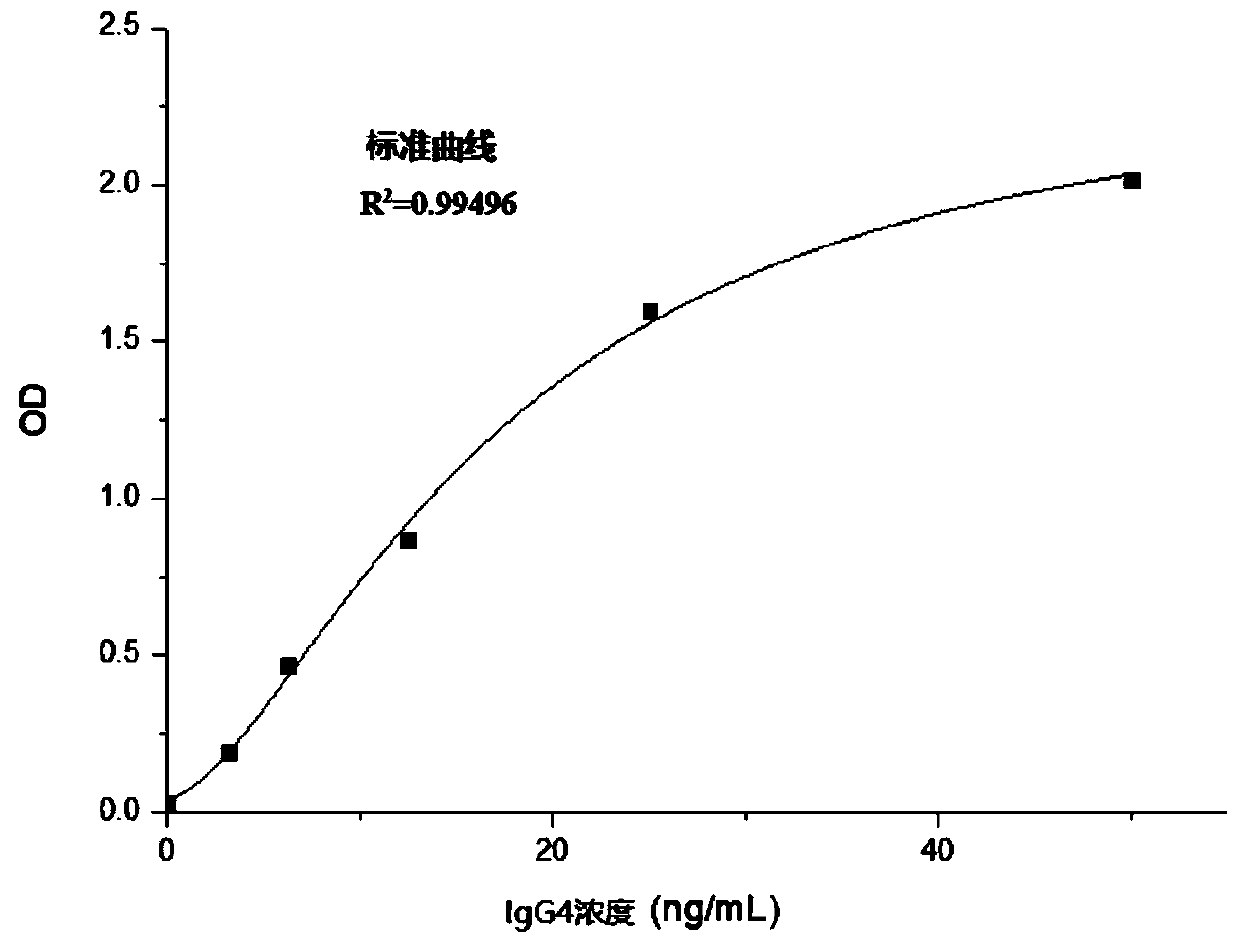
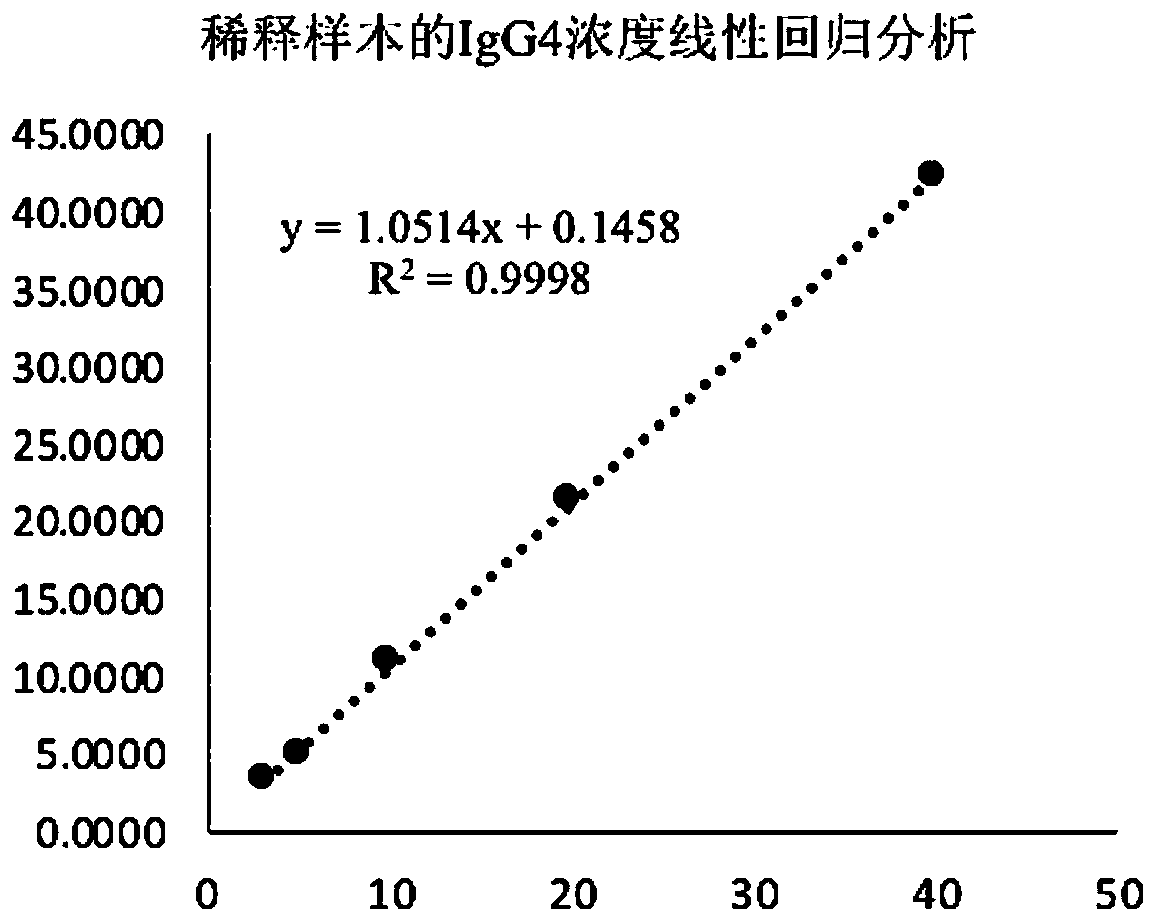
Method for detecting human immunoglobulin G4 and kit
A human immunoglobulin and kit technology, applied in the field of medical testing, can solve problems such as insufficient specificity of detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Embodiment 1: A kind of test kit that detects IgG4 antibody concentration
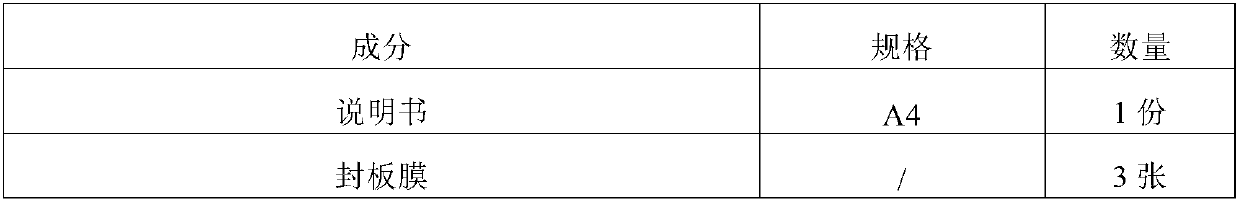
[0057] 1. Kit composition:
[0058] Table 1: Kit Composition
[0059]
[0060]
[0061] 2. Preparation method
[0062] 1. Preparation of ELISA plate
[0063] 1.1 Preparation of coating solution
[0064] Table 2
[0065]
[0066] *The IgG4Fc antibody in Table 2 is the first IgG4 monoclonal antibody, produced by Abcam, and the product numbers are ab1930.
[0067] 1.2 Coating of ELISA plate
[0068] Add 100 μL of coating solution to each well of the 96-well ELISA plate with a coating machine in the coating room of the 100,000-level area, and place it at 4°C overnight.
[0069] 1.3 Preparation of blocking solution
[0070] table 3
[0071]
[0072] 1.4 Sealing of the microtiter plate
[0073] Discard the coating solution and wash once (add 300uL 1×PBST to each well, let it stand for 2min, shake off the solution in the well, and pat dry). Add 200 μL of blocking solution to each ...
Embodiment 2
[0163] Example 2. Method for detecting human immunoglobulin G4 in a sample
[0164] 1. Materials and methods
[0165] 1. Materials:
[0166] (1) The test kit of embodiment 1
[0167] (2) The sample with known diluted IgG4 concentration is a patient sample, which comes from a male patient in the Department of Gastroenterology, First Hospital of Shanxi Medical University, whose IgG4 concentration was measured by this kit to be 0.401g / L.
[0168] (3) Patient samples to be tested: Serum samples were provided by the Gastroenterology Department of the First Hospital of Shanxi Medical University. Department of Gastroenterology, No. 1 Hospital, Tel: 0351-4639020.
[0169] 2. Method:
[0170] (1) Treat the sample to be tested: dilute the sample to be tested with a volume ratio of 1:10000, take 10 μL of the sample and add it to 1 mL of sample diluent, shake and mix, then take 10 μL of it and add it to 1 mL of sample diluent, shake and mix well ;
[0171] (2) Adding samples: Add di...
Embodiment 3
[0186] Embodiment 3. kit and method specific experiment
[0187] 1. Materials and methods
[0188] 1. Materials
[0189] (1) Example 1 kit
[0190] (2) IgG1, IgG2, IgG3 samples, IgG1 was purchased from Abcam, the product number is ab90283; IgG2 was purchased from Abcam, the product number is ab90284; IgG3 was purchased from Sino.Biological Inc, the product number is 13906-HNAH.
[0191] 2. Method
[0192] Dilute IgG1, IgG2, and IgG3 to the following concentrations of test samples: 1000ng / mL, 500ng / mL, 250ng / mL, 100ng / mL and 50ng / mL;
[0193]Use the kit of the present invention to measure IgG1, IgG2, IgG3 (1000ng / mL, 500ng / mL, 250ng / mL, 100ng / mL and 50ng / mL) with different concentrations respectively, and the method is the same as the detection method in Example 2.
[0194] 2. Results
[0195] The measurement results are shown in Table 9, and it was found that the OD values were all close to the blank wells. From the results, it was confirmed that the method established...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com