Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
50results about How to "Avoid non-specific binding" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
NT-ProBNP detection kit and using method thereof
ActiveCN107656071ADetection speedSimplify operation stepsDisease diagnosisBiological testingChemistryEnzyme
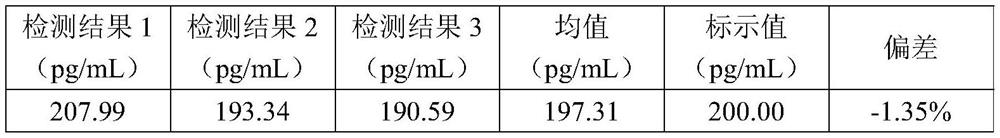
The invention discloses an NT-ProBNP detection kit. The NT-ProBNP detection kit comprises a calibrator, a cleaning solution, a substrate solution, a pretreatment solution, an enzyme conjugate workingsolution and a magnetic bead conjugate working solution; the pretreatment solution contains pyridine, the enzyme conjugate working solution contains NT-ProBNP antibody labeled by enzyme, and the magnetic bead conjugate working solution contains magnetic beads labeled by the NT-ProBNP antibody. The NT-ProBNP detection kit can accurately measure NT-ProBNP in a whole blood sample, the lowest limit detection of the kit is 20 pg / ml, the linearity range is 20-5,000 pg / ml, the detection sensitivity is high, the linearity range is wide, and the result is accurate. The invention further discloses a using method of the NT-ProBNP detection kit. The NT-ProBNP detection kit is simple in using step, the detection time of an NT-ProBNP is shortened, and quick and sensitive detection of the NT-ProBNP is achieved.
Owner:NANTONG EGENS BIOTECH
FISH probe groups for detecting novel coronavirus SARS-CoV-2 and preparation method and application thereof
ActiveCN111254227AImprove the current situation of insufficient detection capabilitiesEarly detectionMicrobiological testing/measurementMicroorganism based processesFluoProbesNucleic acid detection
The invention discloses FISH probe groups for detecting a novel coronavirus SARS-CoV-2. The probe groups comprise at least two fluorescent probe groups among a first fluorescent probe group taking anS gene as a target, a second fluorescent probe group taking an E gene as a target, a third fluorescent probe group taking an M gene as a target, a fourth fluorescent probe group taking an ORF 3a geneas a target, a fifth fluorescent probe group taking an N gene as a target, and a sixth fluorescent probe group taking an ORF 1ab gene as a target. In the at least two fluorescence probe groups, fluorescence molecules labeled by at least one fluorescence probe group have a fluorescence emission spectrum different from that of the other fluorescence probe groups. The FISH probe groups have high specificity and sensitivity, can realize positioning detection of SARS-CoV-2 in a to-be-detected sample, obtain the distribution and relative quantification conditions of the SARS-CoV-2, are an effectivesupplement for novel coronavirus nucleic acid detection, and have important clinical application value.
Owner:SHANGHAI GENEPHARMA CO LTD
Myoglobin detection reagent kit and method for applying same
ActiveCN107907691AAvoid swallowingMeet the needs of rapid diagnosisChemiluminescene/bioluminescenceBiological material analysisMagnetic beadWhole blood sample
The invention belongs to the field of in-vitro diagnostic reagent kits, and particularly relates to a myoglobin detection reagent kit and a method for applying the same. The myoglobin detection reagent kit comprises calibration products, reagents R1, enzyme conjugate working solution R2, magnetic bead conjugate working solution M, cleaning solution and chemiluminescent substrates. The reagents R1contain imidazole components, blood cells in whole blood can be quickly removed by the imidazole components, and accordingly the possibility that magnetic beads are swallowed by the blood cells can beeffectively prevented; the cleaning solution contains sodium lauryl sulfate components, accordingly, cleaning effects can be enhanced, and non-specific binding of the chemiluminescent substrates canbe prevented. The myoglobin detection reagent kit and the method have the advantages that finger peripheral whole blood or anticoagulant venous whole blood can be directly used as a to-be-detected sample for the myoglobin detection reagent kit, the whole blood sample can be directly detected without being pretreated, accordingly, the detection speed can be greatly increased, operation steps can besimplified, the application range of the myoglobin detection reagent kit can be expanded, and large-scale popularization and application can be facilitated.
Owner:NANTONG EGENS BIOTECH
Gene detection product for genetic deafness
ActiveCN107058588AAvoid non-specific bindingHigh detection accuracy and sensitivityMicrobiological testing/measurementGenotypeMass spectrometry
The invention discloses a gene detection product for genetic deafness, and particularly relates to a reagent kit for detecting multiple deafness genes at the same time. The reagent kit uses multiple PCR amplification primers and single base extension primers to simultaneously detect multiple hotspot mutations in the same tube, and has the advantages of quick diagnosis, easy operation and low cost. The invention also discloses a method for combining multiple PCR- single base extension-mass spectrum, and the method can be used to detect the genotype of the deafness gene accurately, sensitively and in high throughput, thereby being helpful for the popularization of deafness gene screening.
Owner:BEIJING CAPITALBIO MEDLAB CO LTD
Photoelectrochemical biosensor as well as preparation method and application thereof
ActiveCN111830104AGood cathode PEC performanceIncrease photocurrentMaterial electrochemical variablesElectrochemical biosensorCancer cell
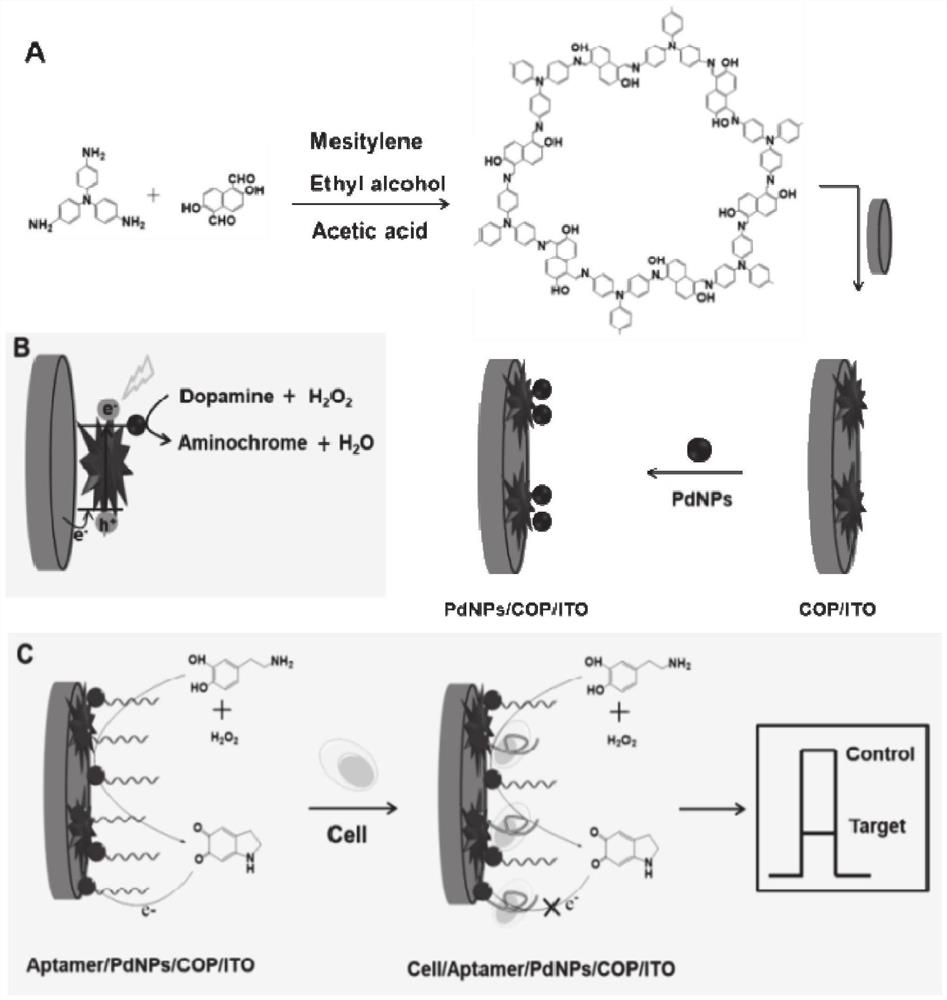
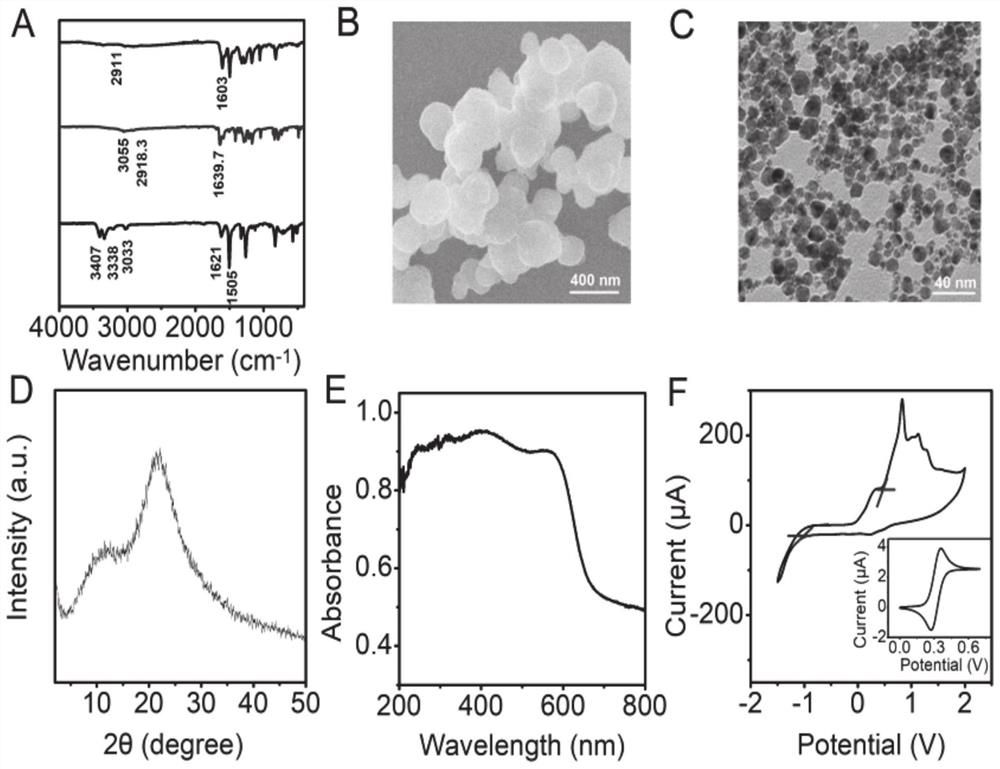
The invention provides a photoelectrochemical biosensor as well as a preparation method and application thereof, and belongs to the technical field of detection and analysis. The photoelectrochemicalbiosensor comprises: an electrode, which comprises an electrode substrate and a covalent organic polymer film coating the electrode substrate, wherein the covalent organic polymer film is modified with palladium nanoparticles and an aptamer; and the covalent organic polymer film is prepared by carrying out a polymerization reaction on 2,6-dihydroxynaphthalene-1,5-diformaldehyde and tris(4-aminophenyl)amine. The cathode photoelectrochemical cell sensor is successfully constructed by in-situ synthesis of the covalent organic polymer film, can be used for detecting various cells such as cancer cells by changing the type of the aptamer, and has high sensitivity and strong specificity, so that the cathode photoelectrochemical cell sensor has a good practical application value.
Owner:SHANDONG NORMAL UNIV
Method for detecting microRNA in lung cancer cells based on non-substrate and non-labeled electrocatalytic amplification biosensor
ActiveCN109722481AImprove electrocatalytic performanceImprove electrocatalytic activityMicrobiological testing/measurementMaterial electrochemical variablesCancer cellFerrous salts
The invention discloses a method for detecting microRNA in lung cancer cells based on a non-substrate and non-labeled electrocatalytic amplification biosensor. The preparation method of the adopted biosensor comprises the following steps of: heating dicyandiamide and ferrous salt to 490-510 DEG C in an inert gas atmosphere to self-assemble to form a precursor, heating the precursor to 890-910 DEGC in the inert gas atmosphere, and performing calcining to obtain iron-containing nitrogen-rich carbon nano-tubes; and modifying the iron-containing nitrogen-rich carbon nano-tubes and gold nano-particles to a glassy carbon electrode to obtain an AuNP / FeCN / GCE electrode, and fixing a thiolated capture probe on the AuNP / FeCN / GCE electrode through a gold-sulfur bond, wherein the capture probe issingle-stranded DNA capable of hybridizing with target microRNA. In the invention, under the condition of no H2O2, a large amount of thiophen introduced by electrocatalytic reduction greatly enhanceselectrochemical signals.
Owner:SHANDONG NORMAL UNIV
Preparation method for polyclonal antibody to salmonella effect protein SopB
InactiveCN103204936AGood for western blot analysisFavorable for immunohistochemical analysisHydrolasesSerum immunoglobulinsStainingMicroscopic observation
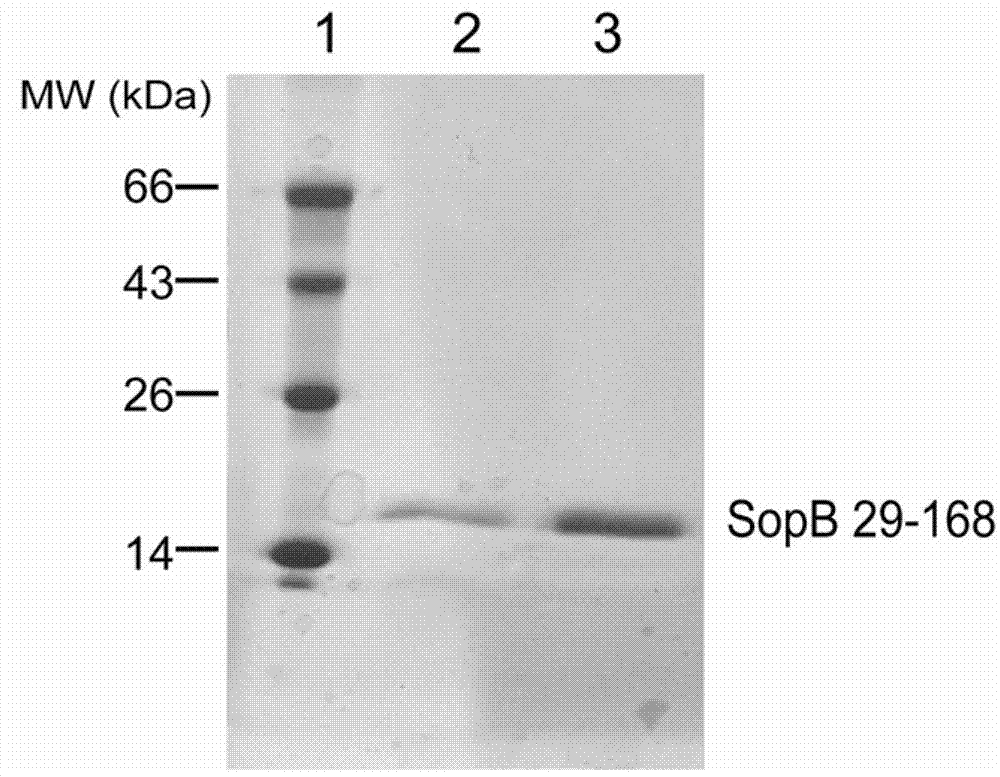
The invention discloses a preparation method for a polyclonal antibody to salmonella effect protein SopB. The invention aims to provide the preparation method for the rabbit-derived polyclonal antibody with good specificity, high sensitivity and capability of specifically binding to the endogenous salmonella effect protein SopB secreted in the process of salmonella infection of a host cell. A protein sequence as represented by SEQ ID No. 2 is used as immunogen, wherein the protein sequence consists of 139 amino acids located between position 29 and position 168, counted from terminal N, of a full-length amino acid sequence as represented by SEQ ID No. 1 of the salmonella effect protein SopB, and a conventional preparation method for a polyclonal antibody is used for preparation of antiserum; then the antiserum is purified so as to obtain the polyclonal antibody to the salmonella effect protein SopB. According to the invention, the polyclonal antibody to the salmonella effect protein SopB is prepared by using the method, and the polyclonal antibody is beneficial for immunoblotting analysis and immunofluorescent staining laser confocal microscopic observation of the salmonella effect protein SopB and for in-depth research on the infection mechanism of enteropathogens.
Owner:TIANJIN UNIV OF COMMERCE
Detection kit for monkey pox virus antigen and preparation method thereof
PendingCN114755418AShorten the windowReduce the risk of invisible transmissionMaterial analysisPox virusIncubation period
The invention relates to a monkey pox virus antigen detection kit and a preparation method thereof, and belongs to the technical field of in vitro diagnostics, the monkey pox virus antigen detection kit comprises a detection card and a sample extracting solution, the detection card comprises an upper cover, a lower cover and a monkey pox virus antigen detection test strip, the monkey pox virus antigen detection test strip comprises a sample pad, a silicon core gold shell combination pad, a solid-phase antibody reaction membrane, absorbent paper and a PVC plate, a sample adding hole and an observation window are formed in the upper cover, and the preparation method of the monkey pox virus antigen detection kit comprises a preparation method of the silicon core gold shell combination pad, a preparation method of the sample pad and a preparation method of the solid-phase antibody reaction membrane. The kit can detect various samples including oropharynx swabs, body fluid, blood and skin focus tissues (including vesicular fluid, pustule fluid and scab), is high in sensitivity, and can effectively shorten the window period of virus diagnosis so as to reduce the hidden transmission risk caused by the incubation period and effectively control the large-scale transmission of epidemic situations.
Owner:山东康华生物医疗科技股份有限公司
Novel 2019 coronavirus antigen detection reagent and preparation method thereof
The invention provides a novel 2019 coronavirus antigen detection reagent and a preparation method thereof. The detection reagent comprises a sample pad, a conjugate pad, an NC membrane and an absorption pad. The product is simple and convenient to operate, short in detection time, high in sensitivity and very suitable for large-scale preliminary screening of primary medical institutions and centralized outbreak areas of epidemic situations.
Owner:北京倍肯恒业科技发展股份有限公司
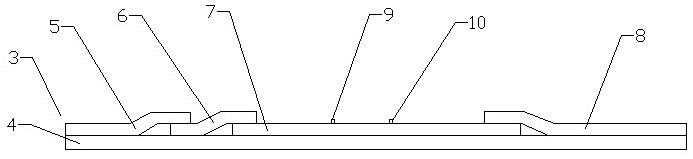
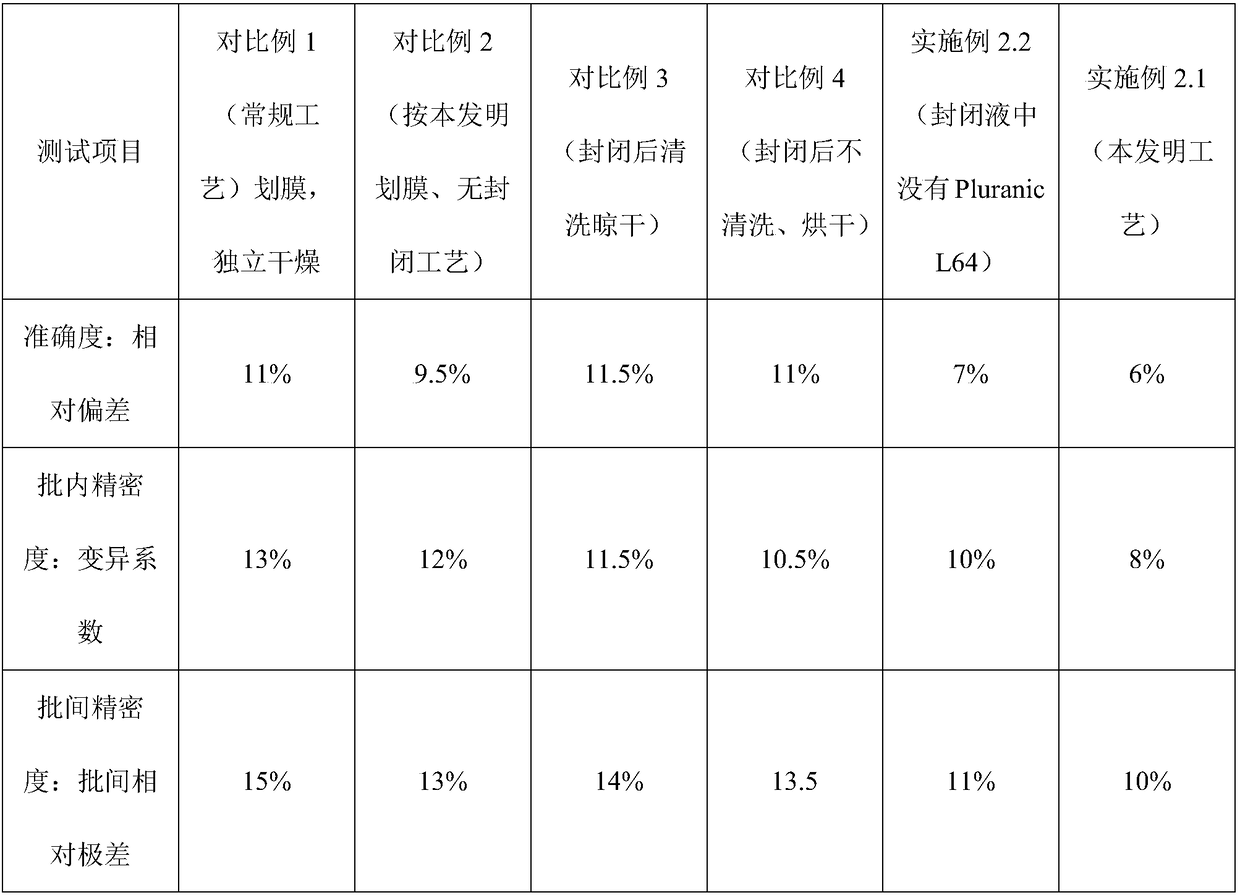
NC (Nitrocellulose Filter) membrane and production technology for preparing NC membrane by lineation
The invention discloses a production technology for preparing an NC (Nitrocellulose Filter) membrane by lineation. The production technology comprises the following working procedures: operating membrane lineation in a continuous state, and drying after membrane lineation; after the working procedure of drying after membrane lineation is carried out, sealing a membrane which operates under a continuous state, and drying after sealing, wherein the operation time of the working procedure of drying after membrane lineation is 2-4 min, and the operation time of membrane sealing and working procedure of drying after sealing is 3-6 min. The NC membrane obtained by the technical scheme of the invention can be used for effectively avoiding the diffusion and the widening of an antibody line, the detection fluorescence of the antibody line is centralized, meanwhile, the specificity combination of the antibody line is improved so as to obviously lower the intra-batch differences and the inter-batch differences of production, and the accuracy and the repeatability of a detection result are improved.
Owner:HEBEI TEWENTE BIOTECH DEV CO LTD
Antibody diluent for enhancing immune imprinting detection signal
InactiveCN110297085AImprove bindingHigh strengthMaterial analysisSignal-to-noise ratio (imaging)Polyvinyl alcohol
The invention relates to an antibody diluent for enhancing an immune imprinting detection signal. Each 1L of antibody diluent comprises the following components in percentage by weight: 0.25-2.5g of polyvinyl alcohol, 0.25-2.5g of polyvinylpyrrolidone, 0.1-2g of surfactant, 0.015-0.75mL of Kathon preservative and the balance of 0.01-0.1moL / L TBS buffer solution, wherein the pH of the TBS buffer solution is 7.2-7.6. The antibody diluent for enhancing the immune imprinting detection signal can be used for antibody dilution of immune imprinting detection, so that a relatively high background generated by pollution caused by protein is avoided; a relatively strong signal-to-noise ratio is displayed; and meanwhile, the strength and the sensitivity of the immune imprinting signal are enhanced.
Owner:武汉博士德生物工程有限公司
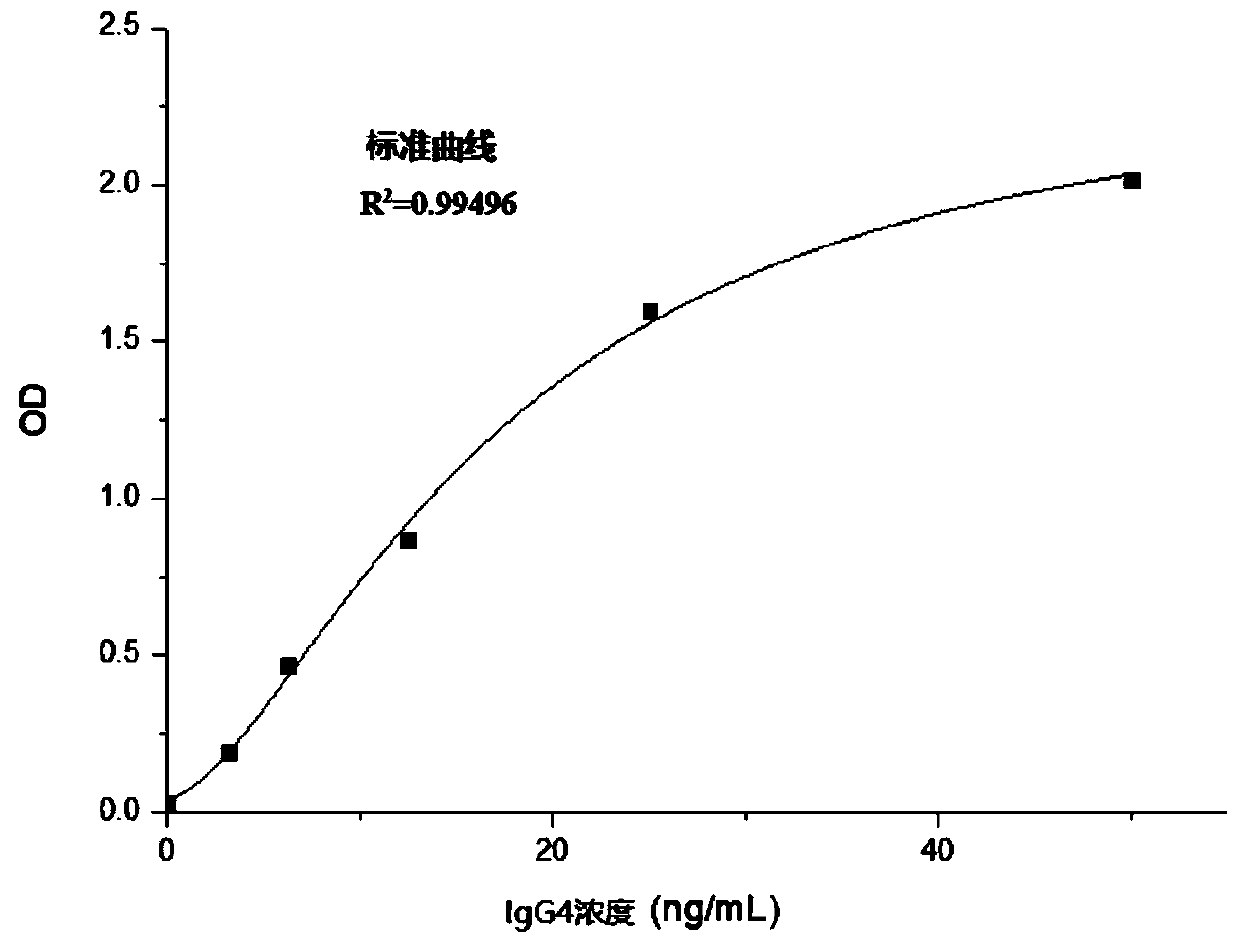
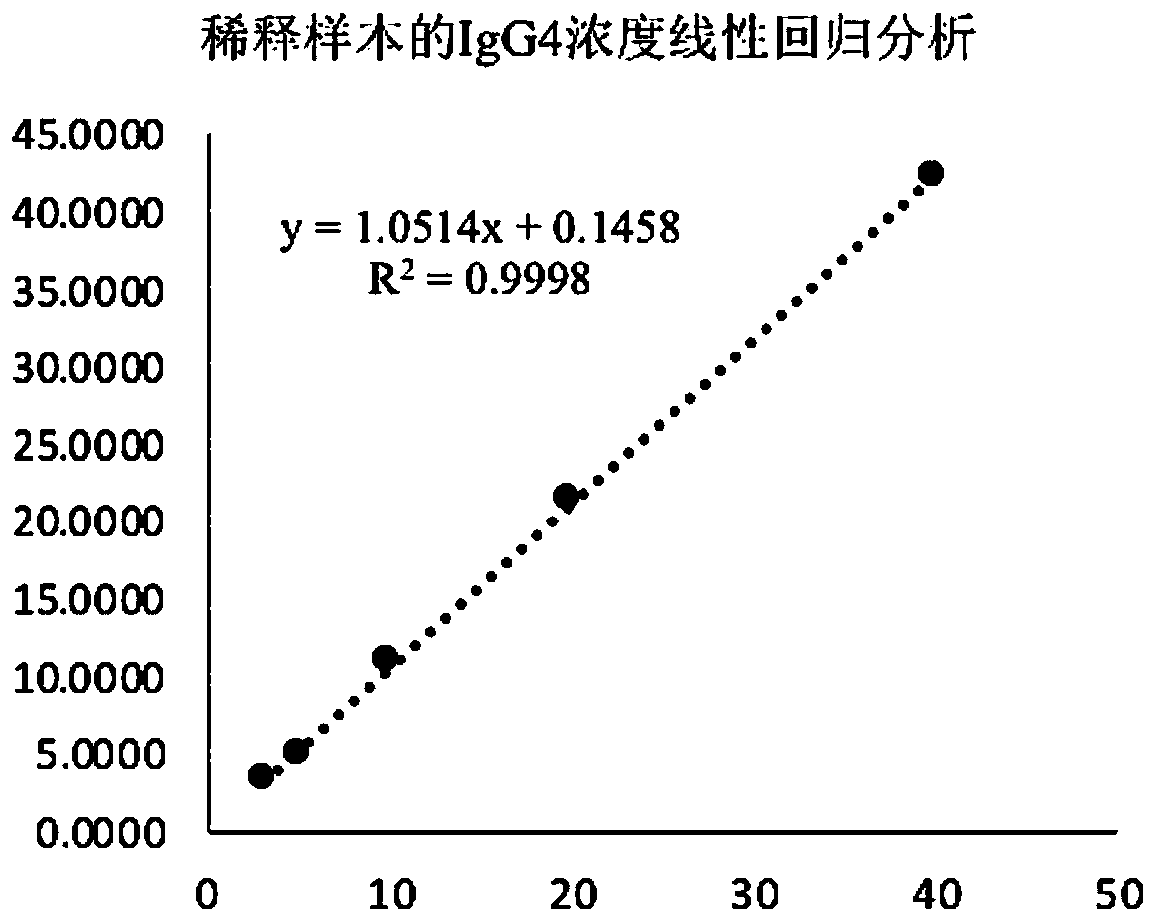
Method for detecting human immunoglobulin G4 and kit
ActiveCN110297093AAvoid non-specific bindingAccurate quantification of concentrationBiological testingAbzymePeroxidase
The invention relates to a method for detecting human immunoglobulin G4 and a kit, and belongs to the technical field of medical inspection. According to the method for detecting the human immunoglobulin G4 (IgG4), two monoclonal antibodies with high-specificity anti-IgG4Fc fragments are used, wherein the first monoclonal antibody specific to IgG4 is combined with a solid-phase carrier while the second monoclonal antibody specific to IgG4 is marked by a peroxidase. By virtue of a bridging effect of IgG4 in a to-be-tested sample, a compound of first IgG4 monoclonal antibody-IgG4 enzyme marked second IgG4 monoclonal antibody is formed, and the compound is fixed on the solid-phase carrier. According to the method, the non-specific interfering substance components in the sample are removed byadopting a high-efficiency cleaning fluid. According to the method for detecting the content of the biological sample IgG4, the high stability and the high specificity of the quantitative detection ofthe IgG4 in the biological sample are realized, and accurately quantifying of the concentration of the to-be-detected object IgG4 in a human biological sample is realized.
Owner:山西瑞豪生物科技有限公司
Kit for identifying circulating tumor cells by combining TCPP with CEP probe and application
InactiveCN110927369AImprove accuracyLow costPreparing sample for investigationBiological testingAntigenTissue fixing
The invention belongs to the field of oncology and medical examination, and relates to a kit for identifying circulating tumor cells by combining TCPP with a CEP probe and application. The kit comprises a TCPP stock solution, a subacid buffer solution, a cell cleaning solution, a cell fixing solution, a tissue fixing solution, a CEP probe solution, a cell membrane rupturing solution, a DAPI staining solution and PBS. The kit is applied to non-disease diagnosis and treatment purposes, and the method for identifying the circulating tumor cells by the kit comprises a solid-phase staining method or a liquid-phase staining method. The method does not depend on antigen expression on the surfaces of tumor cells as a detection means. False negative caused by antigen loss of tumor cells in the EMTprocess due to some existing antigen-dependent detection means is avoided, various epithelial tumors can be identified, and the defect that no unified tumor marker is provided for identifying circulating tumor cells due to heterogeneity of different types of tumor cells is avoided.
Owner:安徽安龙基因科技有限公司
Diagnostic methods
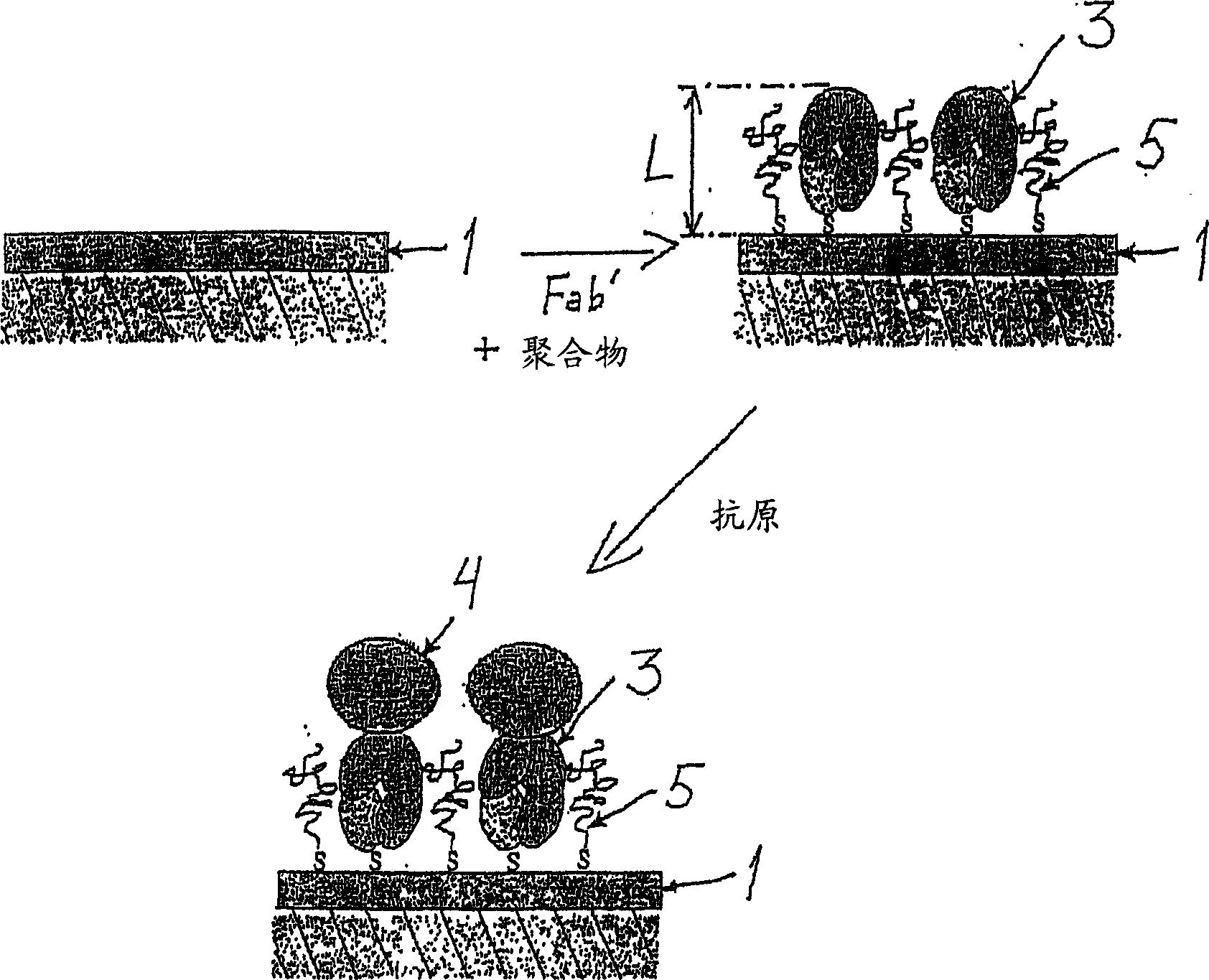
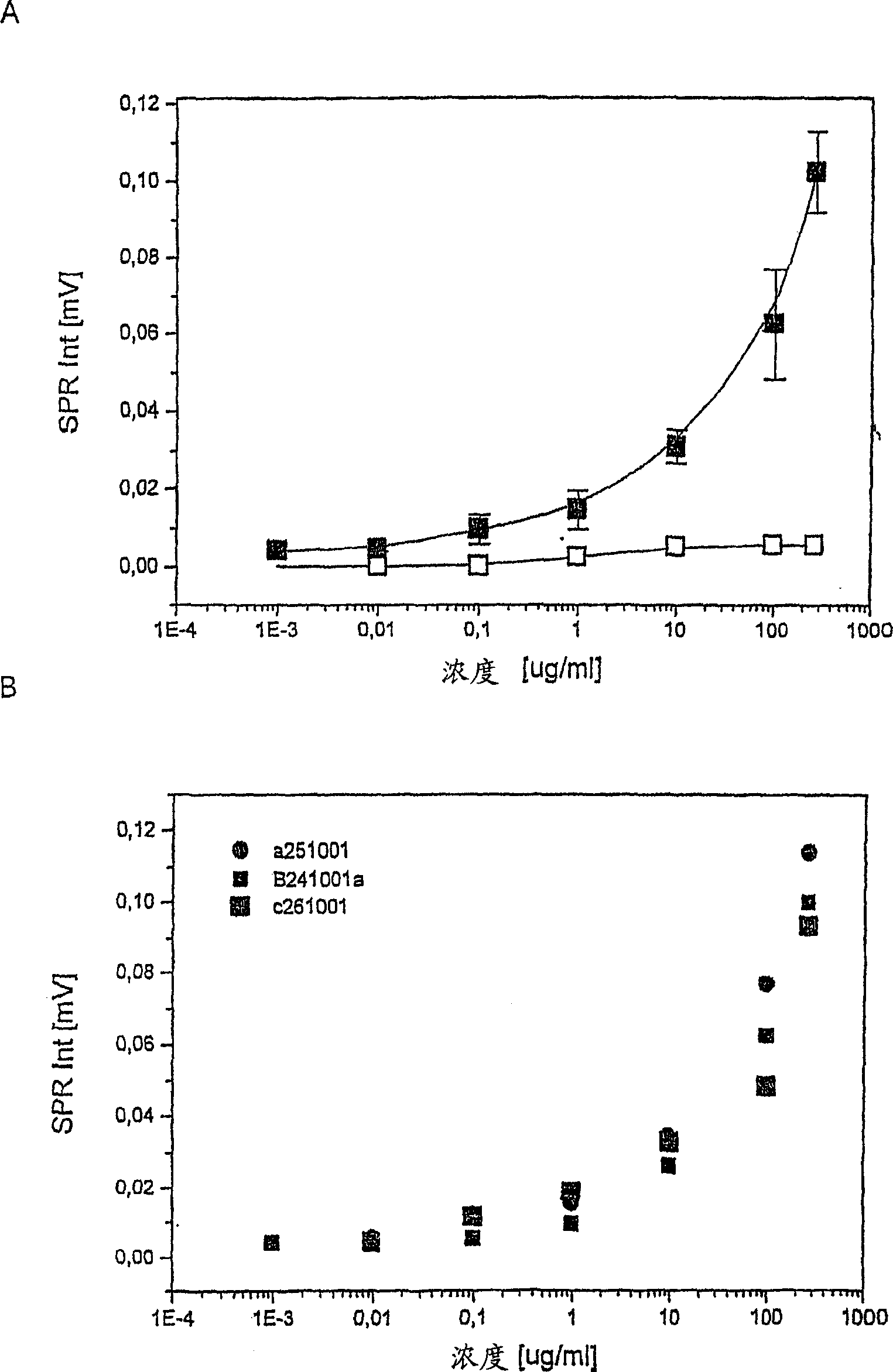
InactiveCN1623092AAvoid non-specific bindingEnzymologyDepsipeptidesMetaboliteAntigen Binding Fragment
The present invention relates to a new method for the diagnosis of Helicobacter pylori infection. In particular, the present invention relates to novel non-invasive methods for detecting the presence in biological samples of H. pylori antigens or metabolites produced by said bacteria using biosensor-based measurements. The present invention also relates to the use of a biosensor comprising a Helicobacter pylori-specific antibody or an antigen-binding fragment thereof immobilized thereon and a biomolecule-repelling polymer that prevents non-specific binding. The invention also relates to detection kits used in the method.
Owner:比恩诺尔股份公司
Preparation method for polyclonal antibody to salmonella effect protein SopB
InactiveCN103204936BGood for western blot analysisFavorable for immunohistochemical analysisHydrolasesSerum immunoglobulinsStainingMicroscopic observation
The invention discloses a preparation method for a polyclonal antibody to salmonella effect protein SopB. The invention aims to provide the preparation method for the rabbit-derived polyclonal antibody with good specificity, high sensitivity and capability of specifically binding to the endogenous salmonella effect protein SopB secreted in the process of salmonella infection of a host cell. A protein sequence as represented by SEQ ID No. 2 is used as immunogen, wherein the protein sequence consists of 139 amino acids located between position 29 and position 168, counted from terminal N, of a full-length amino acid sequence as represented by SEQ ID No. 1 of the salmonella effect protein SopB, and a conventional preparation method for a polyclonal antibody is used for preparation of antiserum; then the antiserum is purified so as to obtain the polyclonal antibody to the salmonella effect protein SopB. According to the invention, the polyclonal antibody to the salmonella effect protein SopB is prepared by using the method, and the polyclonal antibody is beneficial for immunoblotting analysis and immunofluorescent staining laser confocal microscopic observation of the salmonella effect protein SopB and for in-depth research on the infection mechanism of enteropathogens.
Owner:TIANJIN UNIV OF COMMERCE
Antibody screening reagent for enhancing immunosuppression turbidimetry and preparation and use method thereof
PendingCN110133270AAvoid non-specific bindingReduce agglutinationColor/spectral properties measurementsBiological testingTurbidimetryTurbidity
The invention discloses an antibody screening reagent for enhancing immunosuppression turbidimetry and a preparation and use method thereof. The method for enhancing immunosuppression turbidimetry overcomes the defects of the method for enhancing immune turbidimetry in the prior art in the principle of immune reaction. When no antigen exists in a measurement reaction system, the antigen connectedto latex particles generates agglutination reaction with the limited amount of antibody in the system, and the agglutination effect is maximized. When an antigen exists in the measurement reaction system, since the amount of antibody is limited, free antigen is excessive antigen which can inhibit the agglutination effect of the antigen-antibody complex, therefore, as the amount of free antigen inthe reaction system increases, the agglutination effect of the antigen-antibody complex is decreased. When the amount of antigen in the measurement system reaches a certain amount, the free antigen will completely inhibit the binding of the antibody and the antigen on the latex, and if the nonspecific binding of excess antigen and other substances in the latex and sample can be overcome, the turbidity change during the reaction will be zero.
Owner:长沙文瀚生物技术有限责任公司
Rapid noninvasive female corpus luteum function monitoring technology
ActiveCN106146651AControl the number of connectionsHigh sensitivityOvalbuminSerum albuminMetaboliteProgesterones
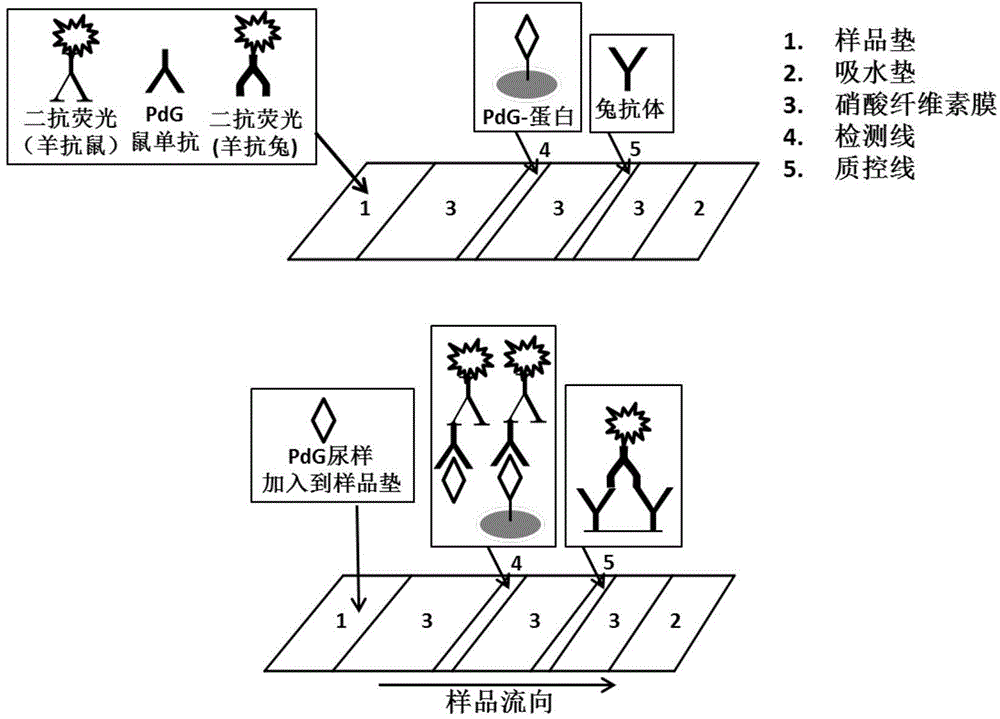
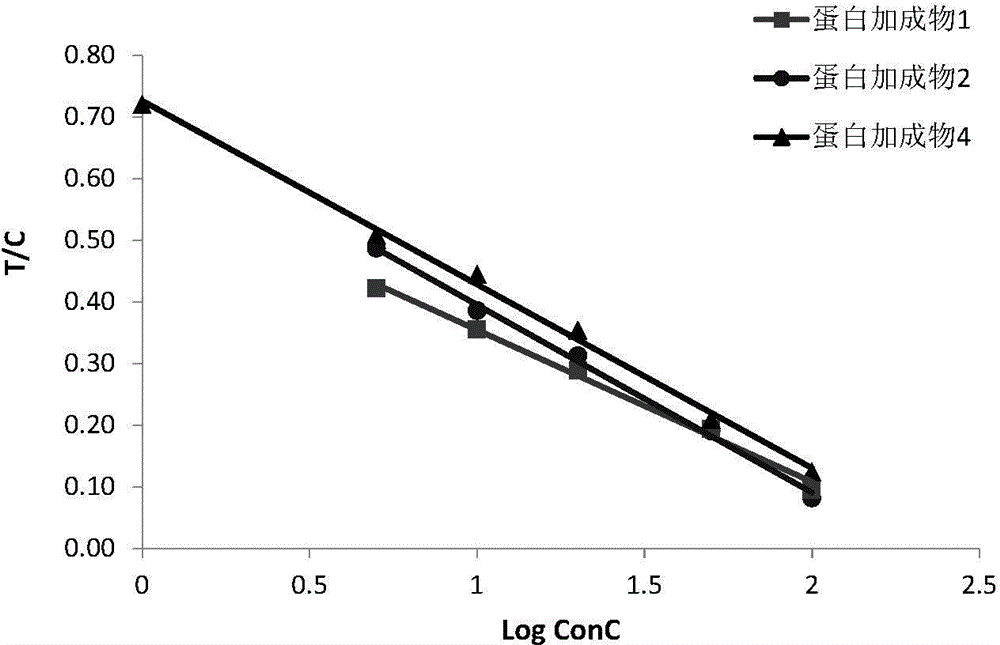
The invention relates to a noninvasive female corpus luteum function monitoring technology, in particular to a full-quantitative or semi-quantitative rapid noninvasive urine test technology for determination of cyclic production amount of progesterone metabolites in women's urine so as to monitor and evaluate a female corpus luteum function, judge different types of ovulation cycle, determine whether ovulation is really performed or not and impregnation can be achieved or not after ovulation. According to the first implementation scheme, a protein hybridized couplet for determining pregnanediol glucuronide is related and contains pregnanediol glucuronide and a coupling protein. The protein hybridized couplet is characterized by further containing a junctional complex that connects the two parts. PdG of the hormone protein couplet is detected without an unsaturated labelled antibody, the sensitivity can be up to 0.1 ng / ml, and the linear range can be controlled to be 0.1-50 ng / ml.
Owner:NANJING JILANG BIOTECH CO LTD
Novel coronavirus detection kit and detection method thereof
PendingCN112048574AAvoid Card Issue StructuresAvoid non-specific bindingMicrobiological testing/measurementAgainst vector-borne diseasesViral testNasopharyngeal aspirate
The invention provides a novel coronavirus detection kit and a detection method thereof, and belongs to the technical field of molecular biological detection. A series of primer probe groups for detecting the novel coronavirus are redesigned, an existing detection method is improved, and detection targets are further increased, so that the detection sensitivity of the novel coronavirus is improved, false negative results can be remarkably reduced especially for detection of low-copy-number samples, the sensitivity and the accuracy of detection are effectively improved, and high-efficiency, high-specificity and low-cost detection is realized. The kit can be used for qualitatively detecting the novel coronavirus genes in pneumonia suspected cases and suspected aggregated case patients infected by the novel coronavirus in vitro and samples such as nasopharyngeal swabs and sputum of other patients needing novel coronavirus infection diagnosis or differential diagnosis.
Owner:北京吉检医疗科技有限公司
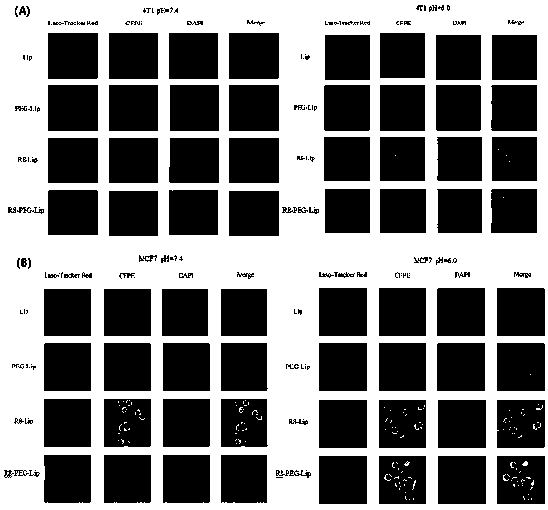
Biotin and cell-penetrating peptide co-mediated breast cancer targeted intelligent liposome material
InactiveCN111166892AAchieving Targeted TherapyHigh membrane penetration efficiencyOrganic active ingredientsEmulsion deliveryPhospholipidBiotin
The invention discloses a biotin and cell-penetrating peptide co-mediated breast cancer targeted intelligent liposome material. The intelligent liposome material is prepared by the following steps: 1,modifying biotin on a PEG long chain, and connecting the biotin with cholest through an acid-sensitive bond (a general formula I, a ligand material a); and 2, connecting cell-penetrating peptide R8 with phospholipid through Michael addition reaction (a formula II and a ligand material b). After the two ligand materials are combined to prepare a liposome, the biotin exposed on the surface of the liposome can specifically recognize an overexpressed SMVT transporter on the surface of a breast cancer cell; and after the liposome reaches the breast tumor part, a PEG long chain connected with a hydrazone bond is broken and separated, and the R8 connected with a short chain is mediated, passes through membrane and enters the cell, so that the effect of strongly treating the breast cancer is realized. The novel intelligent liposome material can be used for different dosage forms including liposome, nanoparticles, micelles and the like, and a prepared paclitaxel-loaded liposome has strong breast cancer penetrability and treatment effect and has a wide application prospect.
Owner:SICHUAN UNIV
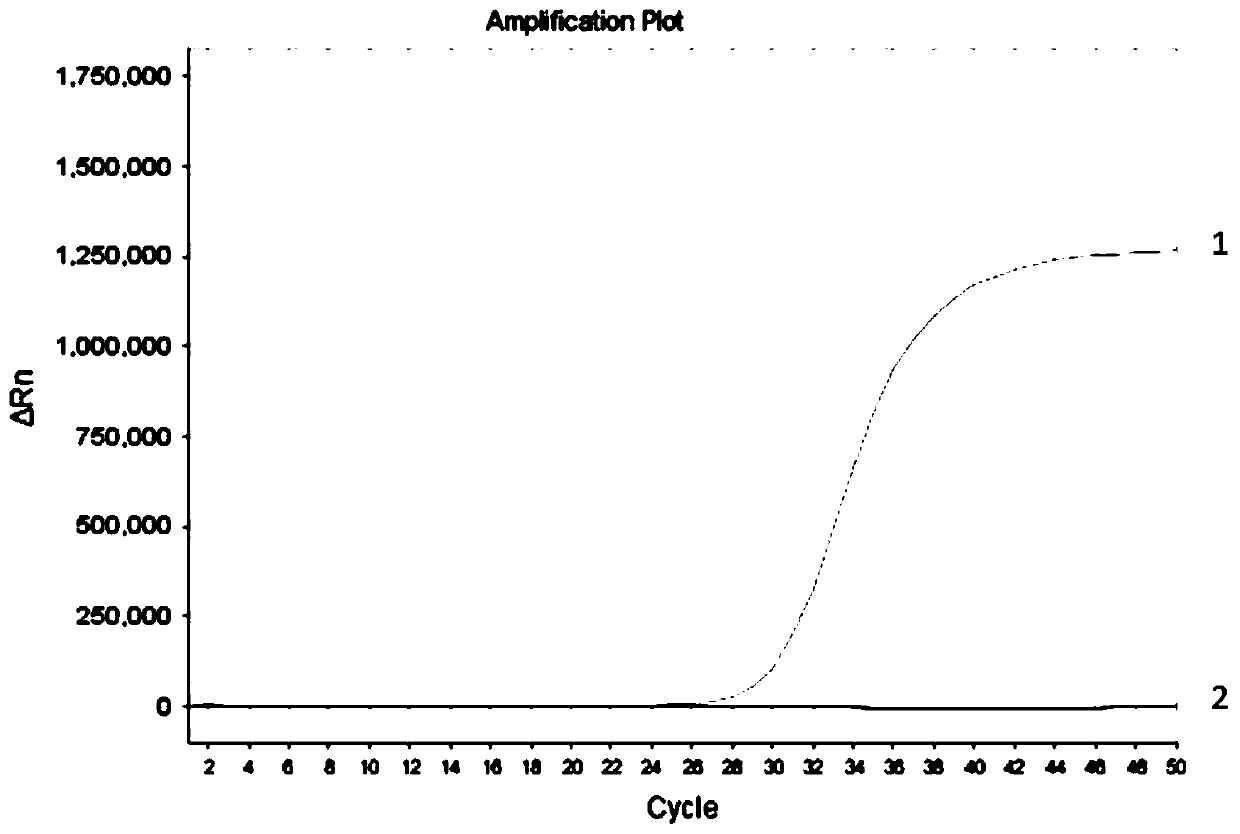
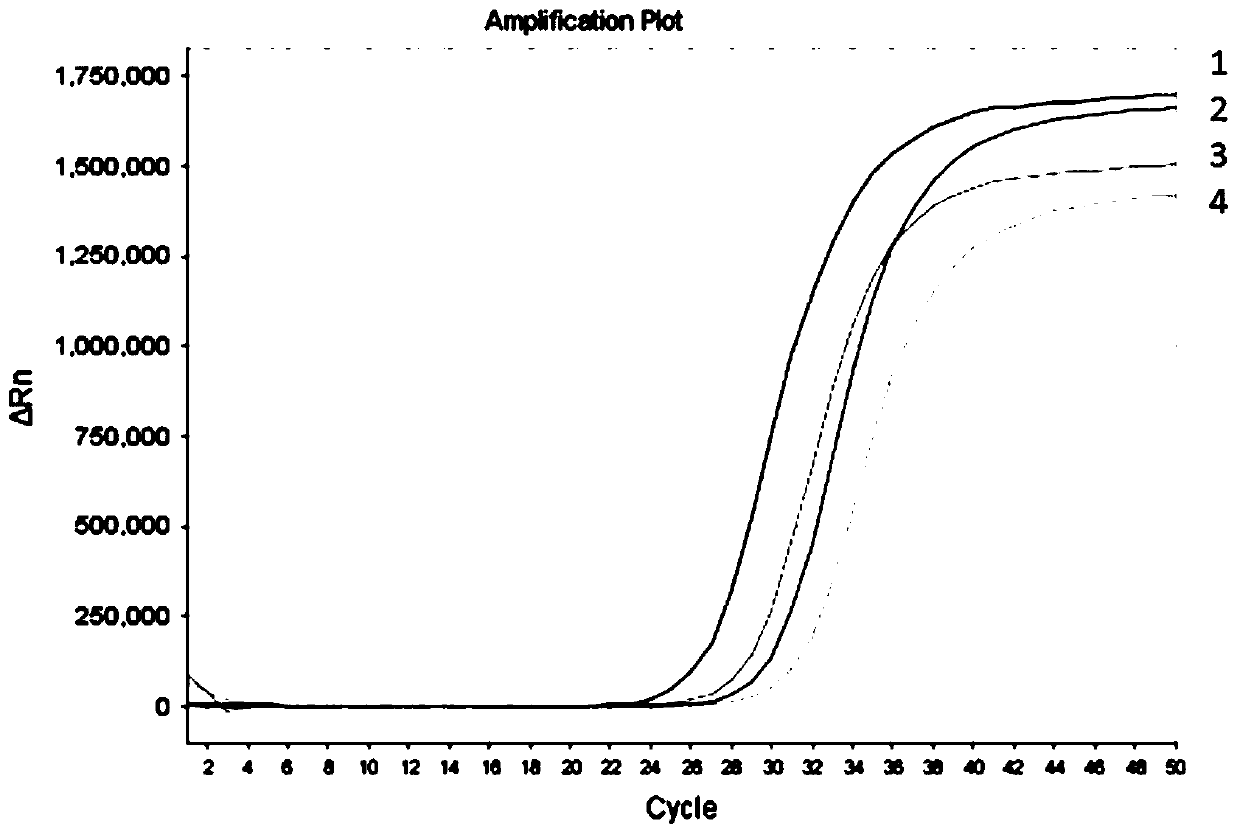
Methylation detection kit for nasopharyngeal carcinoma related genes DACT1, NFAT1 and SHISA3
ActiveCN110643709AEliminate pollutionAvoid False Positive ResultsMicrobiological testing/measurementFluorescent pcrEnzyme
The present invention provides a methylation detection kit for nasopharyngeal carcinoma related genes DACT1, NFAT1 and SHISA3. The kit comprises an alkaline solution and a fluorescent PCR reaction solution, and the alkaline solution and the fluorescent PCR reaction solution are packaged in a same PCR amplification tube by a hot-melt material in a layering manner; the alkaline solution is a NaOH solution with pH value of 12.5-13.3 and concentration of 50-100 mmol / L, and volume of the alkaline solution is less than or equal to 50% of the volume of the PCR reaction solution; and the fluorescent PCR reaction solution comprises primers and probes aiming at the DACT1, NFAT1 and SHISA3 genes and UDG enzymes. A systematic and step-by-step heat treatment is conducted before the fluorescent PCR reaction, the UDG enzymes are effectively applied, and the kit effectively solves a problem of PCR pollution in methylation detection, and also has higher detection sensitivity, specificity and detectionflux.
Owner:SUREXAM BIO TECH
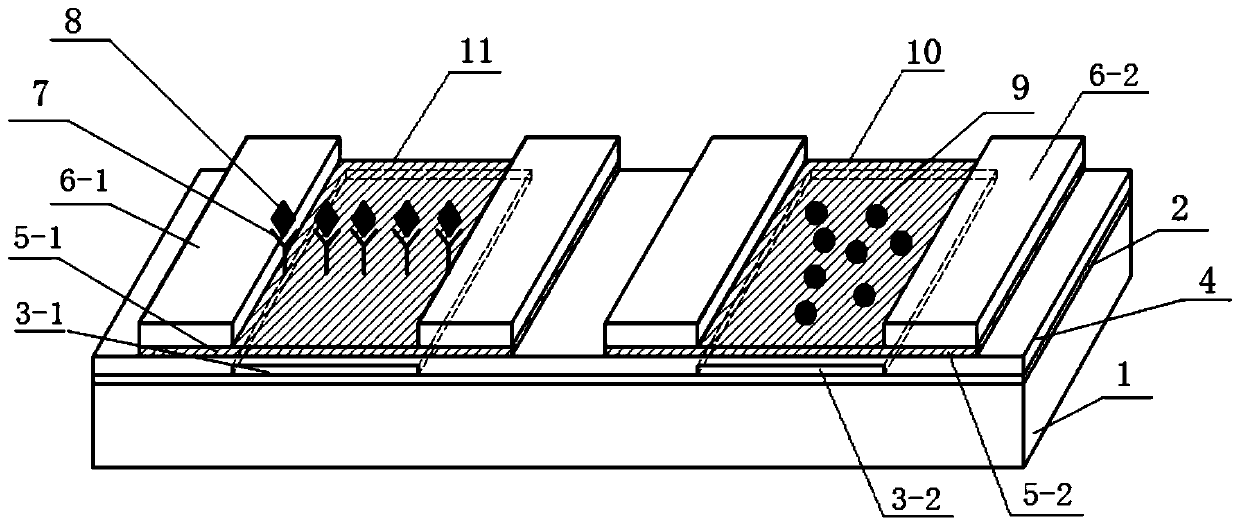
A graphene field effect transistor array biosensor and its preparation method and detection method
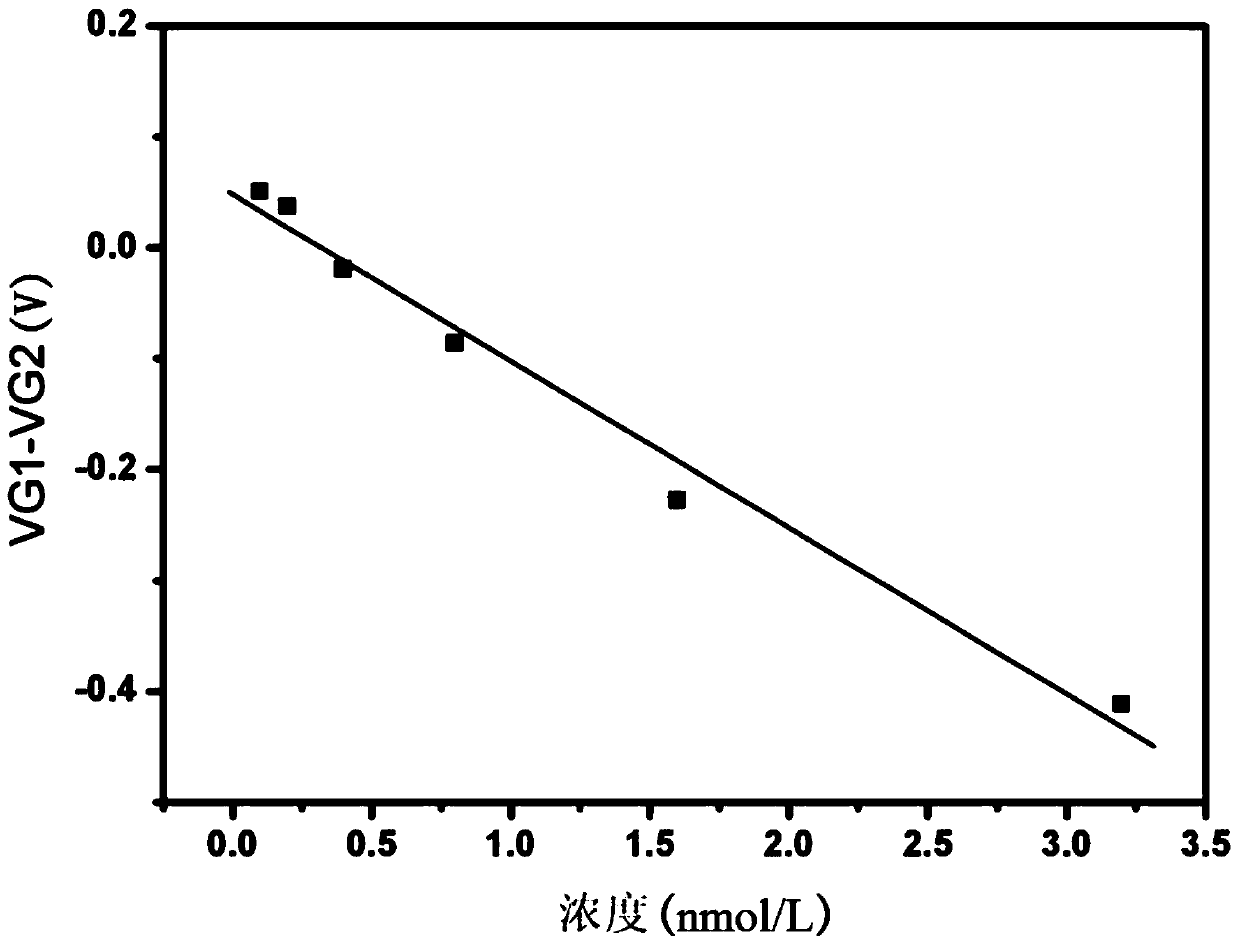
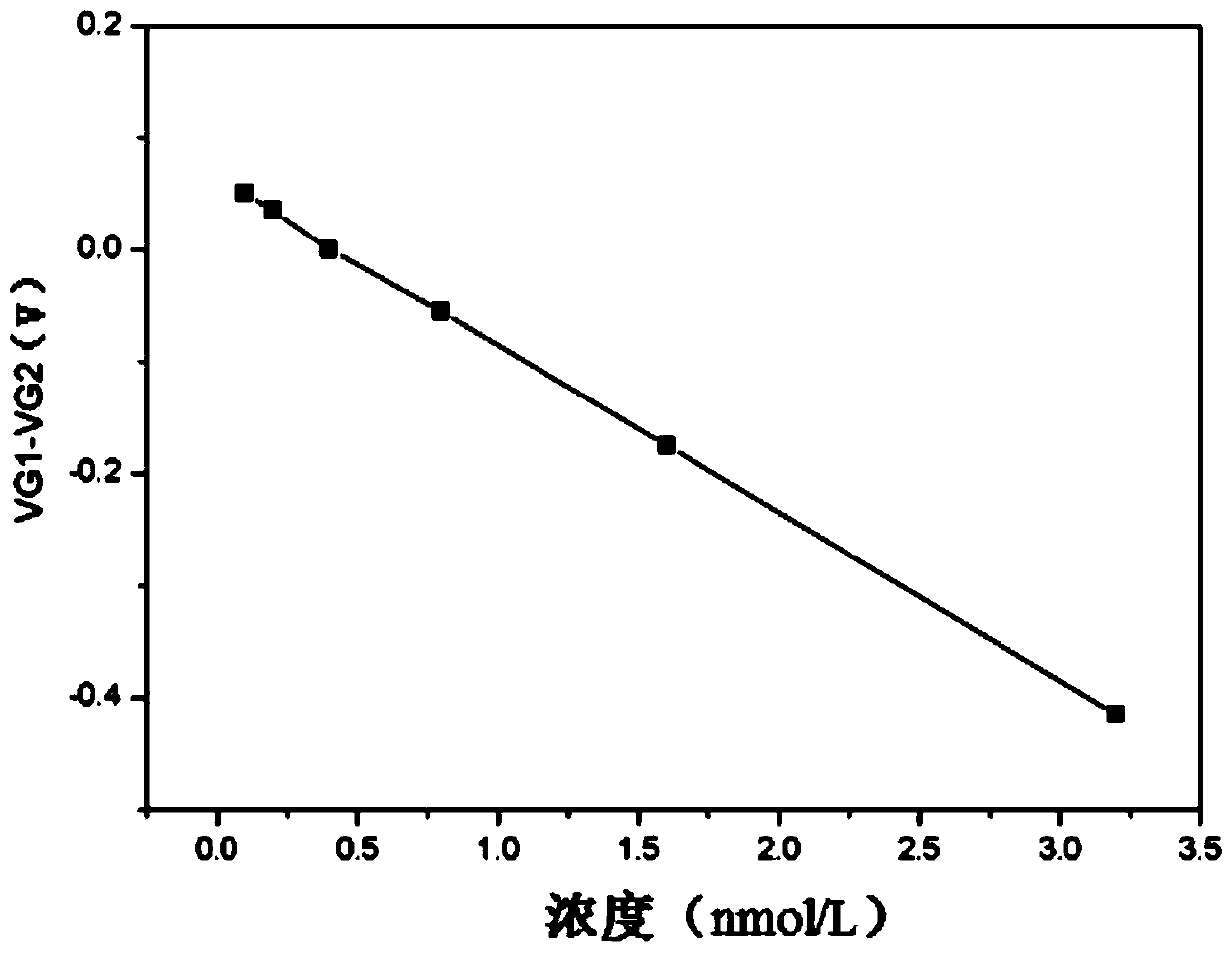
ActiveCN108956742BAvoid non-specific bindingMaterial analysis by electric/magnetic meansAntigenField effect
The invention relates to a graphene field effect transistor array biosensor and a preparation method and a detection method thereof. The invention relates to a biosensor and a preparation method and adetection method thereof, aiming at solving the problem of relatively low accuracy in the detection process of the existing graphene field effect transistor array biosensor. The sensor comprises a silicon substrate, an oxidization layer, a first metal grid electrode, a second metal grid electrode, a gate insulating layer, a first graphene conductive channel layer, a second graphene conductive channel layer, a first group source, a leakage electrode, a second group source and a leakage electrode; the preparation method comprises the following steps: I. making a transistor structure; II. performing aldehyde treatment on the graphene surface; III. modifying a detection unit graphene surface to capture antibody molecules; and IV. closing the remaining active sites on the graphene surfaces ofa detection unit and a reference unit; the detection method comprises the following steps: soaking the sensor in a solution containing be-detected antigen, and detecting the voltage at the graphene dirac point of the detection unit and the reference unit, to obtain the absolute concentration of the to-be-detected substance solution, wherein a difference value corresponds to a standard working curve.
Owner:HARBIN ENG UNIV +1
Human SDC2 gene methylation detection kit
ActiveCN110699437AEasy to operateImprove automationMicrobiological testing/measurementAgainst vector-borne diseasesEnzyme digestionFluorescent pcr
The invention provides a human SDC2 gene methylation detection kit, which comprises an enzyme digestion reaction solution and a fluorescent PCR (polymerase chain reaction) solution, wherein the enzymedigestion reaction solution and the fluorescent PCR reaction solution are packaged in a same PCR amplification tube in layered form through a hot melting material, and the enzyme digestion reaction solution occurs at the bottom of the PCR amplification tube. According to the kit disclosed by the invention, methylation sensitive restriction endonuclease and marking twin primers are utilized to carry out enzyme digestion reaction and fluorescent PCR sequentially in one tube so as to detect the methylation state of the SDC2 gene; the kit has the advantages of high operation speed, good operationsimplicity, high sensitivity, good specificity and good convenience of automation.
Owner:SUREXAM BIO TECH
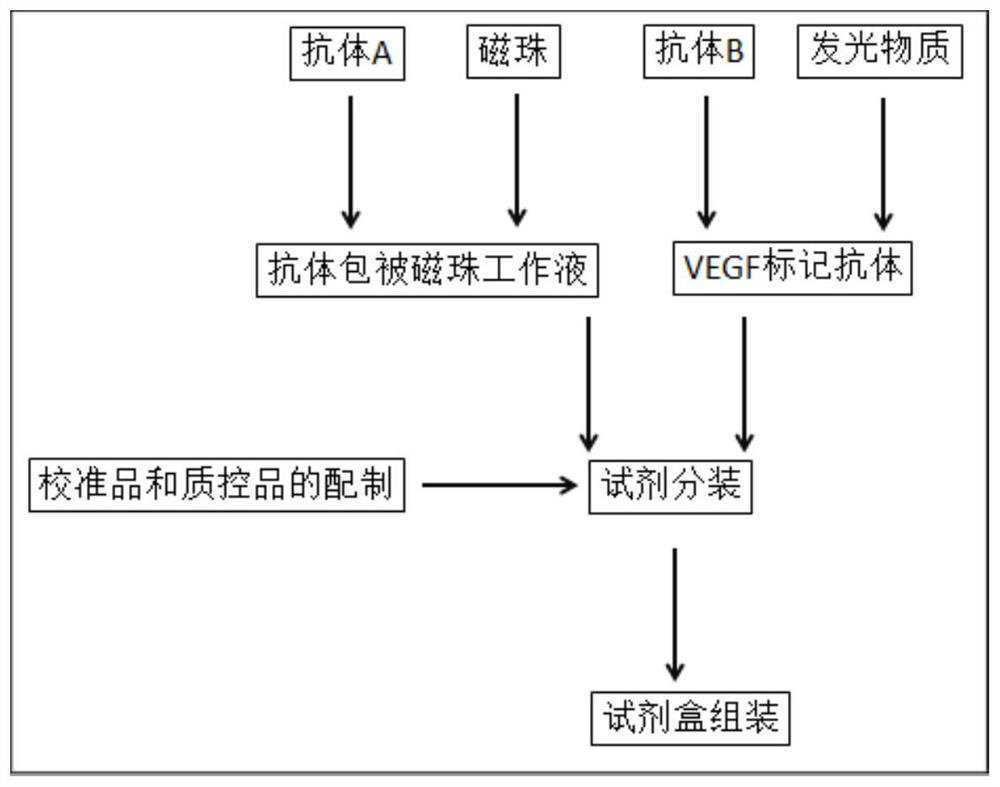
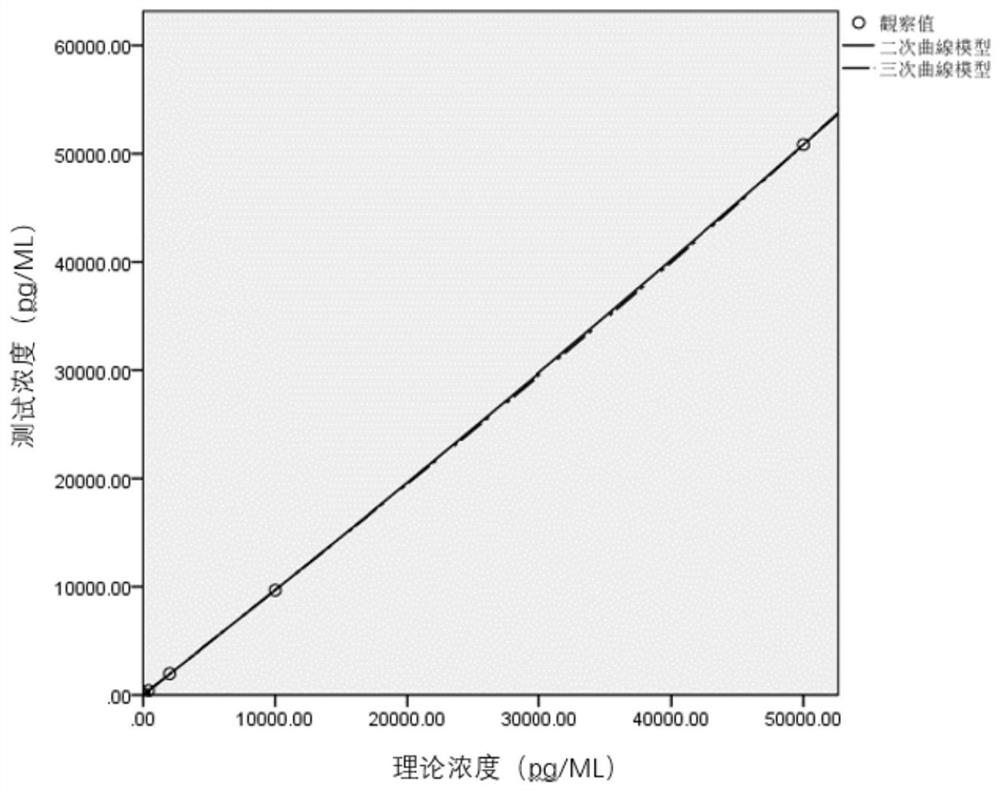
Raw material preparation method and detection method of vascular endothelial growth factor detection kit
InactiveCN113702642AGood water solubilityImprove stabilityChemiluminescene/bioluminescenceBiological material analysisMagnetic beadMicrosphere
The present invention relates to the technical field of vascular endothelial growth factor detection, and discloses a raw material preparation method and a detection method of a vascular endothelial growth factor detection kit. Theraw material preparation method comprises raw material preparation, the raw material preparation comprises an antibody coated magnetic bead working solution, a VEGF labeled antibody, a VEGF calibration product, a VEGF quality control product and a sample diluent; the antibody coated magnetic bead working solution is a magnetic microsphere suspension coated with a Fab segment of a humanized VEGF antibody, and the buffer system is TBS-T (20-200 mM Tris, 0-1 M NaCl, 0.0%-1% BSA, 0.1%-0.5% Tween-20 and 0.1%-1% Proclin-300, and the pH value is 6.5-9.0). According to the present invention, the selected luminous marker is acridine salt NSP-SA-NHS; therefore, luminescence detection is carried out in homogeneous liquid, and the reaction time is shortened. Meanwhile, the bond level of C-N is larger than that of C-O, and the stability of the conjugate of the acridine salt NSP-SA-NHS and the protein is higher. Based on the use of NSP-SA-NHS, the sensitivity, the detection range and the storage time of the detection system are improved, and the reaction time is shortened.
Owner:北京健平金星医疗器械有限公司
A kind of rapid detection test paper and its preparation method and application
ActiveCN110824177BSmooth releaseInhibit refluxBiological material analysisBiological testingAnalyteAntibody conjugate
The invention provides a rapid detection test paper and its preparation method and application. The test paper includes a chromatographic membrane and a binding pad, one end of the chromatographic membrane is connected to the binding pad; the binding pad is pre-sprayed with microsphere marks of different colors Different analytes correspond to antigen / antibody conjugates, and the chromatographic membrane is provided with a detection line and a quality control line in sequence along the direction of liquid chromatography, and the detection line is the antigen / antibody corresponding to different analytes fixed at a certain interval. . The invention also provides the application of the detection test paper in the detection of drugs and early pregnancy.
Owner:RUNBIO BIOTECH CO LTD
A method using liquid chromatography bilayer stationary phase
ActiveCN104655769BAvoid erosionAvoid interferenceComponent separationPolymerLiquid-liquid chromatography
The invention relates to a method using liquid chromatography to prolong the service life of a separated biological substance and to reduce and lower the preparation time and requirements for a separated biological substance sample. According to the method, a series of chemical modifications are performed on the surface of a stationary phase, a bimolecular layer stabilized by a polymer is added to the surface of the stationary phase, and the bimolecular layer cannot generate nonspecific binding with biomass such as a protein, so that the risk that the stationary phase is polluted and absorbed by a complicated macromolecule protein is reduced greatly.
Owner:SHANDONG MEASUREMENT SCI RES INST
Kit and application thereof in detection of vascular endothelial growth factor
ActiveCN114019174AHigh sensitivityExtended storage timeChemiluminescene/bioluminescenceBiological material analysisSulfohydrazideAntiendomysial antibodies
The invention discloses a kit and application thereof in detection of a vascular endothelial growth factor. The kit contains the following components which are independently stored: an antibody-coated magnetic bead working solution, a VEGF labeled antibody, a VEGF calibrator, a VEGF quality control product and a sample diluent. The antibody-coated magnetic bead working solution is a magnetic bead suspension coated with an antibody A. The VEGF labeled antibody is obtained by labeling an antibody B through an acridinium ester modifier. The acridinium ester modifier is acridinium sulfonyl hydrazide amine inner salt or other acridinium ester derivatives, and the antibody A and the antibody B are humanized VEGF antibody Fab segments with different amino acid sequences. The acridinium ester modifier is selected to be coupled with the humanized anti-VEGF antibody Fab segments, so that the stability is high, the sensitivity and the detection range of a detection system are improved, the storage time of the detection system is prolonged, and the reaction time is shortened. The linear range of the kit is 0.5-50000 pg / mL.
Owner:北京健平金星医疗器械有限公司
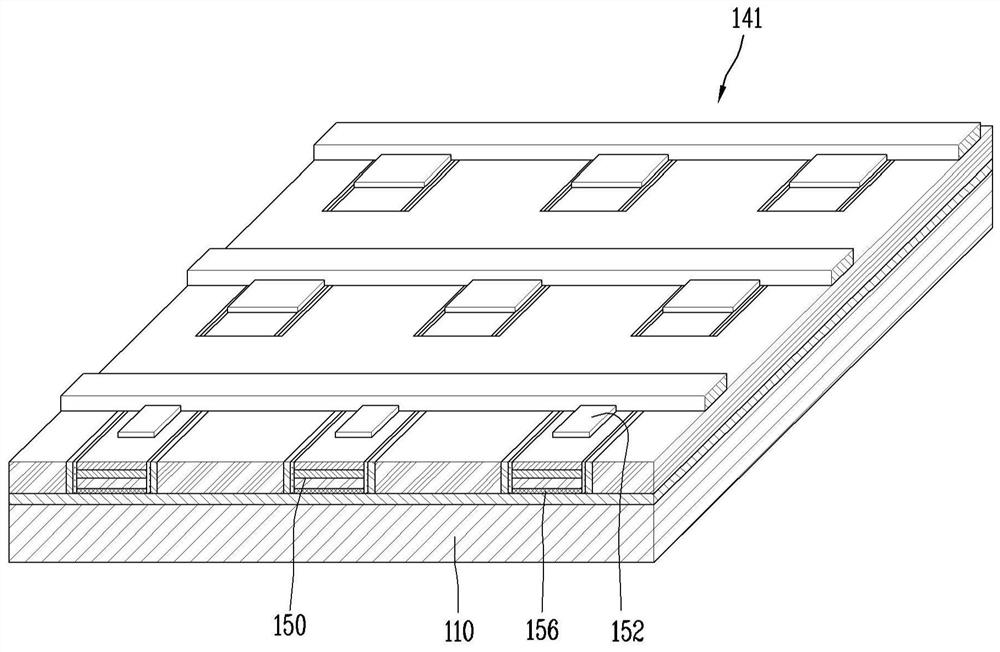
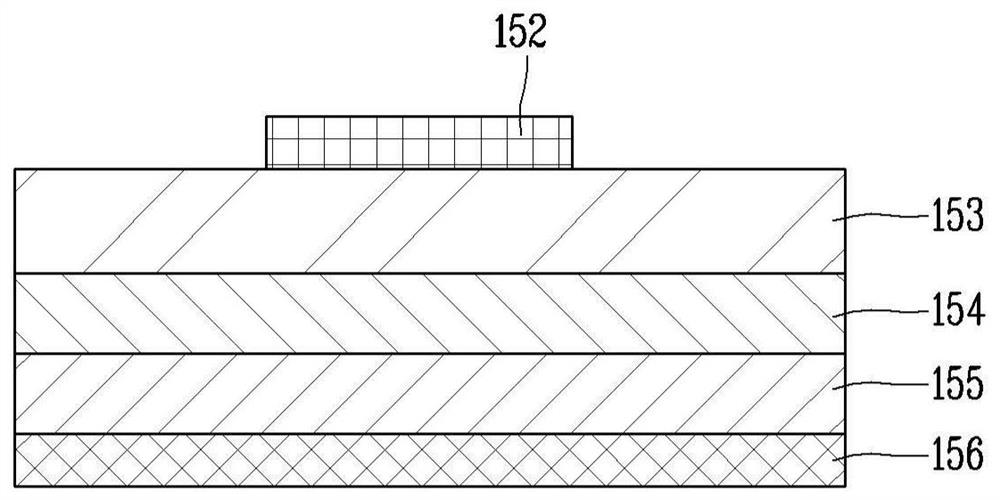
Display device using semiconductor light emitting element and method of manufacturing same
ActiveCN113380848ALow costImprove efficiencySolid-state devicesSemiconductor/solid-state device manufacturingDisplay deviceElectrical connection
The present invention relates to a display device using a semiconductor light emitting element and a method for manufacturing the same, and more particularly, to a display device using a semiconductor light emitting element having a size of several [mu] m to dozens of [mu] m and a method for manufacturing the same. The present invention provides a display device, which is characterized by comprising: a base part; a plurality of transistors disposed on the base part; a plurality of semiconductor light-emitting elements disposed on the base part; a plurality of wiring electrodes disposed on the base part and electrically connected to the plurality of transistors and the semiconductor light emitting element; a partition wall disposed on the base part and formed so as to cover the plurality of transistors; and a connection electrode connecting a portion of the plurality of transistors and a part of the plurality of wiring electrodes, the connection electrode being formed so as to penetrate the partition wall.
Owner:LG ELECTRONICS INC
Device for self-assembling semiconductor light-emitting diodes
PendingCN112531087AChange rotation speedAchieve preparationSolid-state devicesSemiconductor/solid-state device manufacturingCondensed matter physicsSemiconductor
The present disclosure provides a device for self-assembling semiconductor light-emitting diodes, in which the device includes an assembly chamber having a space for accommodating a fluid; a magneticfield forming part having magnets for applying a magnetic force to the semiconductor light-emitting diodes dispersed in the fluid and a horizontal moving part for changing positions of the magnet so that the semiconductor light-emitting diodes move in the fluid; a substrate chuck having a substrate support part configured to support a substrate, a vertical moving part for lowering the substrate sothat one surface of the substrate is in contact with the fluid in a state in which the substrate is supported, and an electrode connection part for applying power to the assembly electrode to generate an electric field so that the semiconductor light-emitting diodes are placed at predetermined positions of the substrate in a process of moving the semiconductor light-emitting diodes by a positionchange of the magnets; and a controller for controlling a movement of the magnetic field forming part and the substrate chuck, wherein the controller controls a depth at which the substrate is submerged in the fluid based on a degree of warping of the substrate.
Owner:LG ELECTRONICS INC
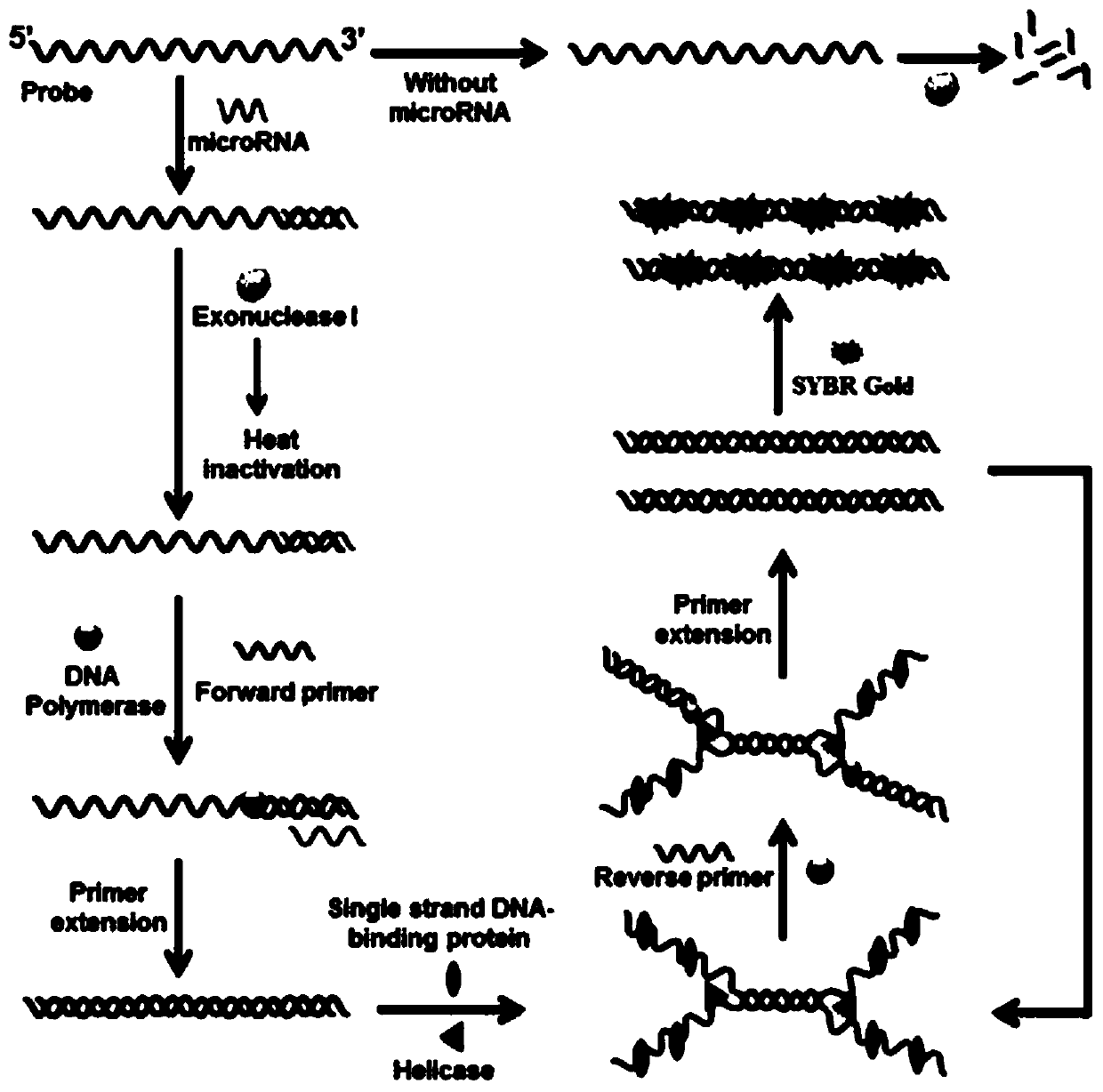
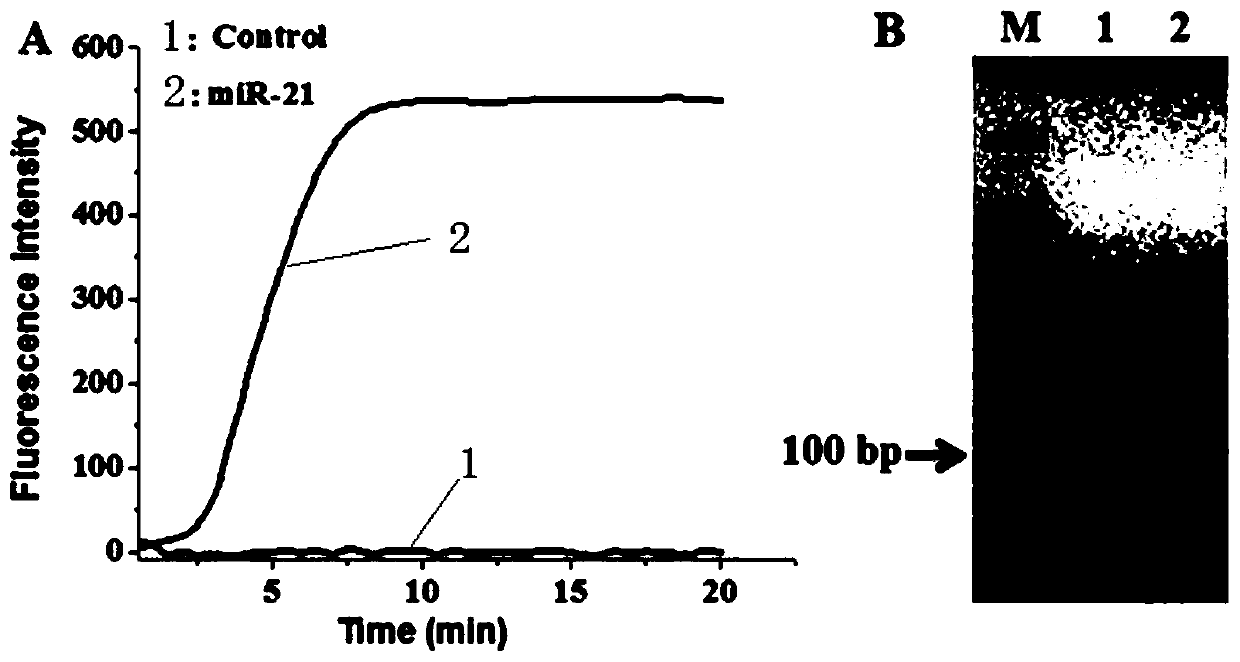
A method for detecting microRNA based on helicase-dependent DNA constant temperature amplification technology
ActiveCN107130024BEfficient detectionReduce background signalMicrobiological testing/measurementTotal rnaExonuclease I
The invention discloses a helicase-dependent isothermal DNA amplification-based method for detecting microRNA. The method comprises the following steps: (1) extracting total RNA in a sample; (2) adding a single-strand DNA probe and excessive exonuclease I into the extracted total RNA, incubating in a reacting buffer solution to realize specific combination of target microRNA and the single-strand DNA probe, and removing residual single-strand DNA probe by utilizing the excessive exonuclease I; and (3) adding upstream primer, downstream primer, single-strand binding protein and helicase to the reaction system, amplifying the target microRNA, and detecting expression of the target microRNA through a fluorescent signal. According to the method, the background is reduced by digesting the exonuclease I, and the signal is amplified by means of exonuclease assisted isothermal amplification (HDA) reaction, so that the microRNA can be quickly and sensitively detected.
Owner:SHANDONG NORMAL UNIV
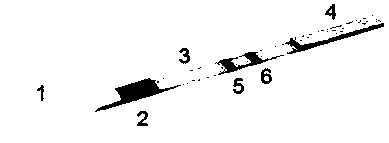
nc film and production process of scribing to prepare nc film
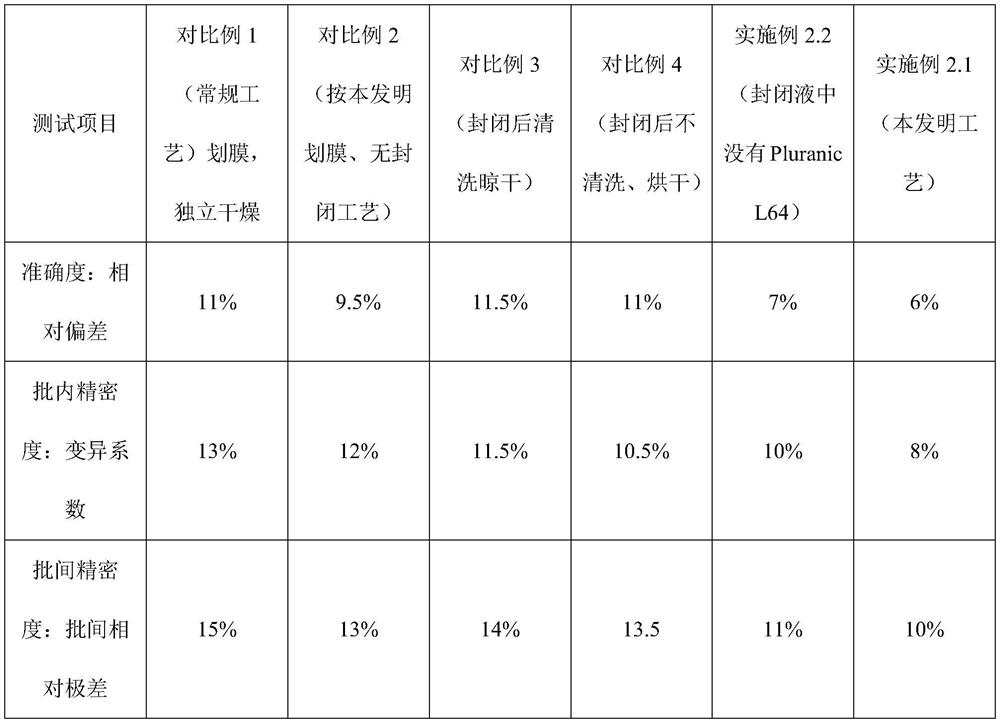
ActiveCN108802364BControl narrownessReduce spreadMaterial analysisNitrocellulose filterProcess engineering
Owner:HEBEI TEWENTE BIOTECH DEV CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com