
[0050] (1) Take by weighing 15mg of buprenorphine hydrochloride, be dissolved in 0.5ml tetrahydrofuran, after fully dissolving, add N,N-diisopropylethylamine equivalent to 2 times the molar amount of buprenorphine hydrochloride wherein, stir overnight. Obtain 30mg / ml (according to buprenorphine hydrochloride) buprenorphine base, standby.
[0051] (2) Weigh 20 mg of polyethylene glycol-polylactic acid-glycolic acid copolymer carrier material and dissolve it in 900 microliters of dichloromethane, and dissolve completely. Measure 100 microliters of the above-mentioned buprenorphine base solution and add it into the carrier material solution, so that the final volume of the organic phase is 1 ml. Ultrasonic dissolved and mixed, and then stirred at 800r / min for 4h to mix thoroughly.
[0052] (3) Under stirring conditions, use a needle to draw the organic phase deep into the bottom of the page and ...
Embodiment 3
[0055] Embodiment 3: Preparation of buprenorphine sustained-release microspheres
[0056] (1) Weigh 20 mg of buprenorphine hydrochloride, dissolve it in 0.5ml N,N dimethylformamide or dimethyl sulfoxide, and add 2 times the molar amount of buprenorphine hydrochloride to it after fully dissolving triethylamine, stirred overnight. Obtain 40mg / ml (according to buprenorphine hydrochloride) buprenorphine base, standby.
[0057] (2) Weigh 20 mg of polyethylene glycol-polylactic acid-glycolic acid copolymer carrier material and dissolve it in 900 microliters of dichloromethane, and dissolve completely. Measure 100 microliters of the above-mentioned buprenorphine base solution and add it into the carrier material solution, so that the final volume of the organic phase is 1 ml. Ultrasonic dissolved and mixed, and then stirred at 800r / min for 4h to mix thoroughly.
[0058] (3) Under stirring conditions, use a needle to draw the organic phase deep into the bottom of the page and slowl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com