Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
54 results about "Venom Protein" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
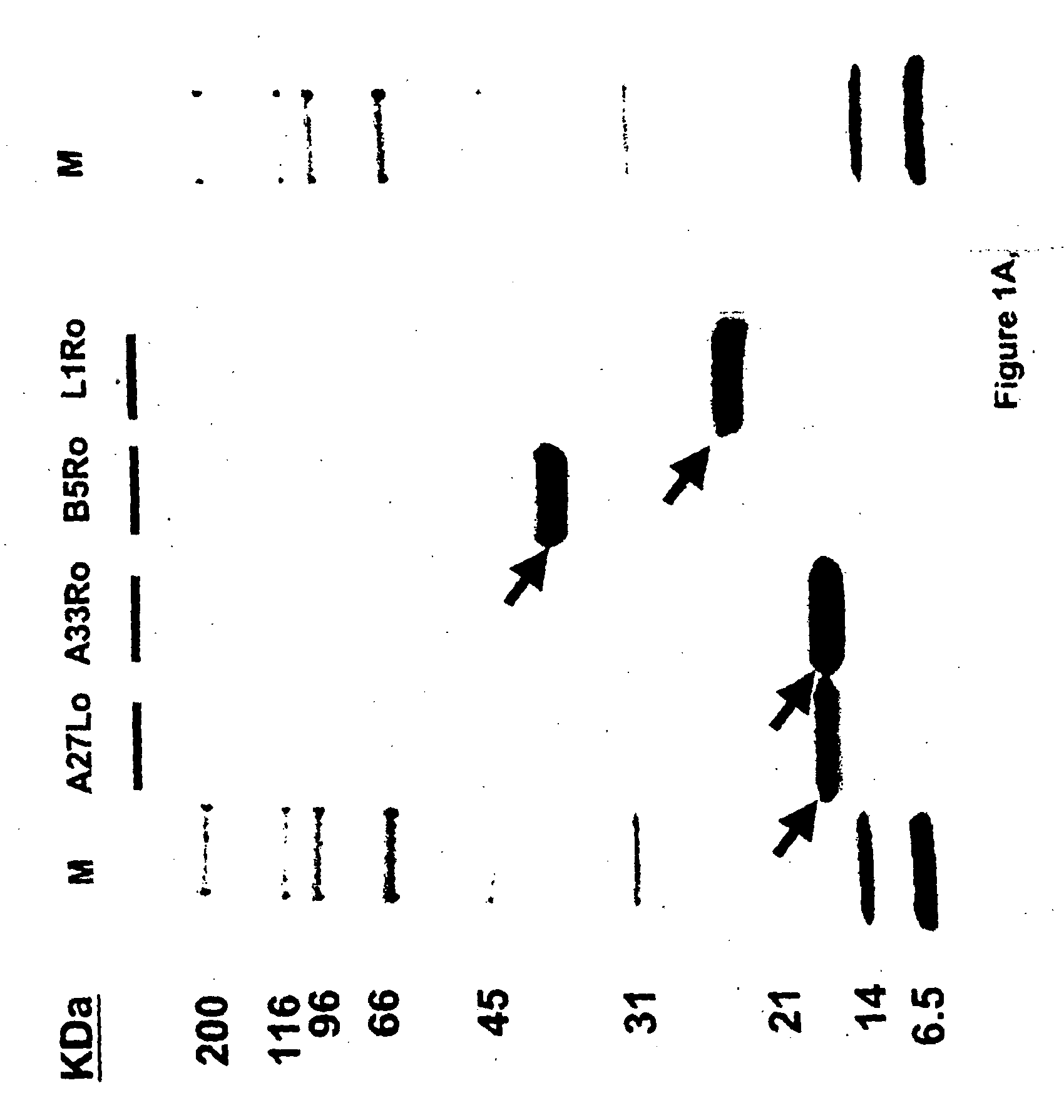
Protein isolated from venom.
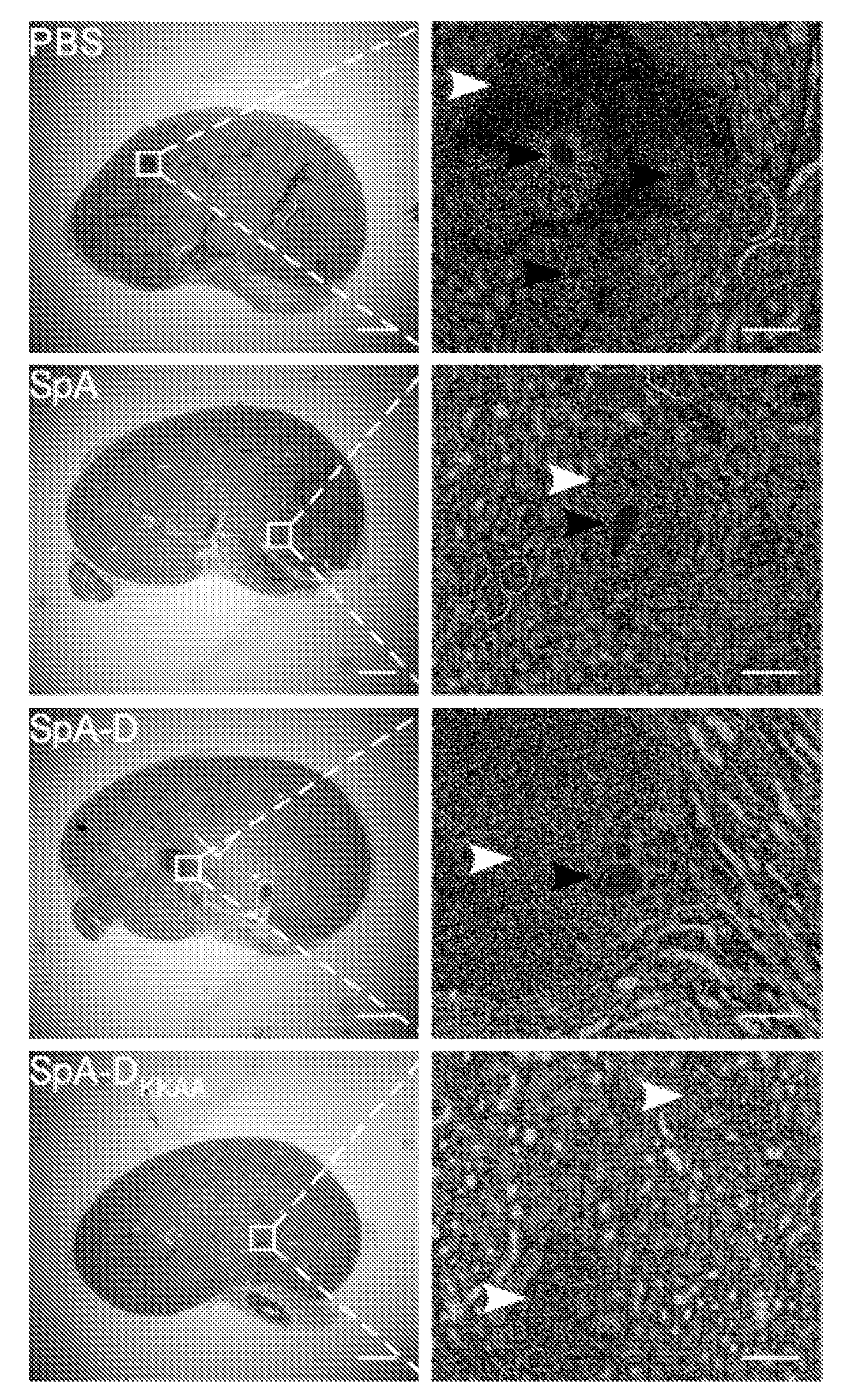
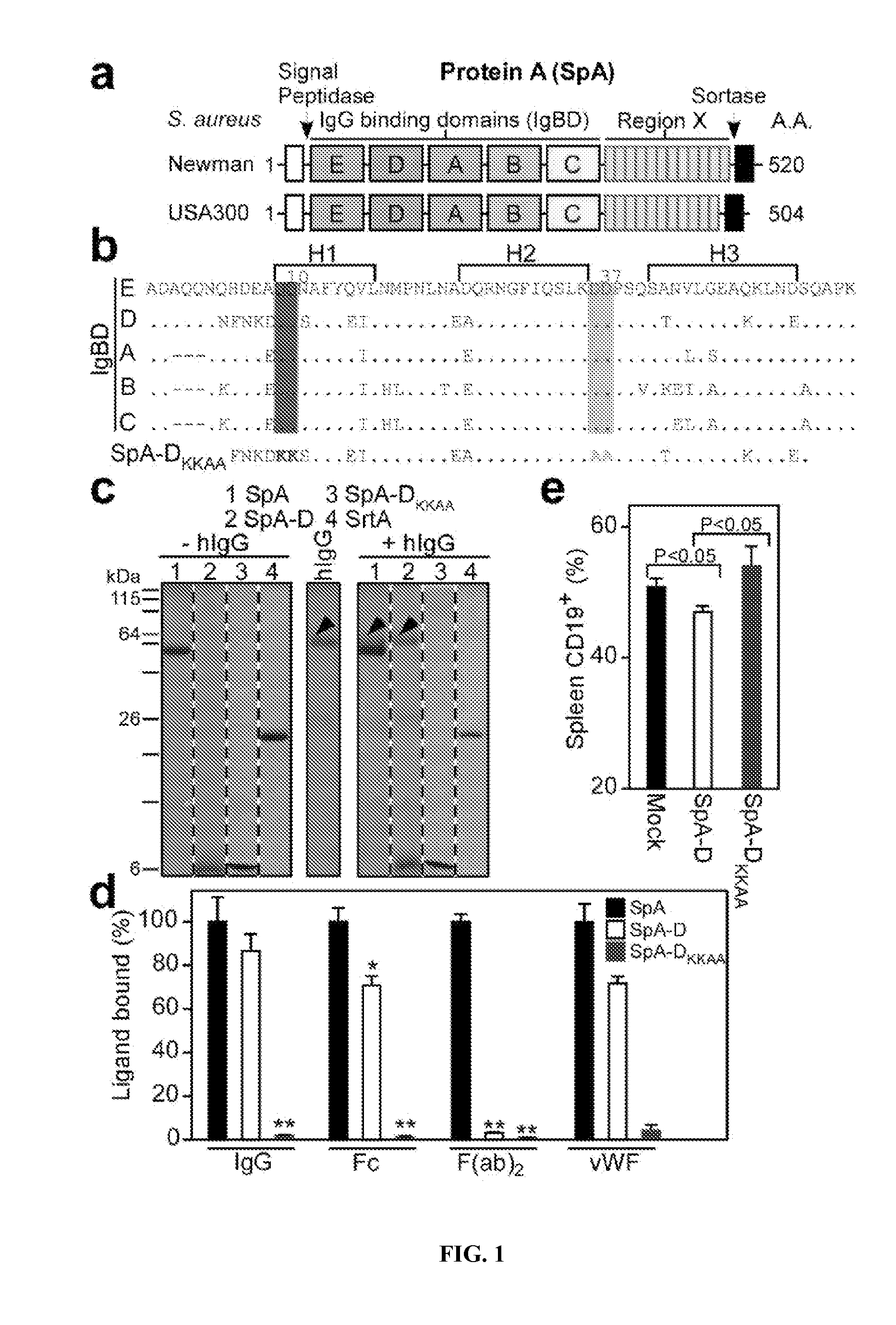
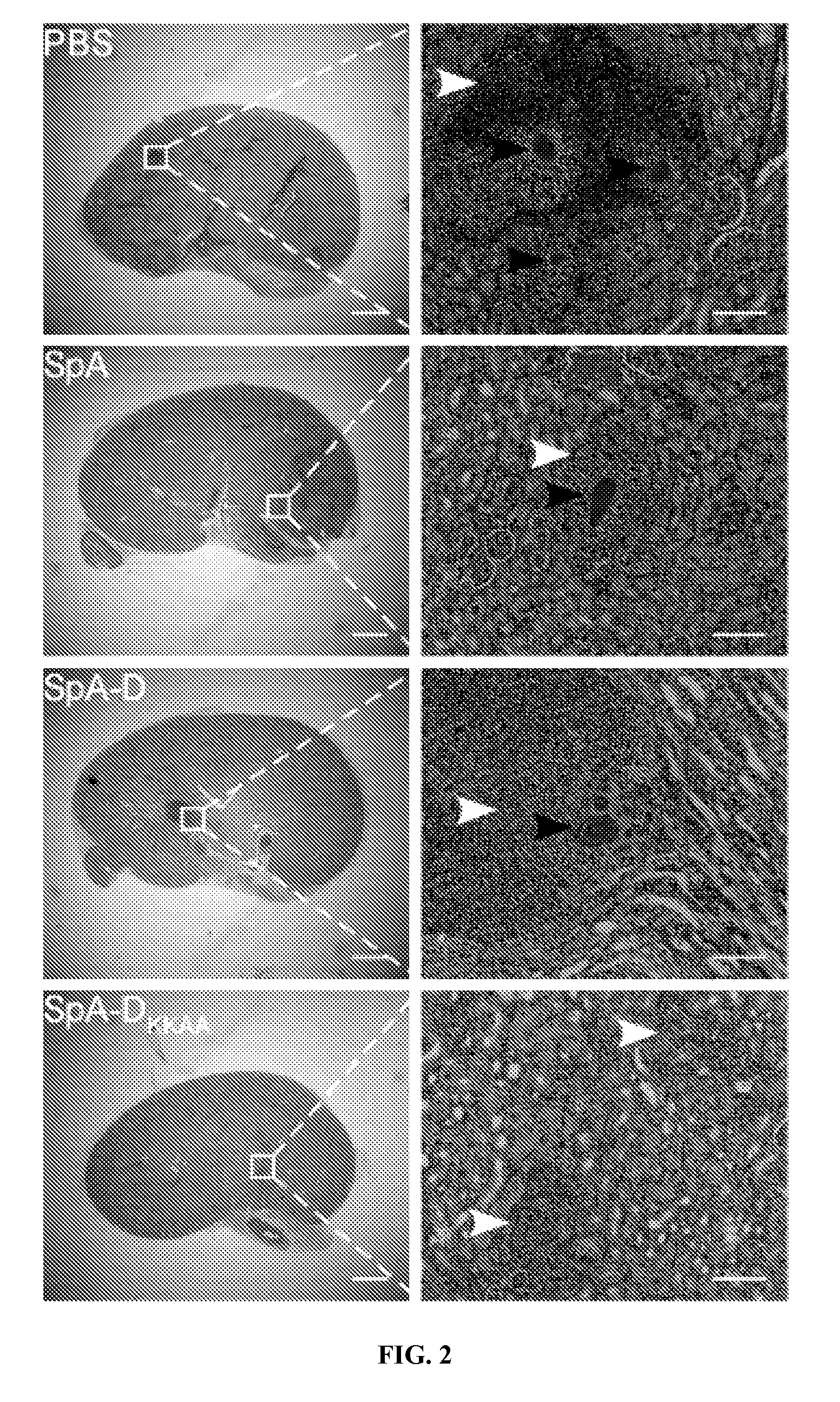
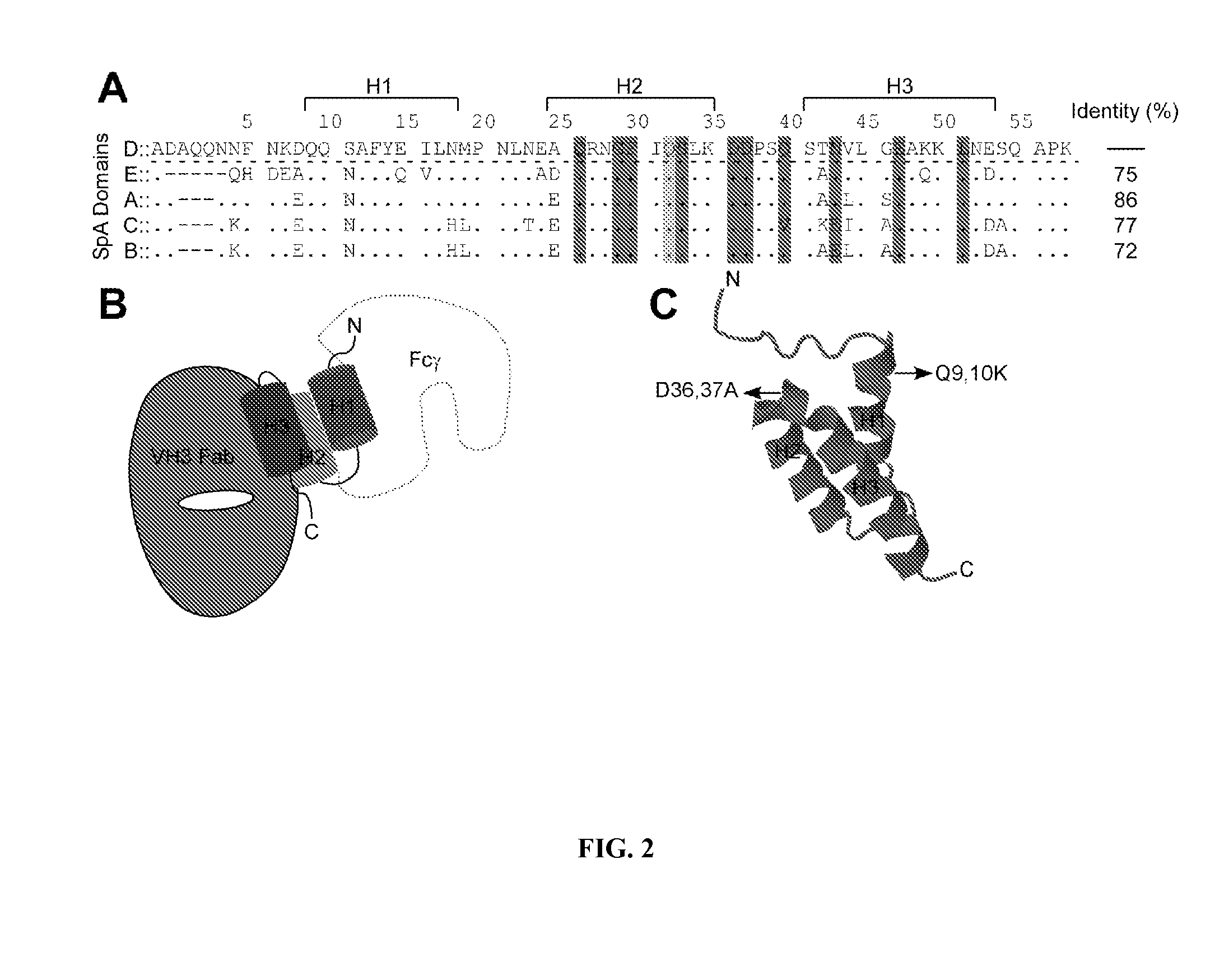
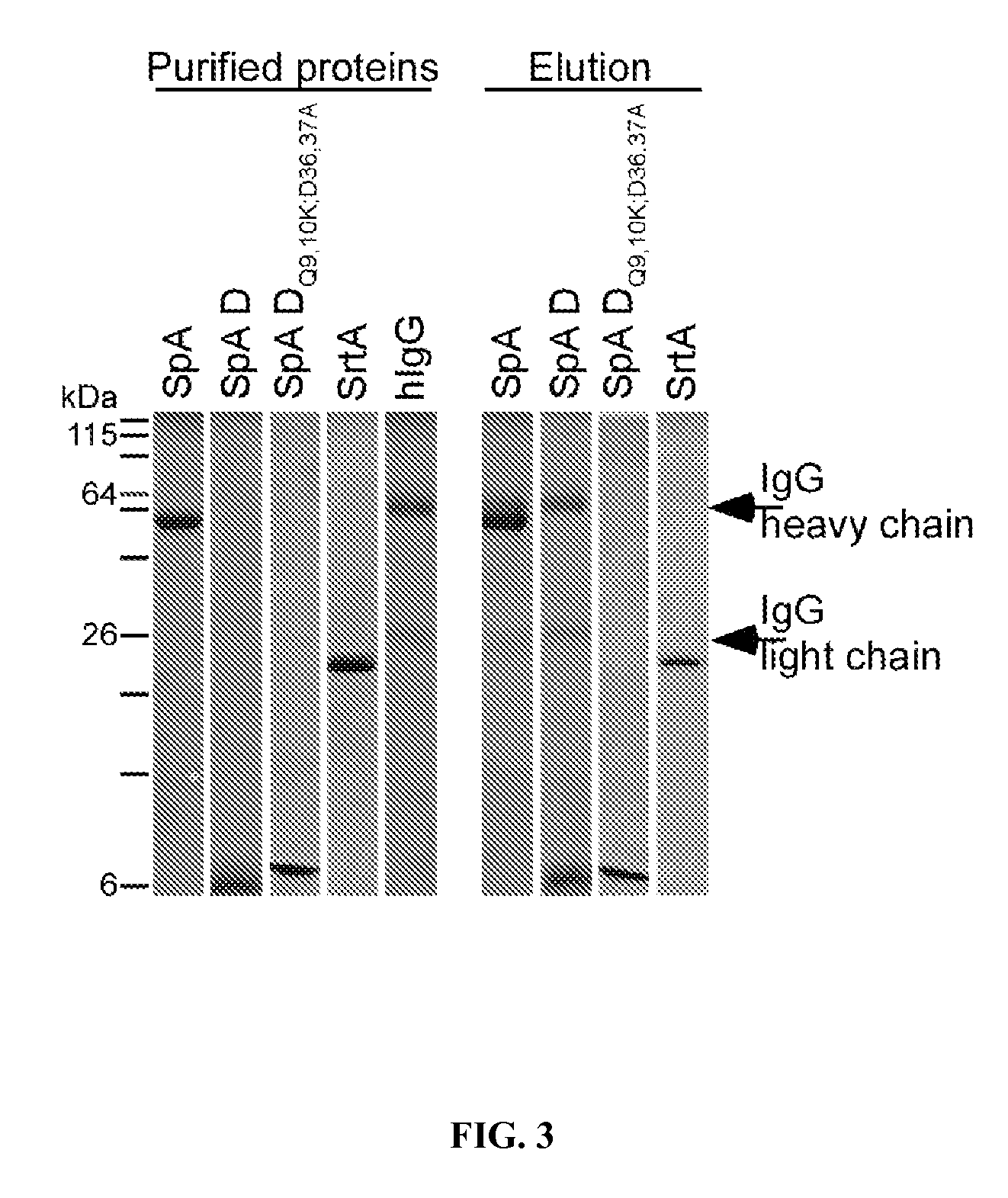
Compositions and methods related to protein a (SPA) variants
ActiveUS20120114686A1Reduced activityPrevents alleviates ameliorates symptomAntibacterial agentsBacterial antigen ingredientsStaphylococcus xylosusProtein A
Disclosed are methods and compositions for treating or preventing a Staphylococcus bacterial infection using a non-toxigenic Protein A (SpA) variant.
Owner:UNIVERSITY OF CHICAGO
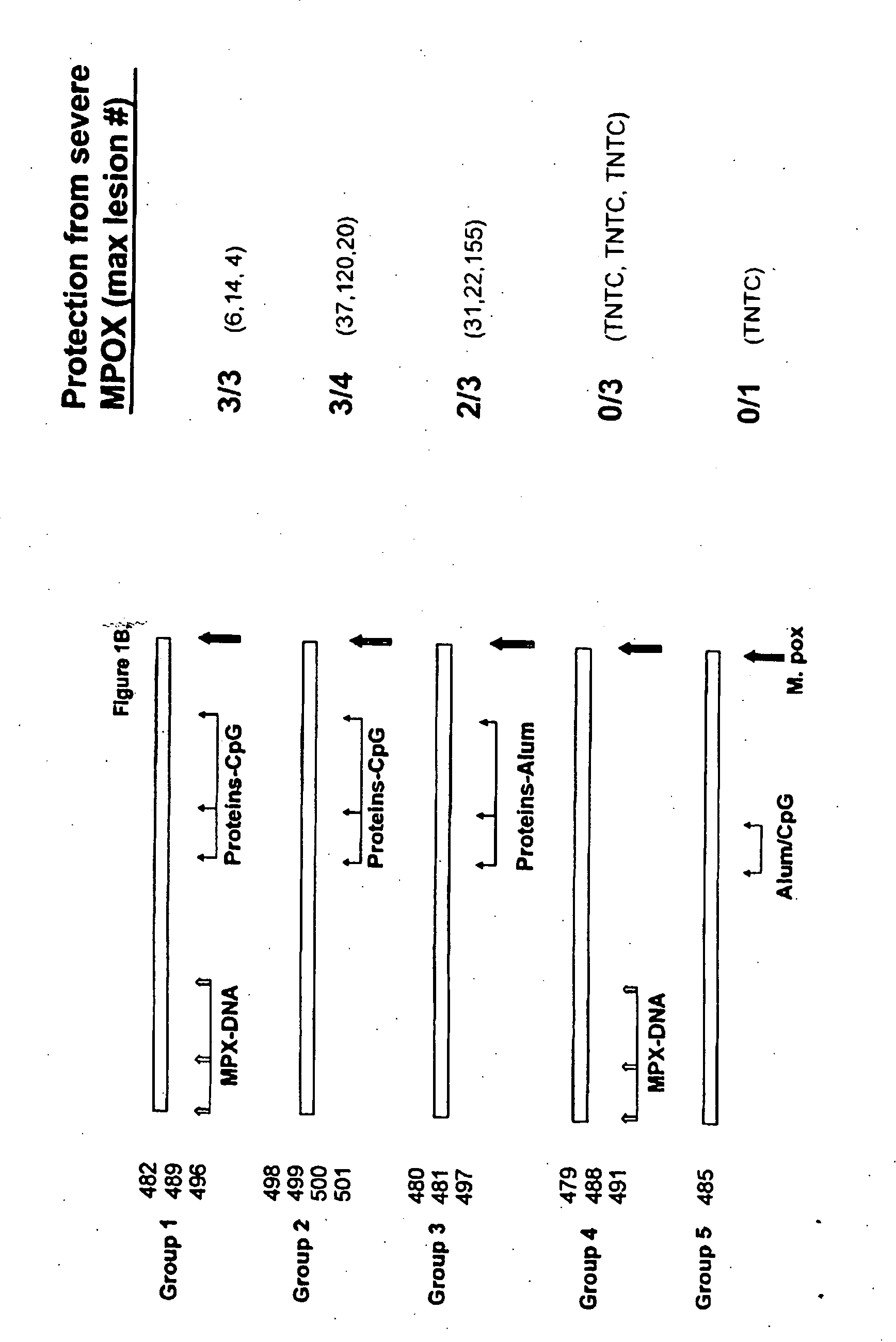
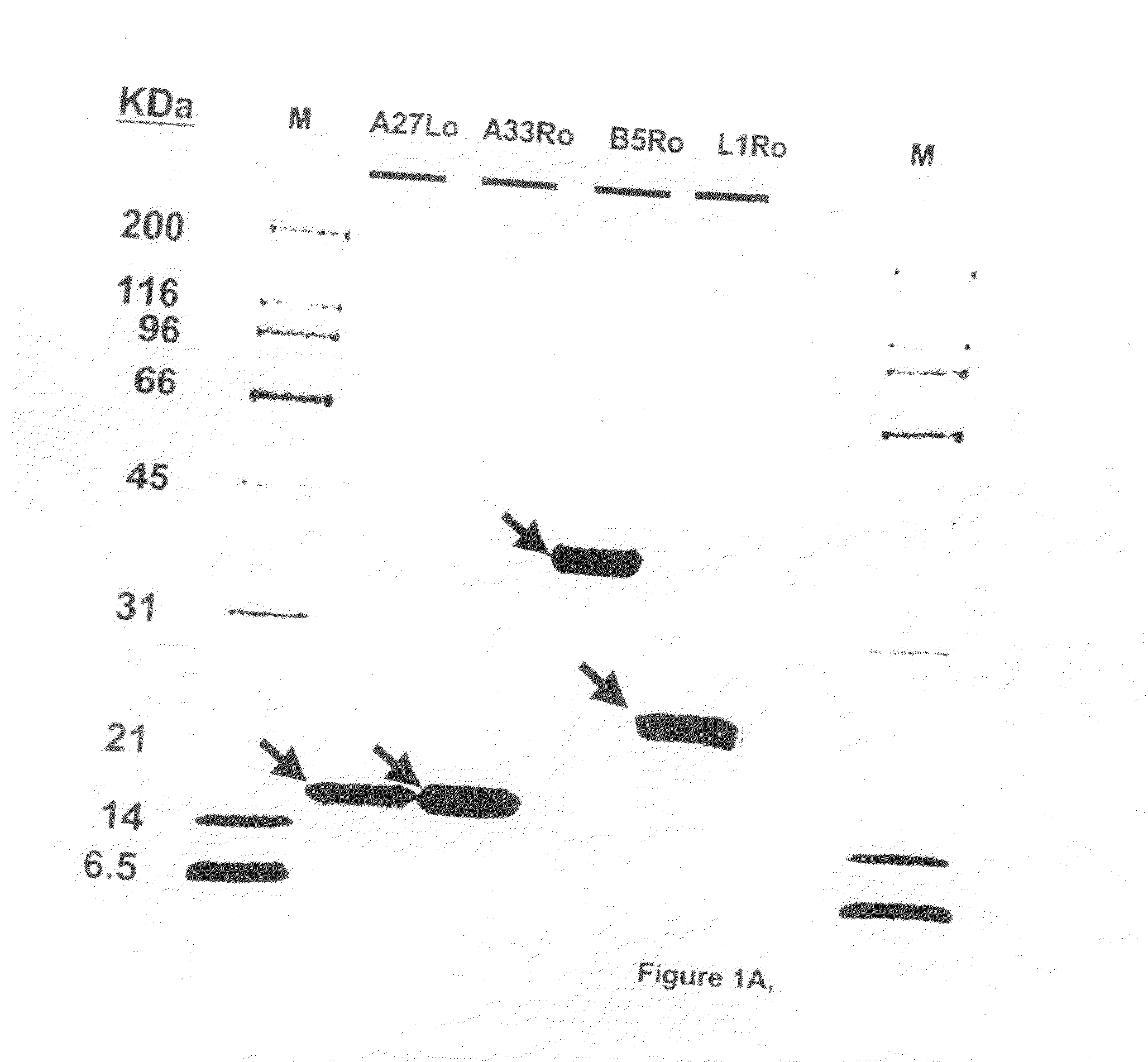
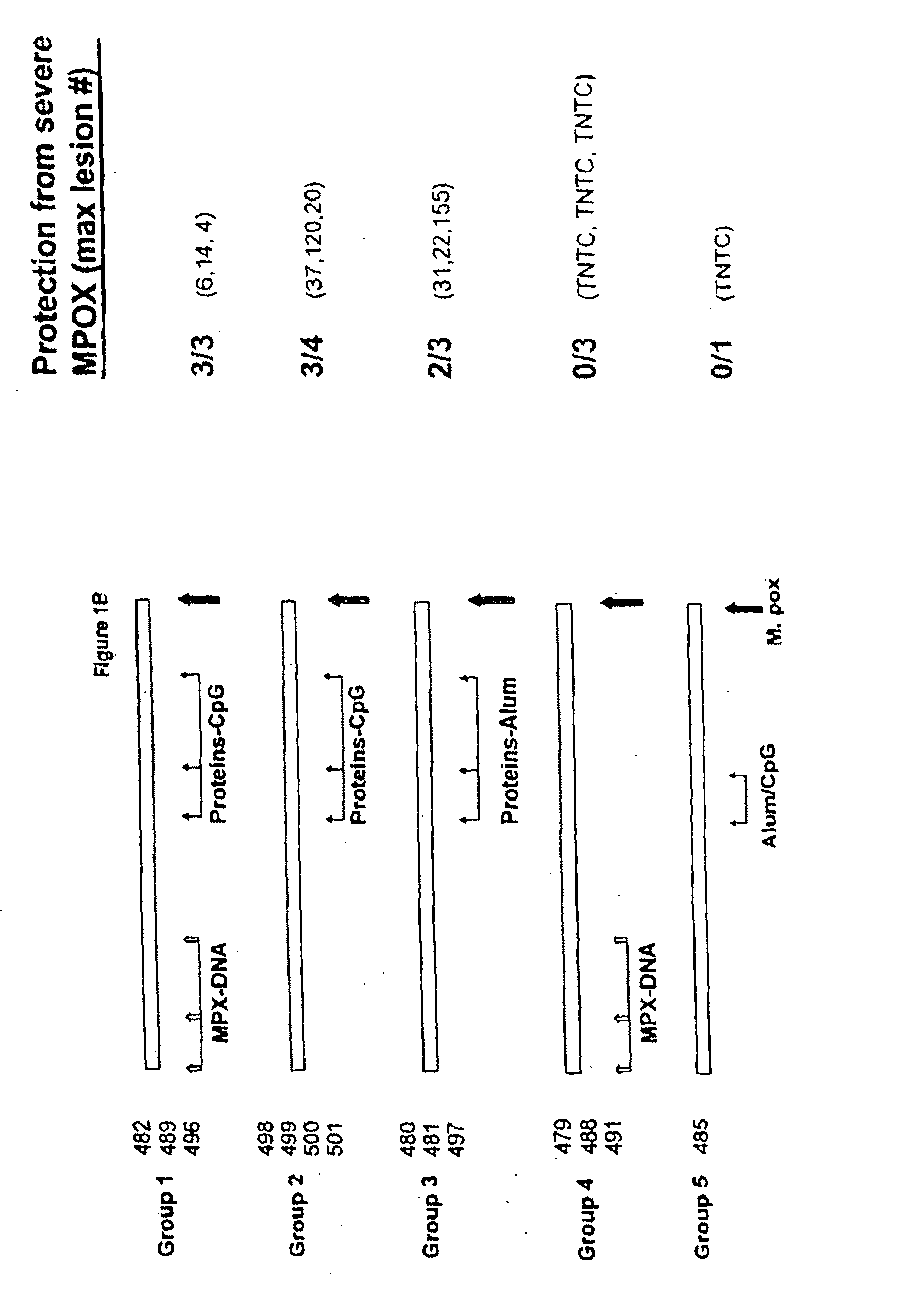
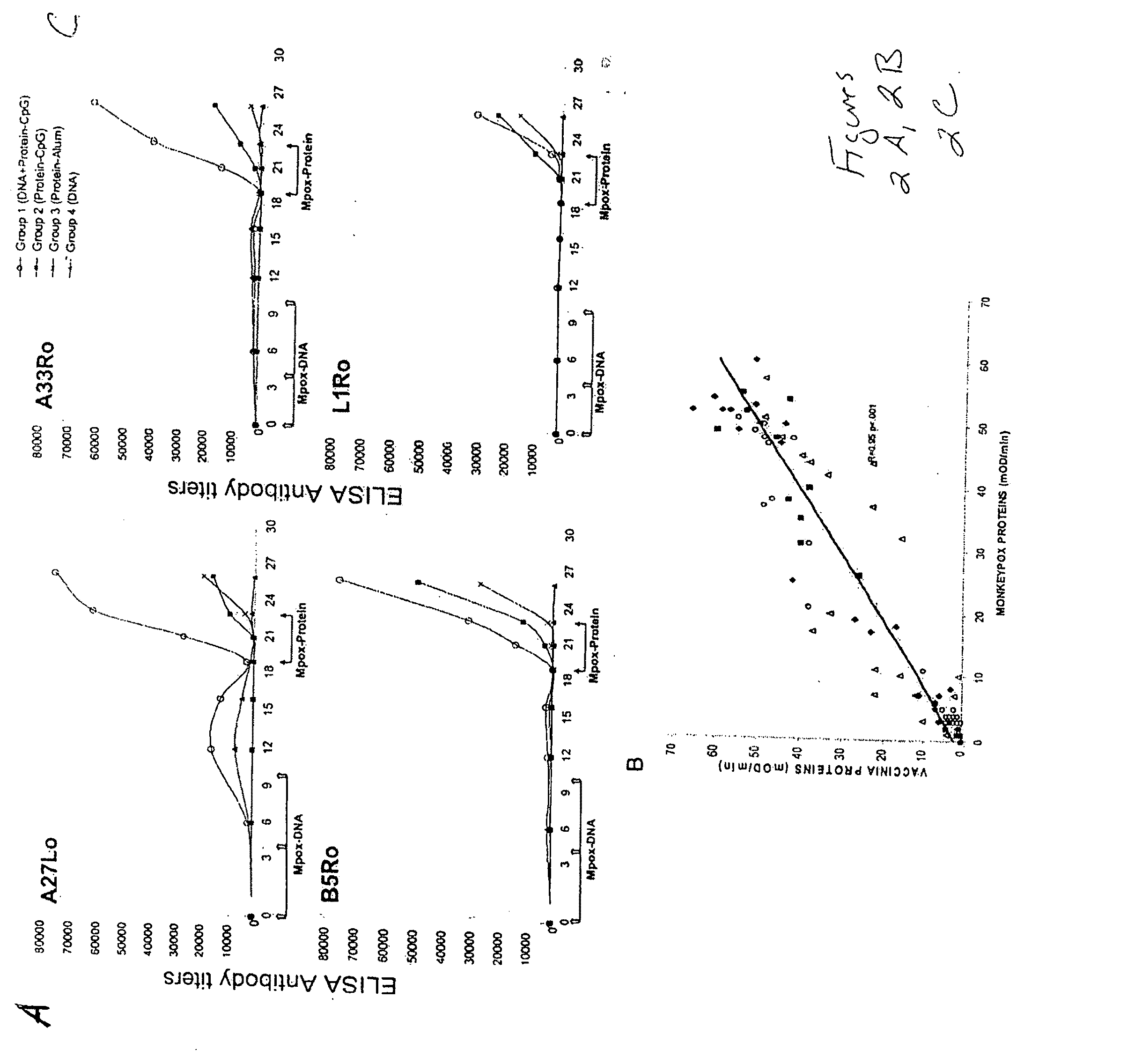
Protein vaccines against poxviruses
InactiveUS20100196491A1High degreeIncreased cross-reactivityPowder deliveryViral antigen ingredientsOpen reading frameMonkeypox
The invention described here entails a protein vaccine against poxviruses which contains at least two purified recombinant monkeypox virus proteins or peptides. The proteins or peptides are encoded by the open reading frames of the monkeypox ortholog genes M1R, A35R, A29L B6R, and orthologs of these proteins or peptides having 90% identity. The invention also entails a vaccine protocol against poxvirus whereby a vaccine is vaccinated with a first vaccine made up of a nucleic acid vaccine of three or more poxvirus virus genes, and subsequently vaccinated with at least one other booster vaccine made up of two or more poxvirus virus proteins.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
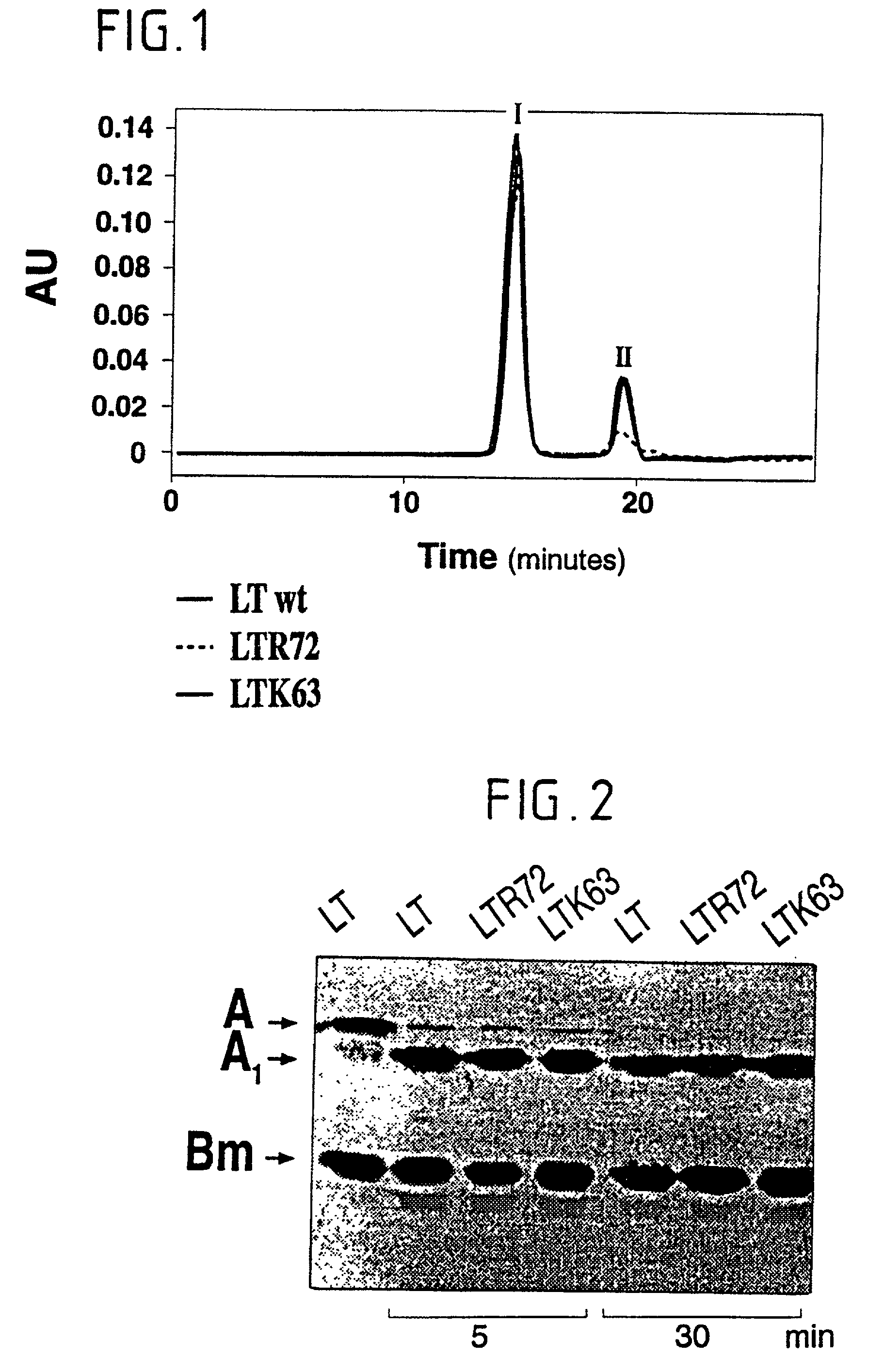
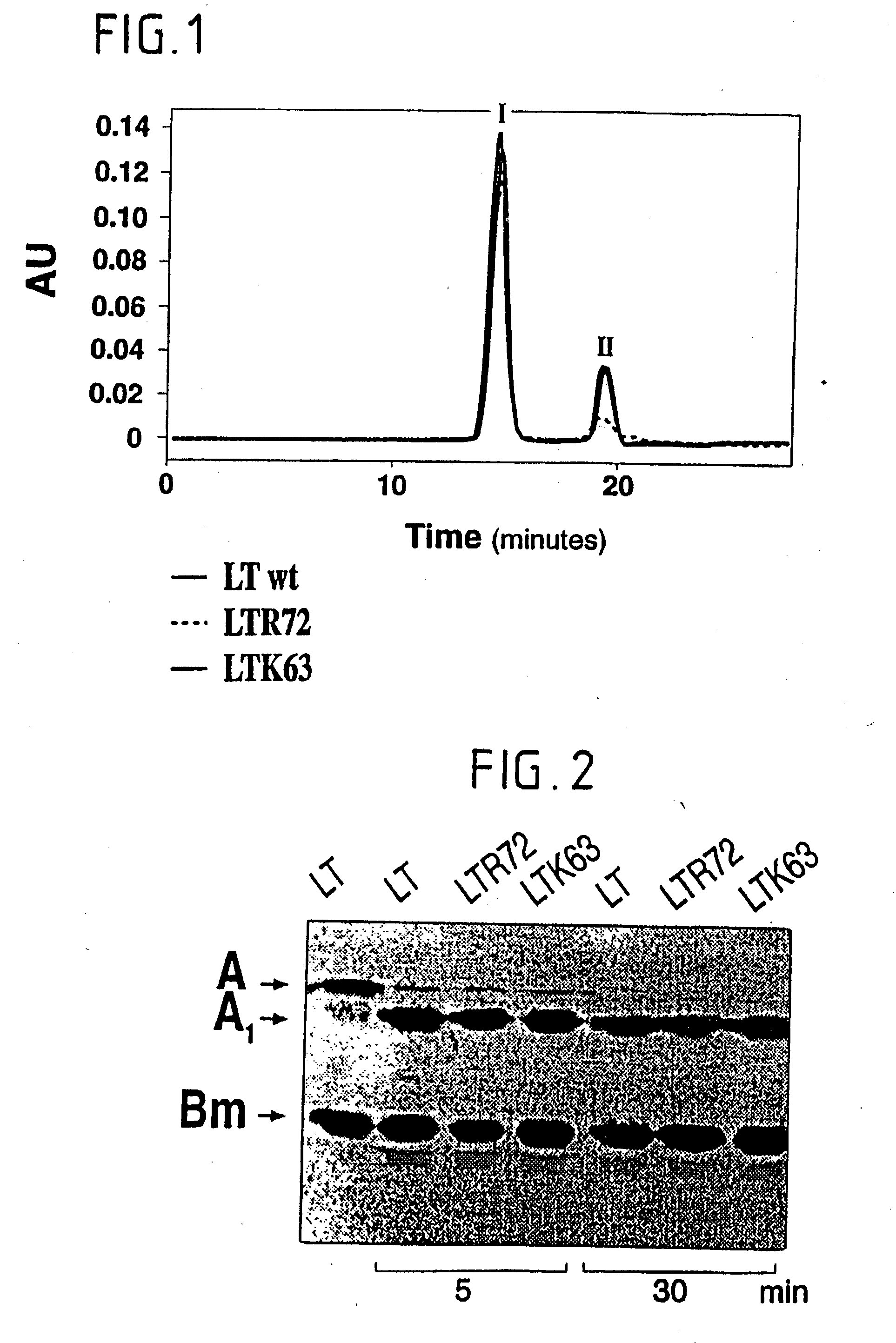
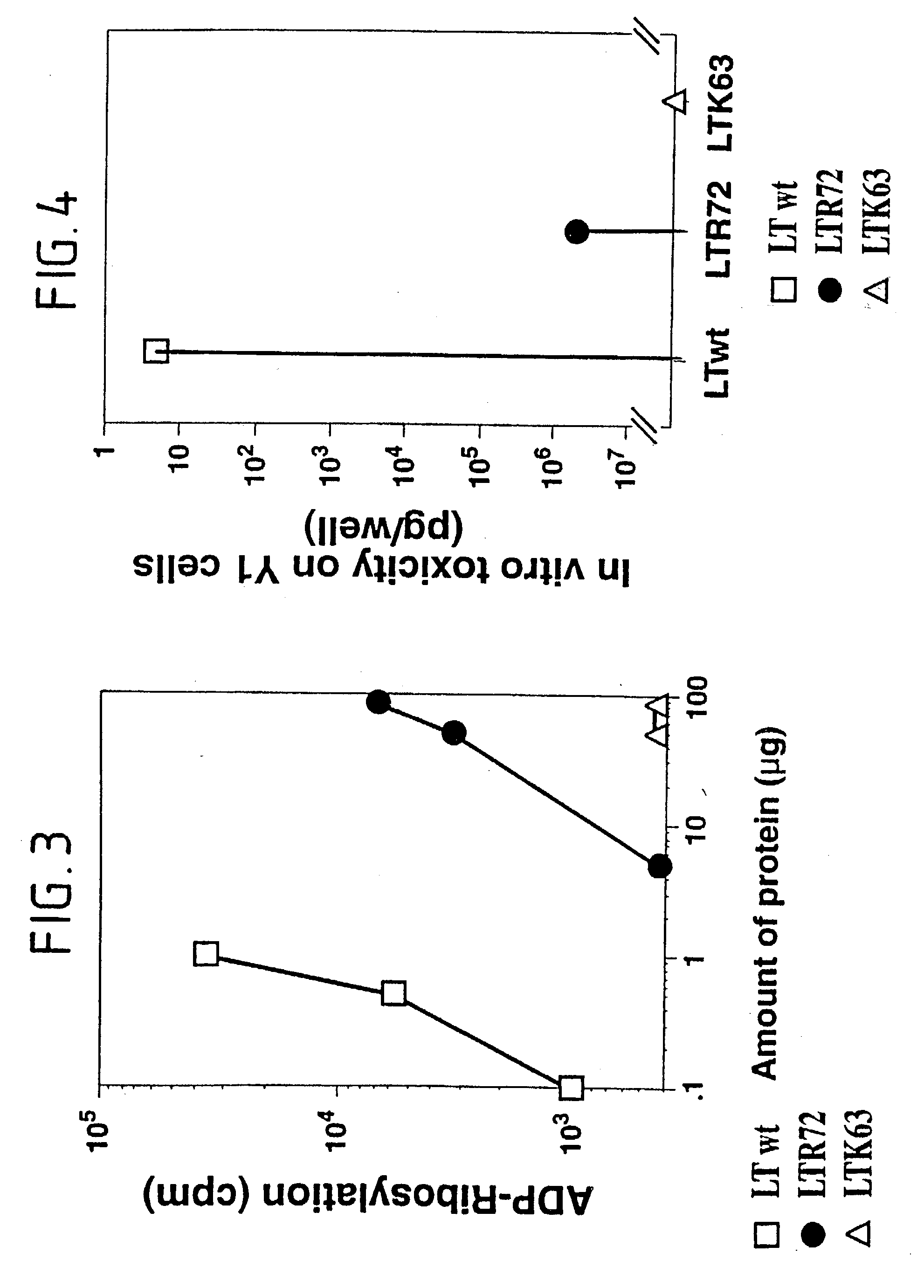
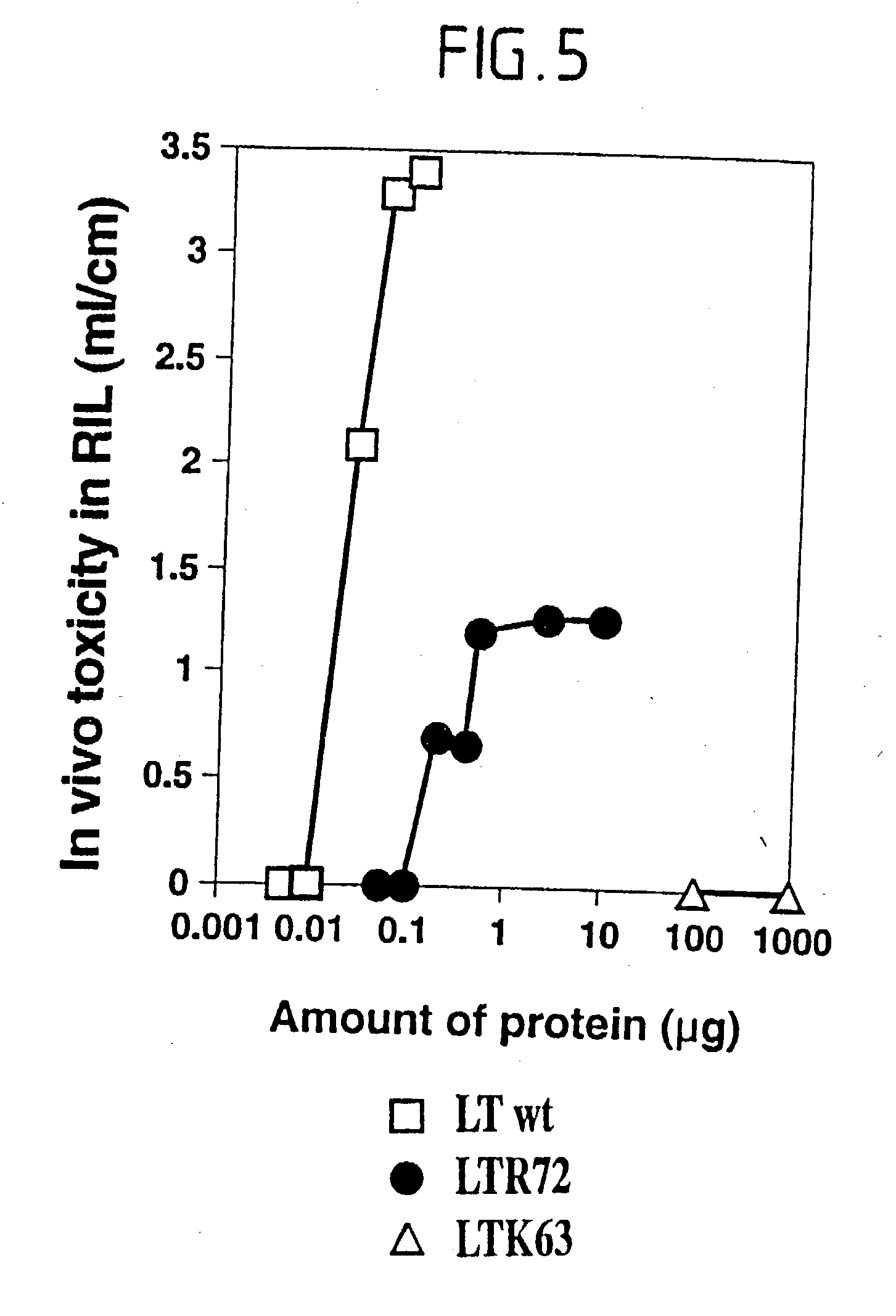
Immunogenic detoxified mutant e. coli lt-a toxin
InactiveUS20030113338A1Maximise adjuvanticityMaximise immunogenicityAntibacterial agentsBiocideEscherichia coliAdjuvant
An immunogenic detoxified protein is provided which comprises the amino acid sequence of subunit A of an E. coli heat labile toxin (LT-A) or a fragment thereof in which at least amino acid Ala-72 of the A subunit is mutated, preferably by substitution with Arg. The toxoid is useful as vaccine against an enterotoxigenic strain of E. coli and is produced by recombinant DNA means by site-directed mutagenesis. It is also an effective adjuvant.
Owner:CHIRON CORP
Katsutoxin extract, preparation method and application thereof
InactiveCN101366733ASuppress generationInhibition of productionAnthropod material medical ingredientsDigestive systemFreeze-dryingAngiogenesis Effect
The invention discloses a scorpion venom extract extracted from East Asia scorpions. A preparation method for the scorpion venom extract comprises the following steps: scorpion venom freeze-dried powder is dissolved, centrifuged, filtered by use of 0.45 mu m and 0.22 mu m microporous membranes and freeze-dried; sephadex is added in; III-IV protein peaks of scorpion venom are collected; cation exchange chromatography is carried out; detection is carried out by use of an ultraviolet spectrophotometer; and the protein peaks are collected, so as to obtain the scorpion venom extract. Experiments prove that the scorpion venom extract has definite functions of inhibiting angiogenesis and hepatoma cell reincrease value. The invention successfully extracts effective components inhibiting angiogenesis and hepatoma cell reincrease value from the East Asia scorpions, which provides a foundation for further research and application in the future.
Owner:INST OF BASIC MEDICINE OF SAMS
Method for separating and purifying cobra neurotoxin protein through dual-ion exchange chromatography, and preparation of cobra neurotoxin protein
ActiveCN103387610AHigh purityIncrease flow ratePeptide/protein ingredientsAntipyreticCobra venomHigh concentration
The invention provides a method for separating and purifying cobra neurotoxin protein through dual-ion exchange chromatography. The method comprises the following steps of: (1) first separation and purification through ion exchange chromatography, namely, a) dissolution of crude cobra venom, and b) separation and purification through an SP-Sephodex-C25 gel column; and (2) second separation and purification through ion exchange chromatography, namely, a) balance of a SourceS(XK5030) column having a column volume of 500 ml by using 1000 ml of liquid A for future use; and b) SourceS(XK5030) column purification, and then collection of the cobra neurotoxin protein purified twice according to a standard purification chromatogram. The method provided by the invention is scientific and rational in process; and no organic reagent, no high-concentration salt and no protein modification method are used in production, so that the biological activity of cobratide can be maintained to an utmost extent. The method provided by the invention is advanced in process and simple to operate; the production period can be greatly shortened, the production time can be saved and the production cost can be reduced; and the method is completely suitable for an industrial production line.
Owner:奔驰生物科技(云南)有限公司
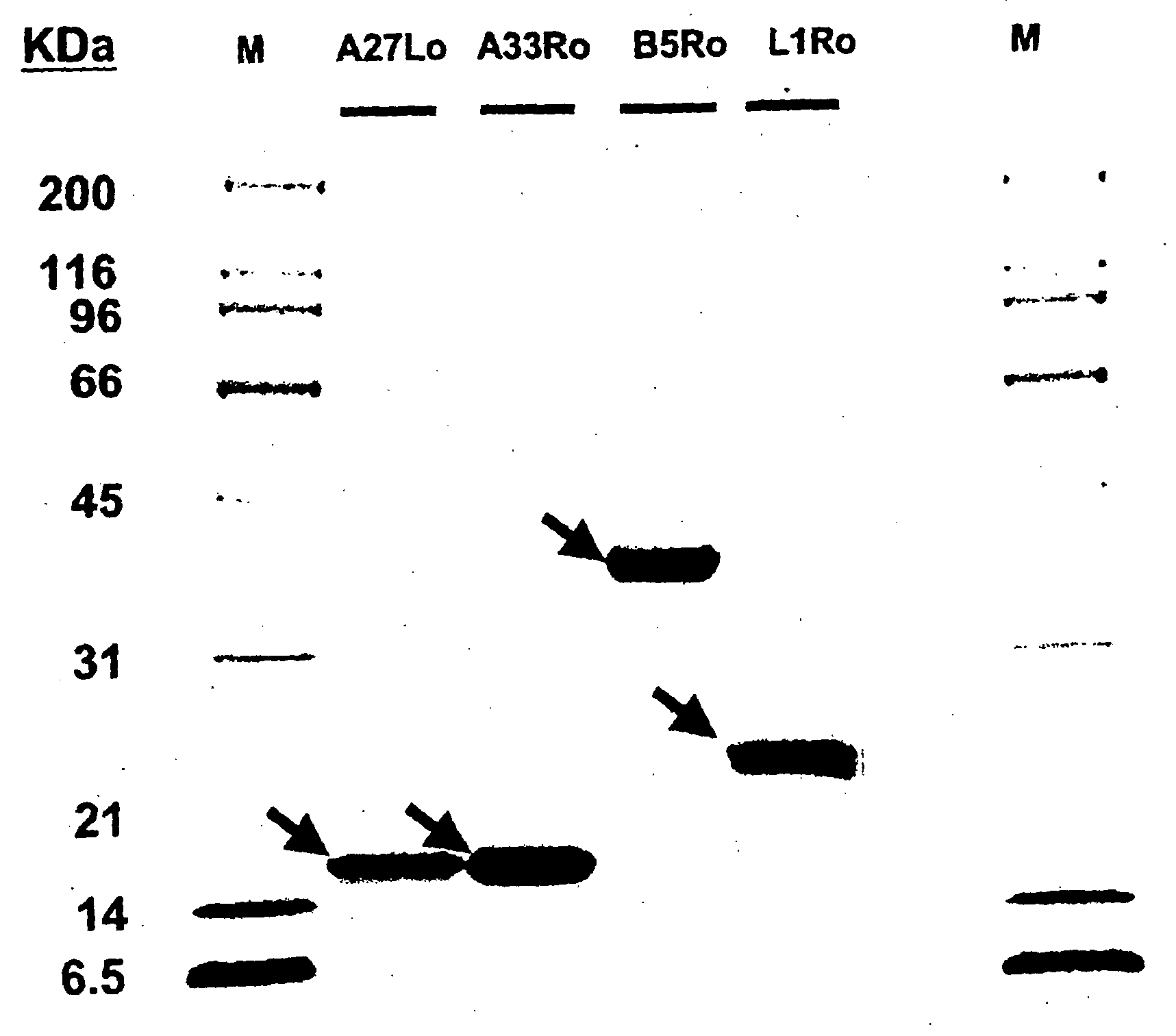
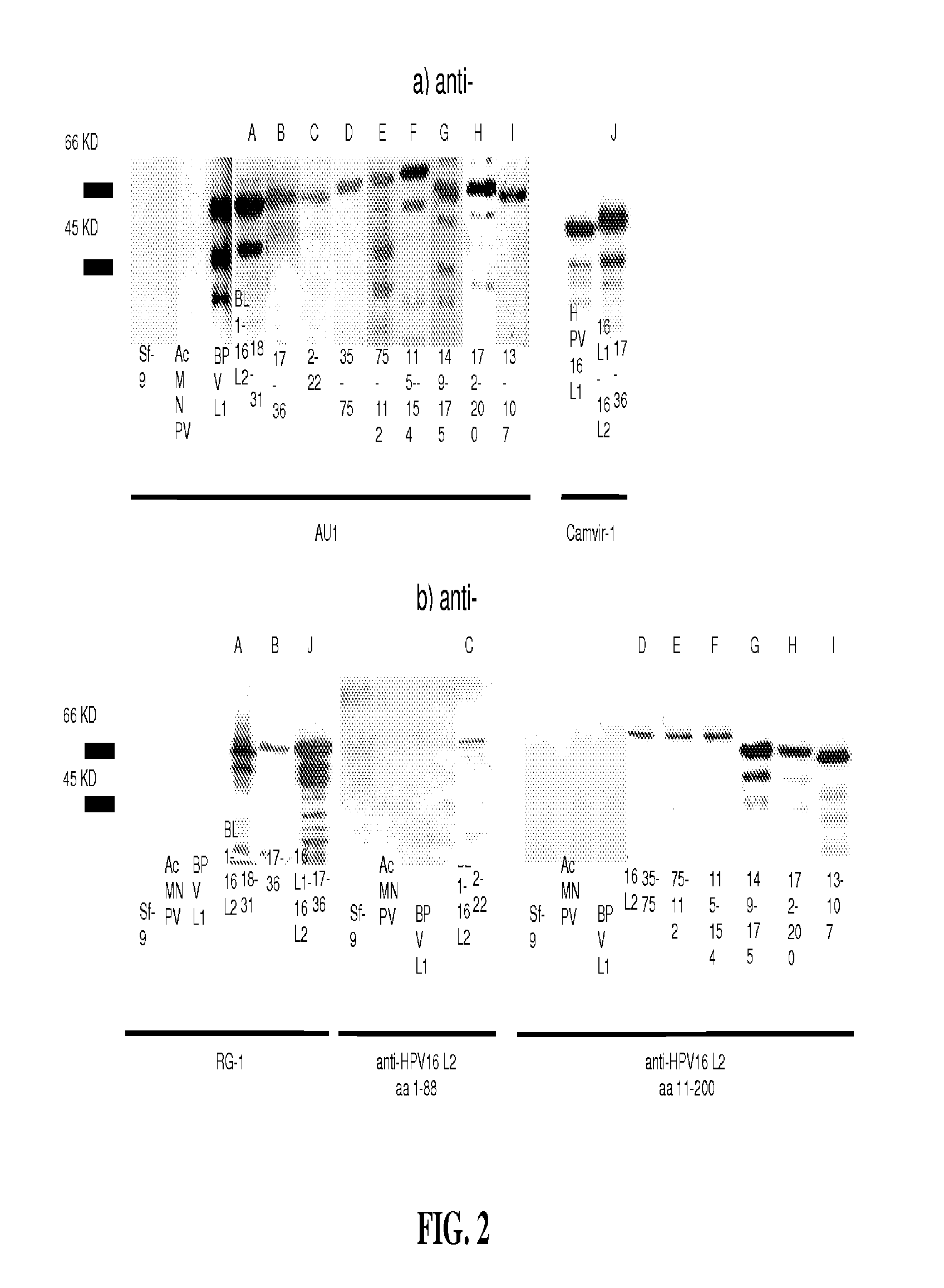
Papillomavirus-like particles (VLP) as broad spectrum human papillomavirus (HPV) vaccines
ActiveUS20120093821A1Induce high-titer anti-L1 antibody levelsImprove toleranceFungiVirusesSurface displayHuman papillomavirus
This invention relates, e.g., to a virus-like particle (VLP) composition assembled from a chimeric polypeptide comprising a papilloma virus (e.g., human papillomavirus, or HPV) L1 major capsid protein, into which is inserted a surface-displayed peptide comprising a neutralizing epitope of a papillomavirus L2 protein. Vaccine compositions comprising the VLP are described, as well as methods for inducing an immune response (e.g., vaccinating) a subject against papilloma virus, using the VLP, and kits comprising the VLP, for carrying out a method of the invention.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Scorpion venom protein polypeptide colon-targeted preparation and preparation method thereof
InactiveCN102764426ASimple production processReduce production requirementsOrganic active ingredientsNervous disorderSmall intestineTherapeutic effect
The invention discloses a scorpion venom protein polypeptide colon-targeted preparation and a preparation method thereof. Low-ester pectin is used as a main carrier and dripped into solution containing calcium ions, pellets are formed after reaction and solidification of the low-ester pectin, the pellets are soaked in polymine solution with a certain concentration and further cross-linked after being dried to form outer layers with higher hydrophobicity, and the scorpion venom protein polypeptide colon-targeted preparation is obtained after drying. After the preparation is taken orally, medicines are concentrated to release at the position of a colon, the concentration of the medicines at the position of the colon can be remarkably improved, the medicines directly contact with a focus of the colon and can be effectively prevented from being damaged by gastric acid or enzyme and the like in a stomach and a small intestine, and therapeutic effects of the medicines are made full use of.
Owner:FUJIAN HEALTH COLLEGE
Immunogenic detoxified mutant E. coli LT-A toxin
InactiveUS20030170262A1Maximise adjuvanticityMaximise immunogenicityAntibacterial agentsBacteriaEscherichia coliAdjuvant
An immunogenic detoxified protein is provided which comprises the amino acid sequence of subunit A of an E. coli heat labile toxin (LT-A) or a fragment thereof in which at least amino acid Ala-72 of the A subunits mutated, preferably by substitution with Arg. The toxoid is useful as vaccine against an enterotoxigenic strain of E. coli and is produced by recombinant DNA means by site-directed mutagenesis. It is also an effective adjuvant.
Owner:CHIRON CORP
Insecticidal protein Cryl A. 301, expression vector and application thereof
ActiveCN102633868ARapid initial detection of toxicityPossesses anti-insect activityBiocideDepsipeptidesBiotechnologyEuropean corn borer
The invention provides an insecticidal protein Cryl A. 301 which has 1) an amino acid sequence shown by SEQ ID No.2; or 2) an amino acid sequence which is obtained by substituting the amino acid sequence shown by SEQ ID No.2 or decreasing and or increasing one or more amino acid(s) of the amino acid sequence shown by SEQ ID No.2, and has the same function. The experiment in vitro proves that the reformed and synthesized Bt gene toxin production protein has remarkable insecticidal effect for european corn borer. The insecticidal protein Cryl A. 301 can be efficiently expressed in monocotyledons, thus being used for producing insect-resistant transgenic plants.
Owner:INST OF CROP SCI CHINESE ACAD OF AGRI SCI +1
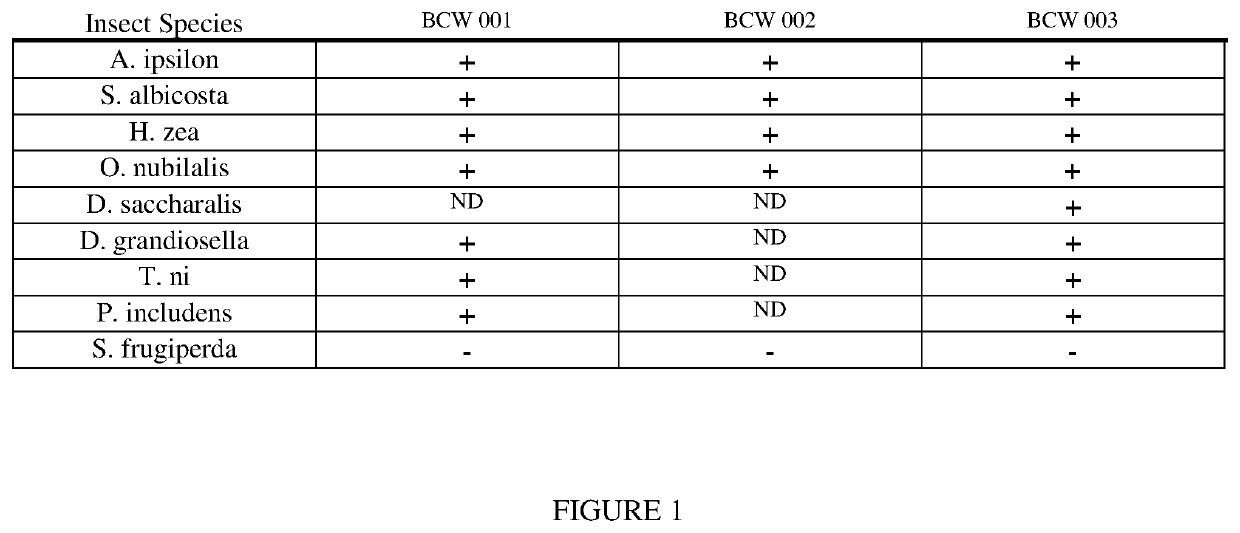
Pesticidal toxin proteins active against lepidopteran insects
Disclosed herein are nucleotide sequences encoding an insecticidal protein exhibiting Lepidopteran inhibitory activity, as well as novel insecticidal proteins referred to herein as a BCW 001, BCW 002, BCW 003, and BCW toxic protein-containing chimeras and BCW toxin insecticide, transgenic plants expressing the chimeras or the insecticide, and methods for detecting the presence of the nucleotide sequences or the insecticide in a biological sample.
Owner:MONSANTO TECH LLC
Method of determining bacillus thuringiensis toxic protein CrylAc
InactiveCN103175962AHigh sensitivityLow detection limitChemiluminescene/bioluminescenceAntigenSpecific immunity
The invention discloses a method of determining bacillus thuringiensis toxic protein CrylAc. The method comprises the steps that: Fe3O4@Au nana-particles combining with a transgenic Bt toxic protein CrylAc primary antibody is modified onto the surface of a magnetic control glassy carbon electrode, and specific immune reaction is carried out on the magnetic control glassy carbon electrode with an antigen and a GOD labeled secondary antibody to form a compound with a sandwich structure; an electrogenerated chemiluminescence immunosensor is developed to be used for detecting the concentration of transgenic Bt toxic protein CrylAc; glucose is catalyzed and oxidized by using GOD to generate H2O2; voltage is applied to a working electrode to excite luminal to be oxidized; a luminal oxide and the H2O2 react to generate an electrochemiluminescence signal; an electrochemiluminescence response is strengthened along with the increase of the concentration of the GOD; and the electrochemiluminescence signal has a linear relation with the concentration of the transgenic Bt toxic protein CrylAc within a range of 0-6ng / mL. The method has high sensitivity and low detection limit, is widely applied and supplies a wide application prospect of reliably and ultra-sensitively detecting the toxic protein of a transgenic plant.
Owner:GUILIN UNIVERSITY OF TECHNOLOGY
Protein vaccines against poxviruses
InactiveUS20110081368A1Effective protectionEasy to optimizeViral antigen ingredientsPharmaceutical delivery mechanismOpen reading frameVirus Protein
The invention described here entails a protein vaccine against poxviruses which contains at least two purified recombinant monkeypox virus proteins or peptides. The proteins or peptides are encoded by the open reading frames of the monkeypox ortholog genes M1R, A35R, A29L B6R, and orthologs of these proteins or peptides having 90% identity. The invention also entails a vaccine protocol against poxvirus whereby a vaccine is vaccinated with a first vaccine made up of a nucleic acid vaccine of three or more poxvirus virus genes, and subsequently vaccinated with at least one other booster vaccine made up of two or more poxvirus virus proteins.
Owner:HOOPER JAY W +1
Immunogenic detoxified mutants of cholera toxin
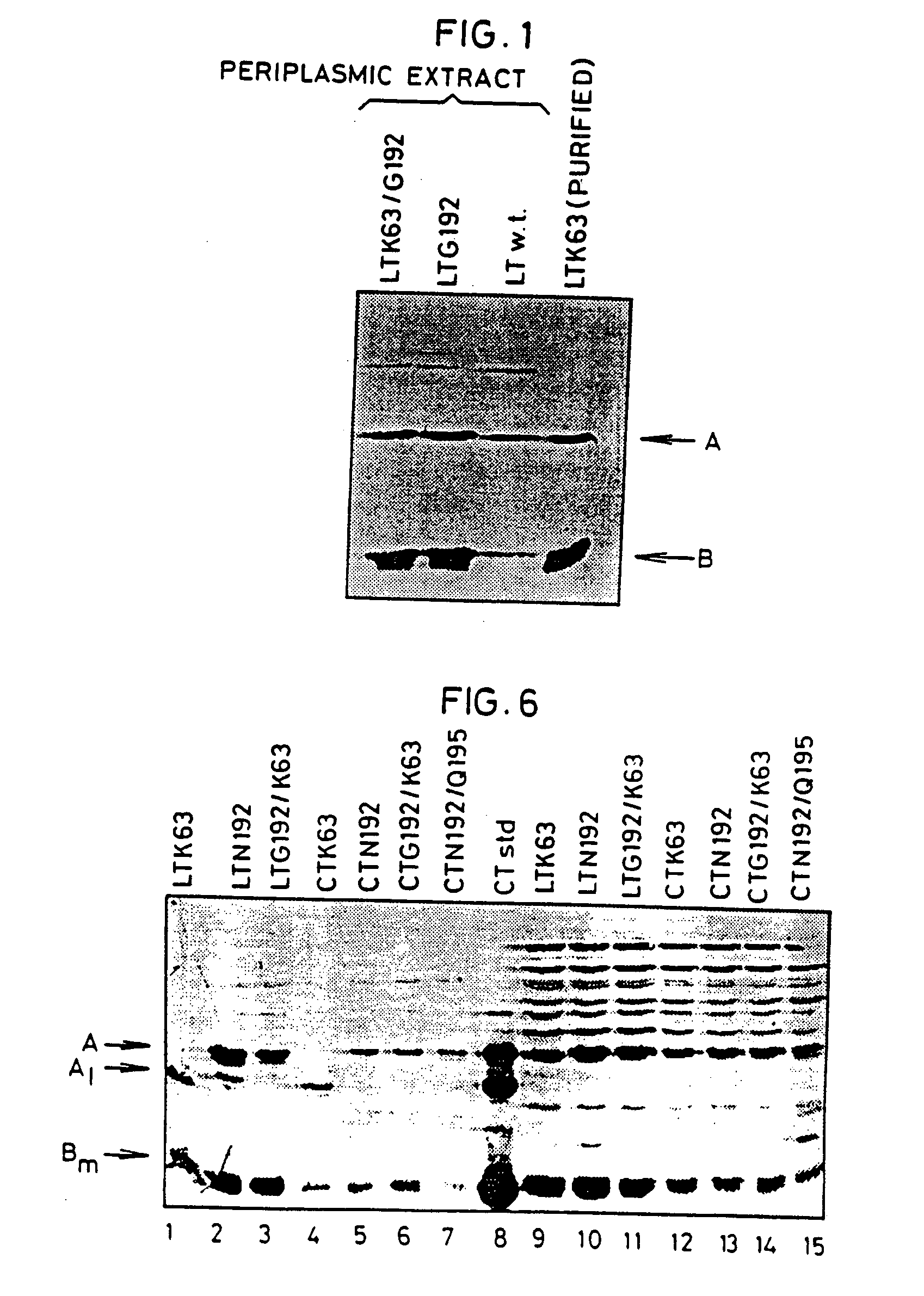
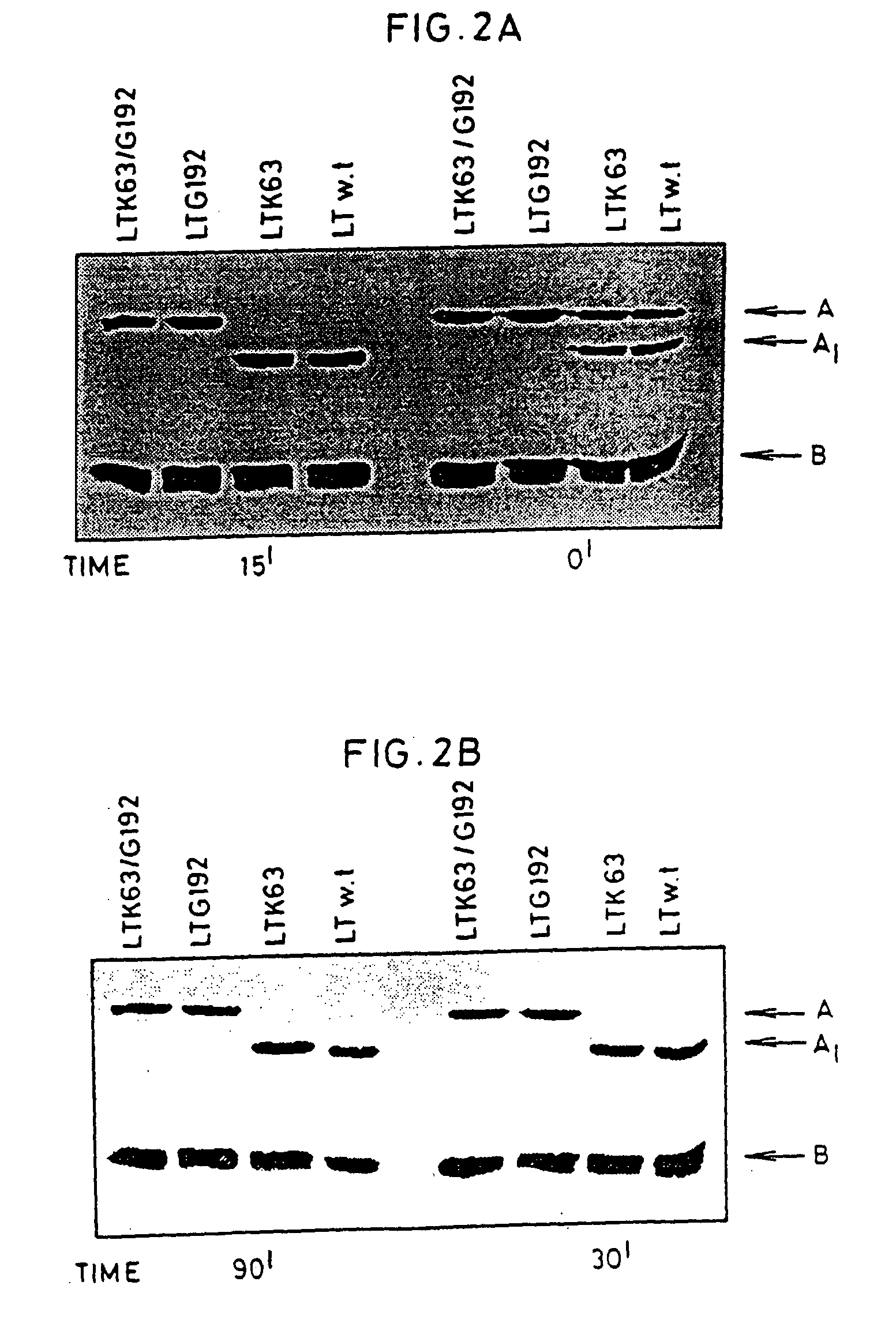
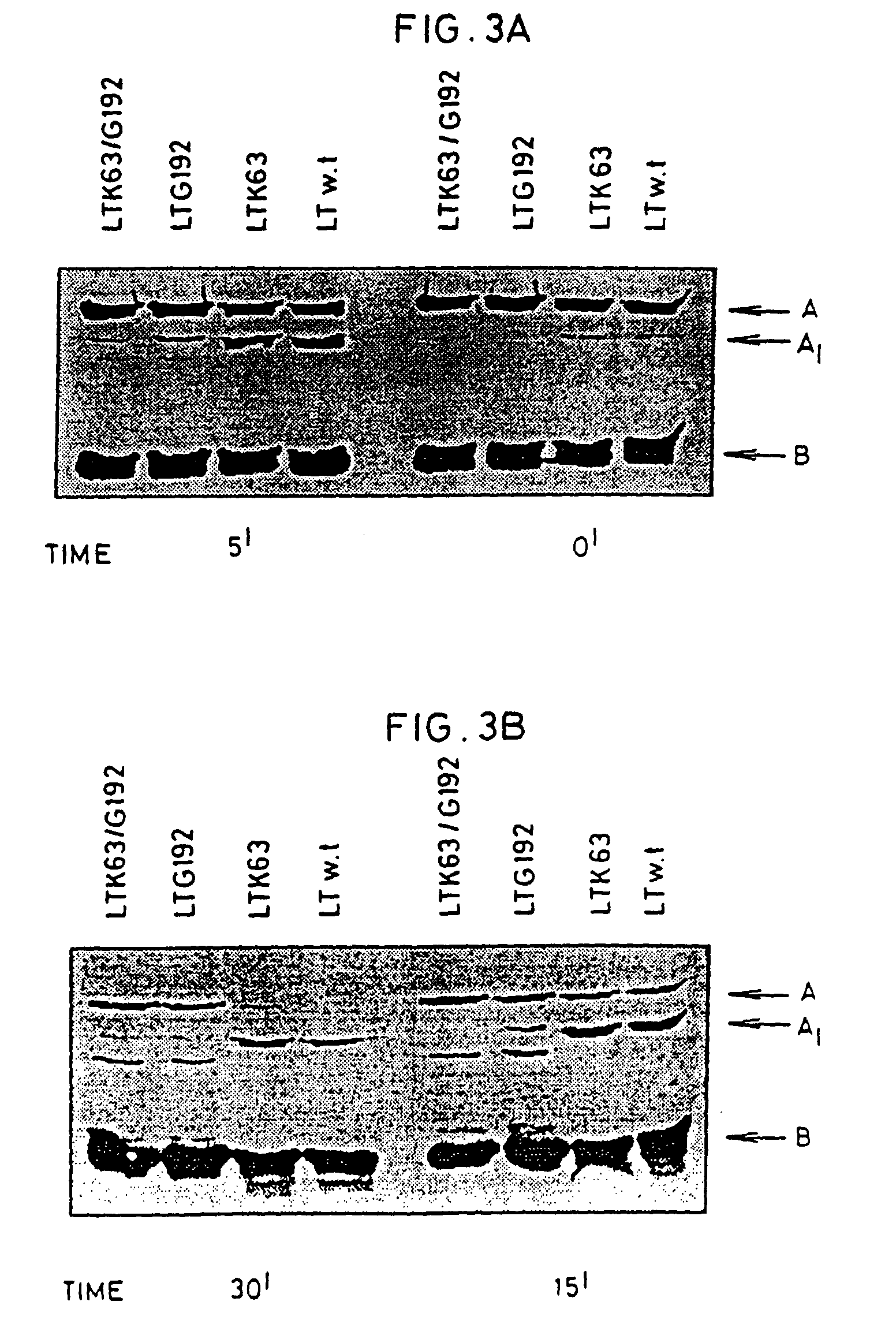
An immunogenic detoxified protein comprising the amino acid sequence of subunit A of a cholera toxin (CT-A) or a fragment thereof or the amino acid sequence of subunit A of an Escherichia coli heat labile toxin (LT-A) or a fragment thereof wherein the amino acids at, or in positions corresponding to Ser-63 and Arg-192 are replaced with another amino acid. The immunogenic detoxified protein is useful as vaccine for Vibrio cholerae or an enterotoxigenic strain of Escherichia coli and is produced by recombinant DNA means by site-directed mutagenesis.
Owner:CHIRON CORP
Methods and compositions involving protective staphylococcal antigens
ActiveUS9095540B2Prevents alleviates ameliorates symptomImprove survivalAntibacterial agentsBacterial antigen ingredientsAntigenMicrobiology
The present invention concerns methods and compositions for treating or preventing a bacterial infection, particularly infection by a Staphylococcus bacterium. The invention provides methods and compositions for stimulating an immune response against the bacteria. In certain embodiments, the methods and compositions involve a non-toxigenic Protein A (SpA) variant. In some embodiments, the methods and compositions involve SdrD, ClfA, and / or FnbpB polypeptides.
Owner:UNIVERSITY OF CHICAGO
Compound, protein, preparation method of compound, and preparation method of protein
InactiveCN107033247AStrong stabilityHigh purityMicroorganism based processesFermentationLong actingDrug
The invention provides a compound, a protein, a preparation method of the compound, and a preparation method of the protein, and relates to the technical field of biomedicines. The compound has high stability, can be orally administered on the basis of the long-acting blood sugar lowering function of glucagon-like peptide-1, lays a foundation for the further development of diabetes related drugs, and deserves subsequent continuous development. The preparation method of the protein is characterized in that physical deoxidation is carried out at 75-85 DEG C, so the purity of the protein is greatly increased; and the protein is prepared through using the preparation method of the protein, and the purity reaches 80% or above. A protein purification technology provided by the present invention can remove the heat toxin proteins and nucleic acid substances introduced by yeast fermentation, and brings convenience for the researches on the safety and the quality of the protein used as a medicinal preparation in the later application stage.
Owner:天津博锐生物科技有限公司
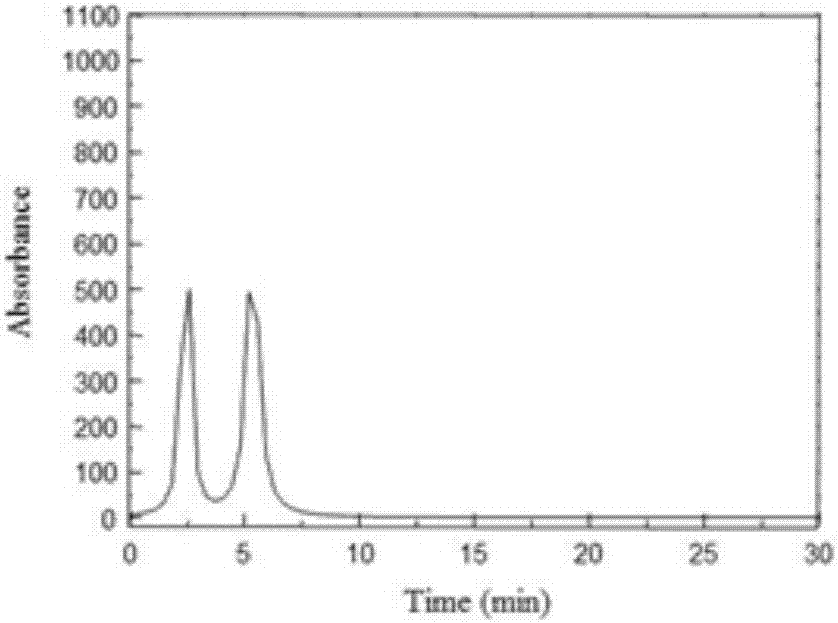
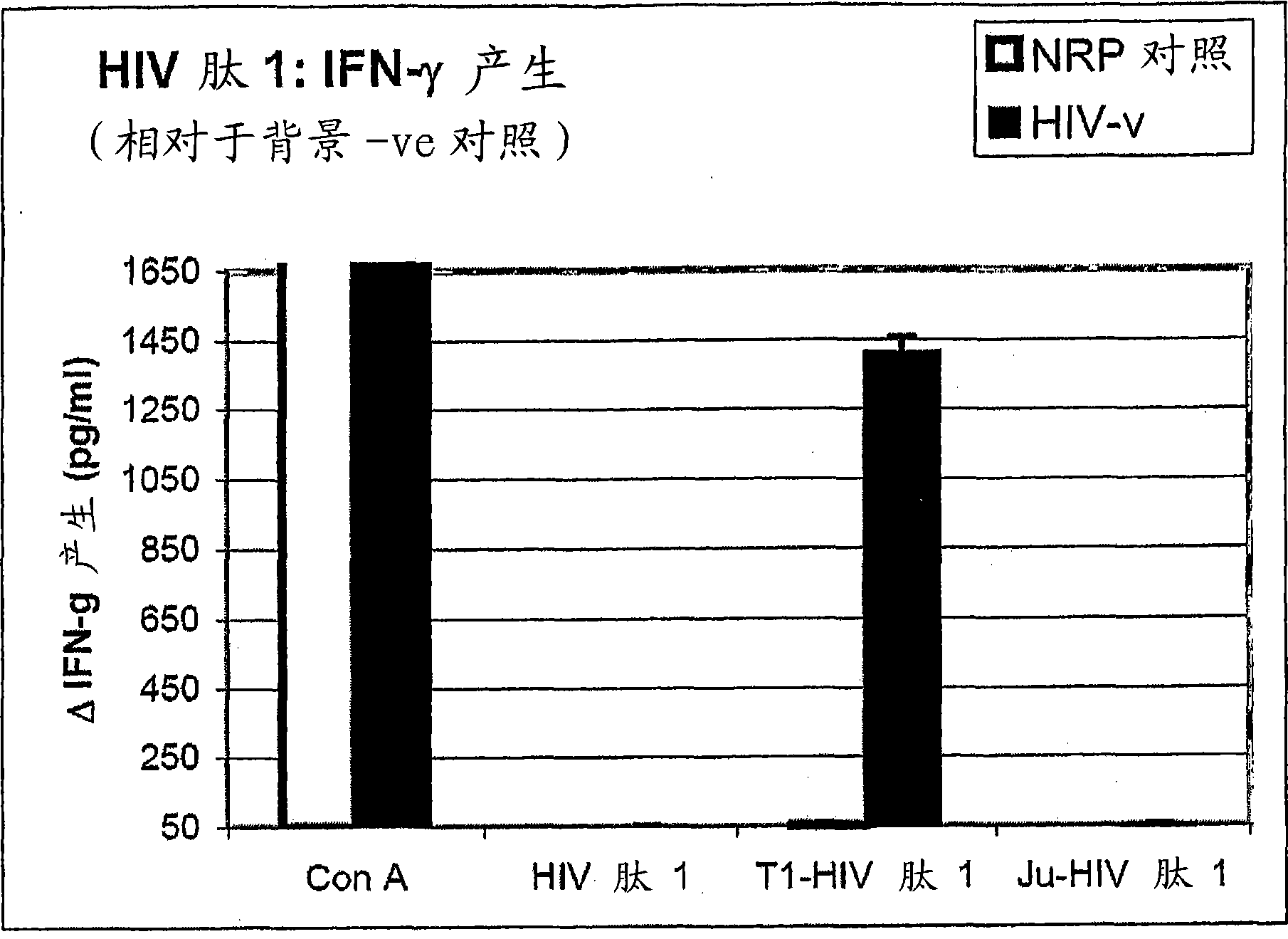
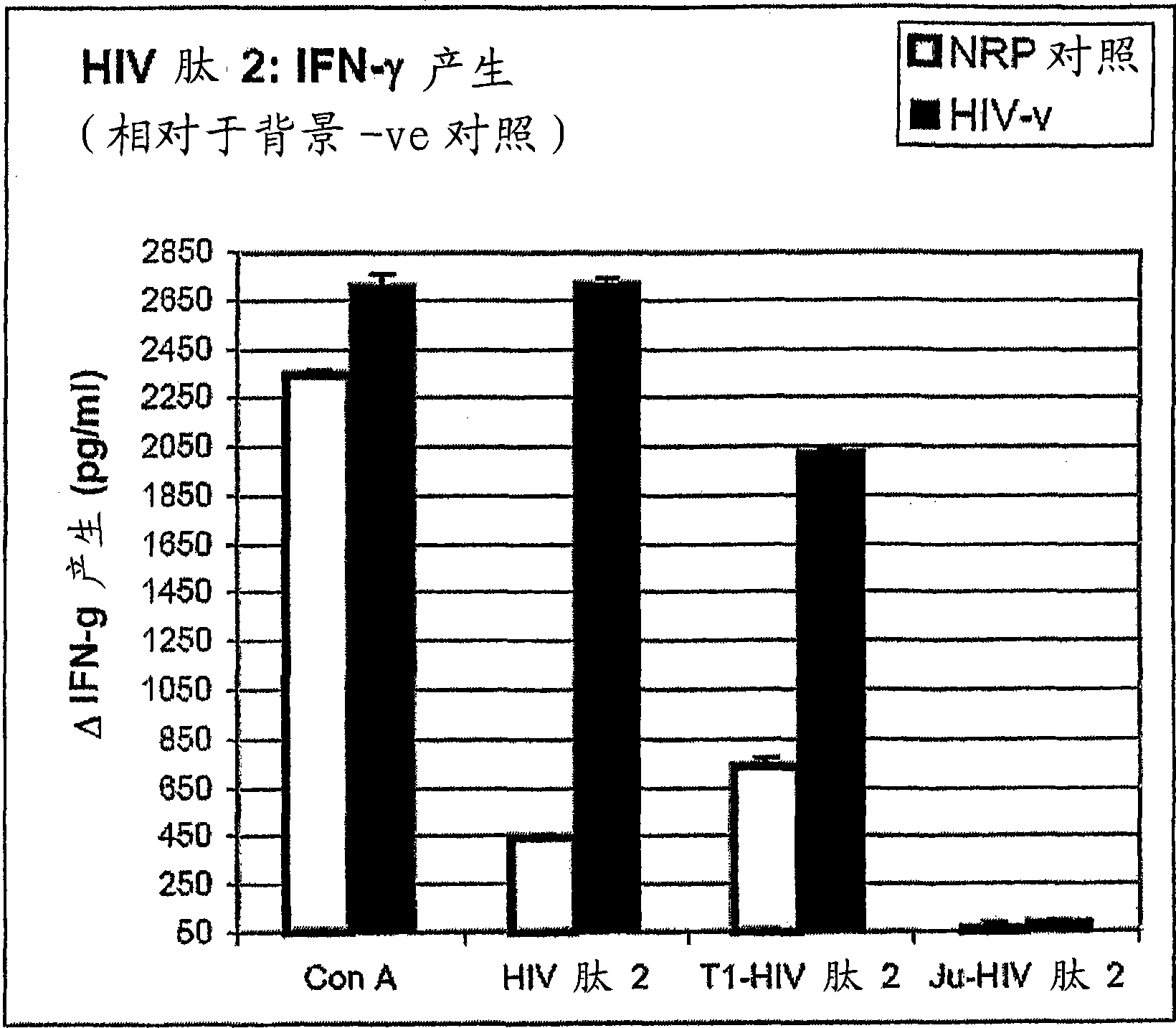
Peptides of regulatory or accessory proteins of HIV, compositions and the utilization thereof
Provided is a polypeptide having no more than 100 amino acids, which polypeptide comprises one or more sequences having at least 60% homology with any of SEQ ID 1-4, or comprises two or more epitopes having 7 amino acids or more, each epitope having at least 60% homology with a sub-sequence of any of SEQ ID 1-4 that has the same length as the epitope: SEQ ID 1 GDTWAGVEAIIRILQQLLFIHFRIGCQHSR; SEQ ID 2 KVGSLQYLALTALITPKKIKPPLPSVKKLTEDRWNKPQKT; SEQ TD 3 EPVPLQLPPLERLTLDCSEDCGTSGTQ; SEQ ID 4 YKGALDLSHFLKEKGGLEGLIYSQKRQDILDLWVYHTQGYFPD wherein, the polypeptide is immunogenic in a vertebrate expressing a major histocompatibility complex (MHC) allele, and wherein the polypeptide is not a complete HIV virus protein.
Owner:PEPTCELL
Method for monitoring tetanus toxoid or diphtheria toxoid
ActiveCN111855826ASolve the problem of lack of monitoring methods for the processQuality improvementComponent separationTetanus toxoidsTarget peptide
The invention discloses a method for monitoring tetanus toxoid or diphtheria toxoid. The method comprises the following steps of: (1) carrying out enzymolysis of a standard substance; (2) carrying outsolid-phase extraction to enrich a target peptide fragment; (3) performing high performance liquid chromatography tandem mass spectrometry analysis; (4) drawing a standard characteristic peptide fragment table to obtain relative response intensity of each characteristic peptide fragment of a standard variety; (5) detecting a sample, and calculating to obtain the relative response intensity and arecovery rate of each characteristic peptide fragment in the sample; and (6) evaluating the detoxification effect according to the number of peptide fragments with good characteristic peptide fragmentrecovery rate. According to the monitoring method of the invention, the method for quantitatively monitoring the process stability of tetanus toxoid and diphtheria toxoid is implemented for the firsttime, the problem that a formaldehyde detoxification protein process lacks a monitoring method is solved, effective quantitative data is provided for production optimization of tetanus vaccine, tetanus-diphtheria bivalent vaccine, pertussis-diphtheria-tetanus triple vaccine, pentavaccine and proteoglycan protein conjugate vaccine and research and development of novel proteoglycan protein conjugate vaccine, and the vaccine quality improvement process and new product research and development are accelerated.
Owner:SHIMADZU (CHINA) CO LTD +1
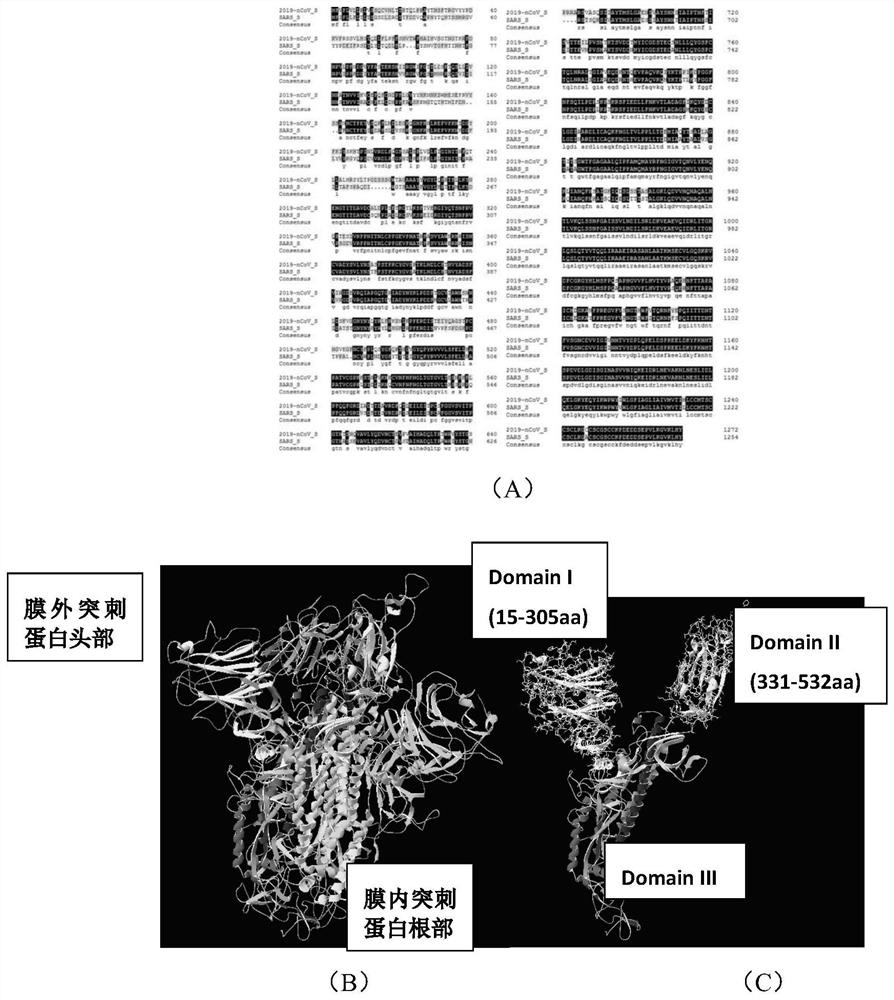
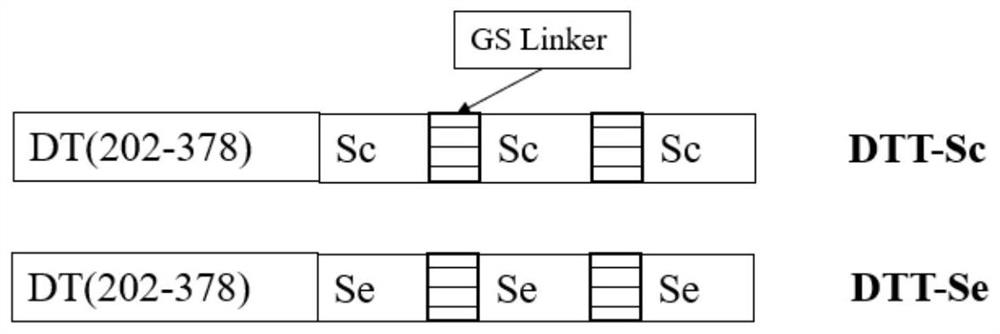
Recombinant protein highly targeting SARS-ConV-2 virus S protein extracellular BD-terminal domain and subunit vaccine thereof
ActiveCN113151331AImprove immunity against stressImprove developmentAntibacterial agentsSsRNA viruses positive-senseAdjuvantImmunity response
The invention provides a recombinant protein highly targeting SARS-ConV-2 virus S protein extracellular BD-terminal domain and a subunit vaccine thereof. A nucleotide sequence for coding the recombinant protein is as shown in SEQ ID NO: 5 or SEQ ID NO: 6. The novel coronavirus subunit vaccine comprises the recombinant protein and a pharmaceutically acceptable adjuvants. According to the invention, the recombinant protein highly targeting SARS-ConV-2 virus S protein extracellular BD-terminal domain is prepared by the following steps: screening S protein extracellular domain surface antigenic determinants to find Sc and Se peptide fragment gene sequences capable of stimulating cells to generate strong immune response, carrying out codon optimization, cloning into a plasmid vector containing a diphtheria toxin T structure, and carrying out secretory expression and purification. Experiments show that the purified recombinant protein can induce specific cell reaction, is safe and efficient, and has good development and application prospects.
Owner:WUHAN UNIV
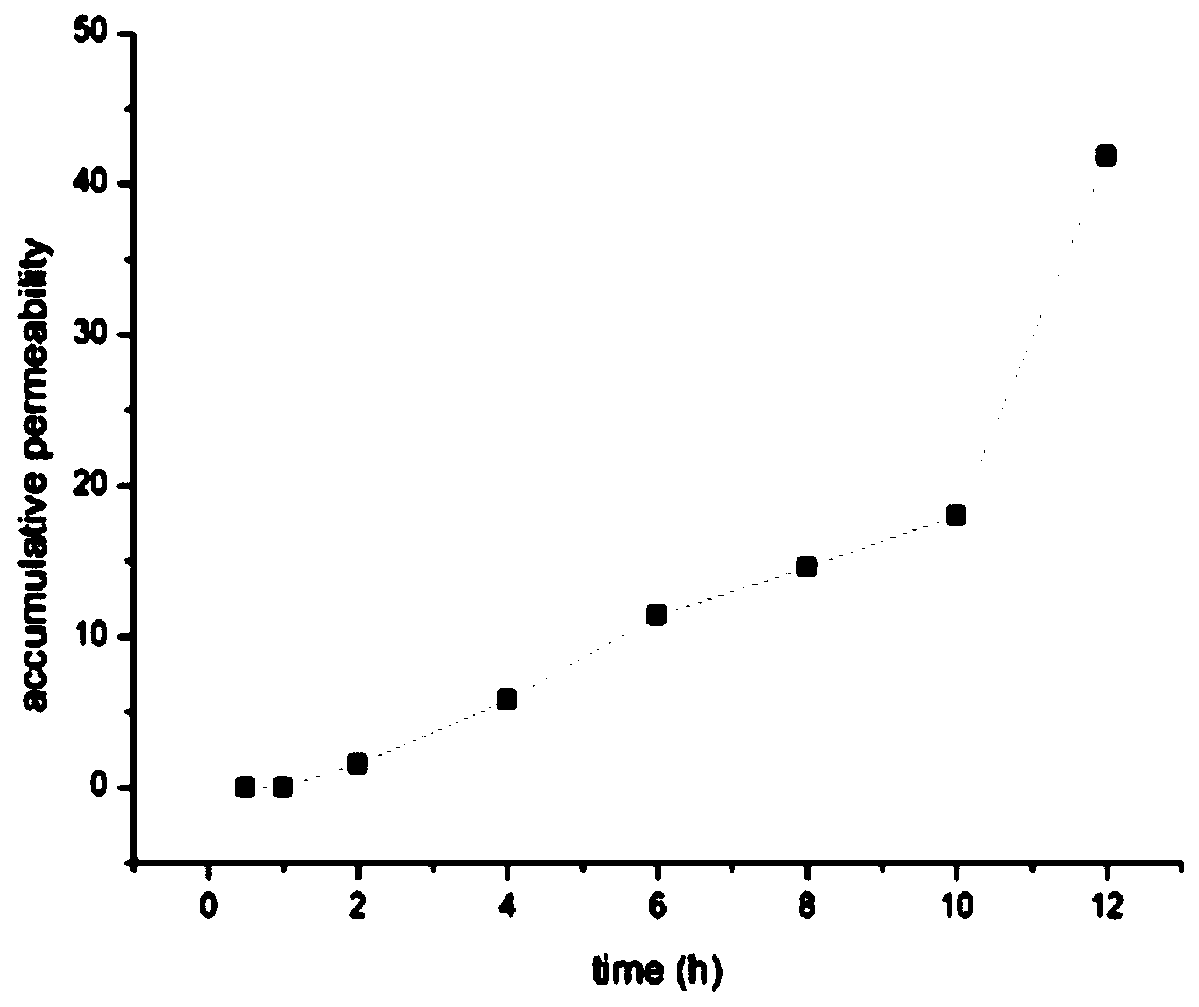
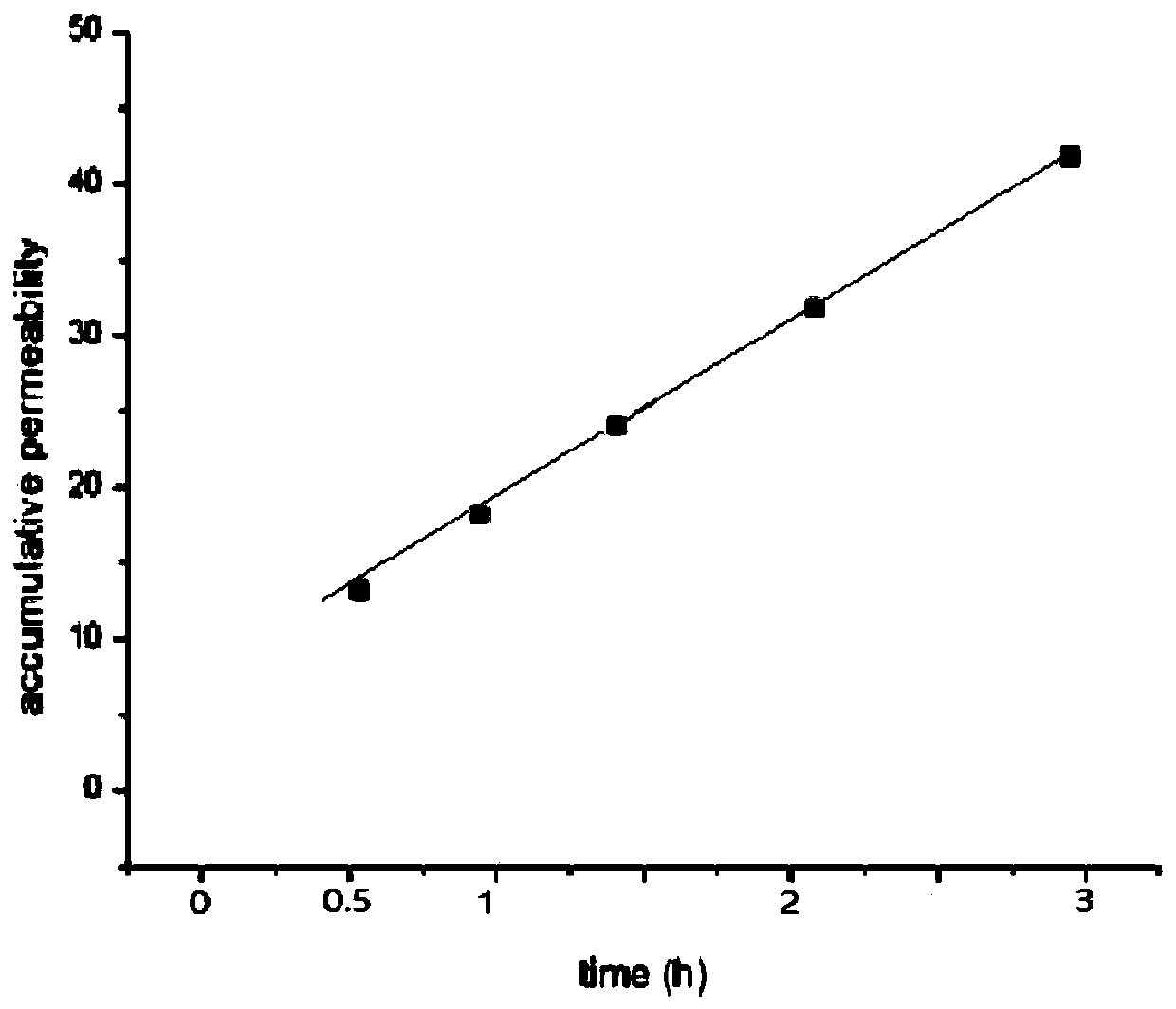
Venom protein gel and preparation method thereof
PendingCN110201146APromote dissolutionHigh viscosityPeptide/protein ingredientsAntipyreticSodium bicarbonateDrug release
The invention discloses a venom protein gel. The venom protein gel is characterized by comprising venom protein dry powder, a gel matrix, a transdermal absorption promoter, a protein protective agent,a pH regulator, a solvent and other pharmaceutically acceptable auxiliary materials; the gel matrix is carbomer 940; the transdermal absorption promoter is essential oil; the protein protective agentis mannitol; and the pH regulator is a compound of triethanolamine and sodium bicarbonate. The venom protein gel has the characteristic of zero-order drug release.
Owner:NANJING ANGGU PHARMA TECH
Ochratoxin detoxification protein, encoding gene thereof and application
InactiveCN106349349AHas detoxification activitySignificant detoxification activityBacteriaMicroorganism based processesBiotechnologyMicroorganism
The invention relates to a microorganism protein and a gene, and particularly discloses an ochratoxin detoxification protein and an encoding gene of the ochratoxin detoxification protein. The amino acid sequence of the protein is shown as SEQ ID NO.1, and the encoding gene sequence is shown as SEQ ID NO.2. The recombinant bacteria structured by gene sequence can express the protein with ochratoxin detoxification activity.
Owner:CHINA AGRI UNIV
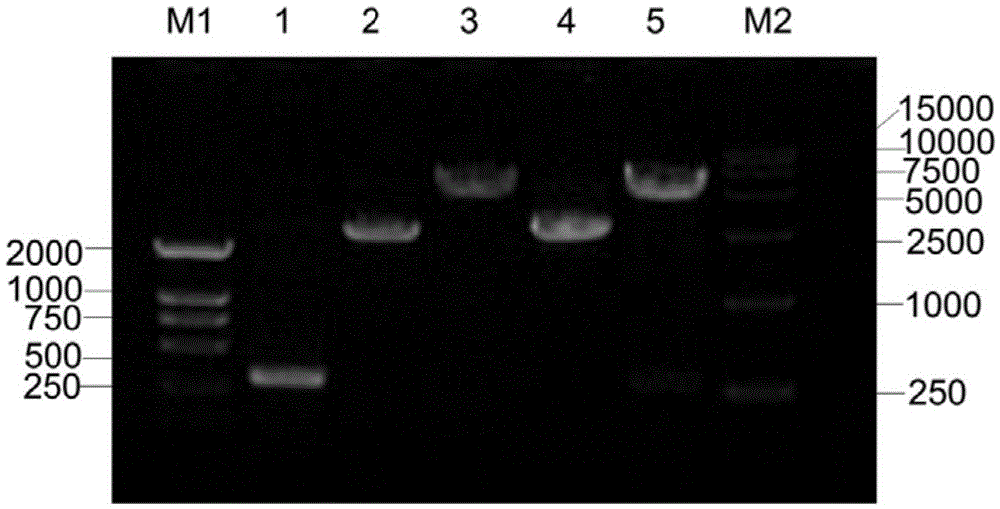
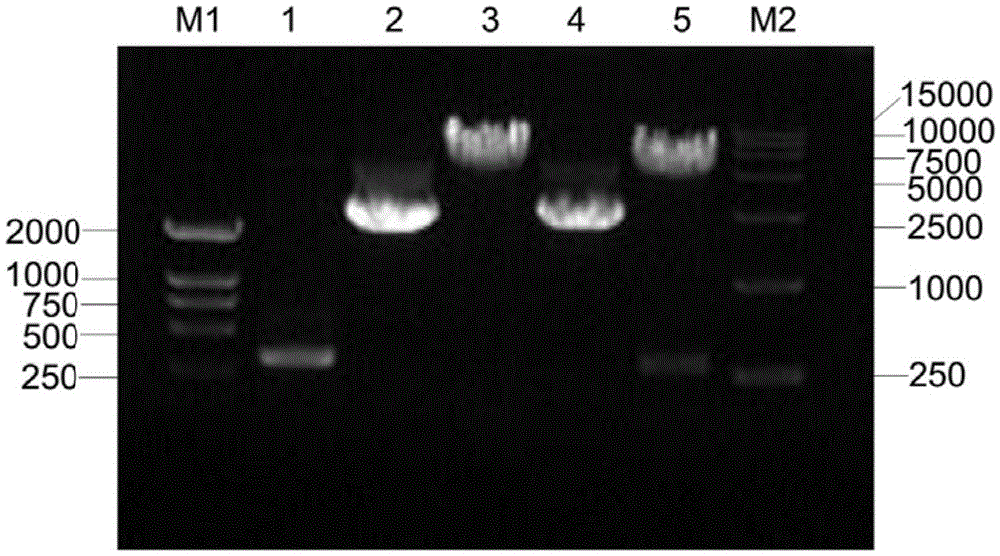
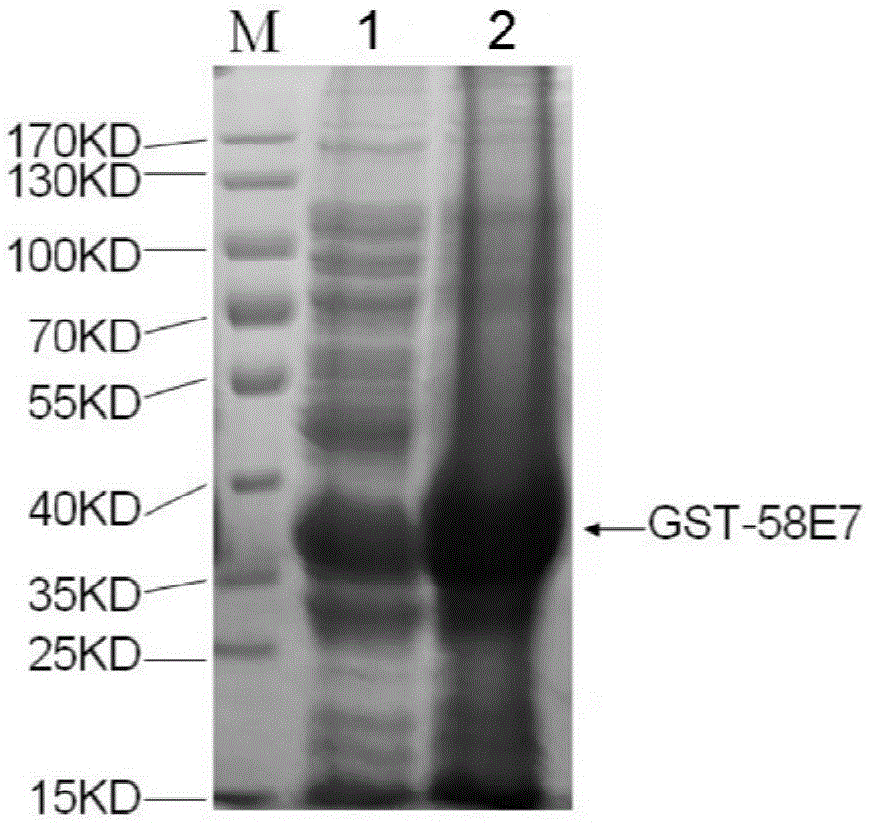
Human papilloma virus 58E7 protein expression and purification method and application
InactiveCN105646677AImprove accuracyVirus peptidesImmunoglobulins against virusesPurification methodsMagnetic bead
The invention provides a human papilloma virus (HPV) 58E7 protein expression and purification method. The method includes: designing an amplification primer of the HPV-58E7, and effectively amplifying genes from HPV-58 positive cells; inserting a HPV-58E7 gene sequence containing different enzyme cutting sites into vectors pGEX-4T2 and pEGFP-C1 according to a sequence to obtain a recombinant vector; combining the recombinant vector with Glutathione-Sepharose 4B magnetic beads, cutting a GST tag to obtain HPV-58E7 protein, and subjecting the HPV-58E7 to PBC dialysis overnight purification. Through the HPV-58E7 protein obtained by the method, blood serum can be obtained through immune animals, and polyclonal antibodies with high specificity and high potency ratio can be obtained through purification; the HPV-58E7 protein can be applied in preparing the polyclonal antibodies.
Owner:ZHEJIANG UNIV
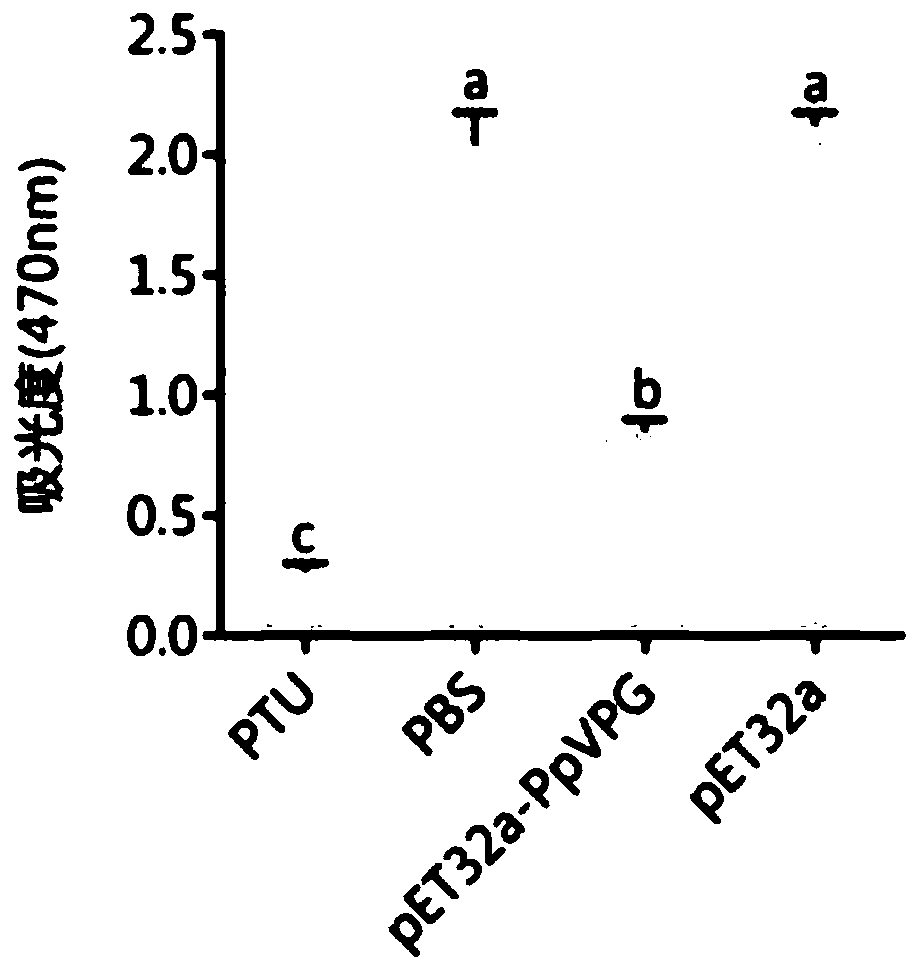
Pteromalus puparum venom protein PpVPG and application thereof
ActiveCN110590925AExtend your lifePolypeptide with localisation/targeting motifGenetic engineeringVenom glandNucleic acid sequencing
The invention relates to the fields of molecular biology, gene engineering and protein engineering in particular to a protein PpVPG expressed in pteromalus puparum venom, and a coded nucleic acid sequence and application thereof. The invention discloses the pteromalus puparum venom protein PpVPG and a gene encoding the pteromalus puparum venom protein PpVPG. The pteromalus puparum venom protein PpVPG can be used for preparing venom protein PpVPG secreted by pteromalus puparum venom glands, and the protein can be used for inhibiting activation of hemolymph PPO of pieris rapae or papilio xuthuslinnaeus.
Owner:ZHEJIANG UNIV
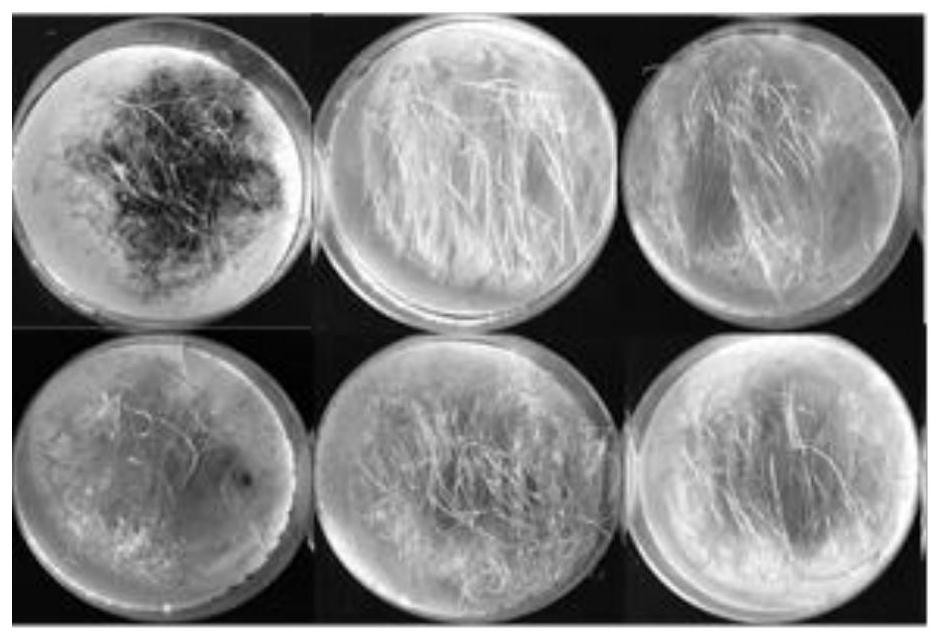
Artificially synthesized insect-resistant protein mCry1Ia2 as well as preparation method and applications thereof
ActiveCN111995690AHigh expressionEfficient and stable expressionBiocideBacteriaBiotechnologyToxin protein
The invention discloses artificially synthesized insect-resistant protein mCry1Ia2. The invention also discloses a nucleic acid molecule of the protein, and a recombinant vector, a host cell or a recombinant bacterium of the nucleic acid molecule. The invention also discloses applications of the protein, the nucleic acid molecule and the recombinant vector of the nucleic acid molecule, the host cell or the recombinant bacteria to regulation and control of plant insect resistance, insect killing or improvement in insect resistance of transgenic plants, and preparation of insecticides or insectcontrol preparations. In vitro experiments show that the modified Bt toxin-producing protein has higher insecticidal activity against ostrinia nubilalis, and has significant insecticidal effect on thepopulation of ostrinia nubilalis resistant to Cry1Ab, Cry1F and Cry1Ie toxin protein. A novel plant expression vector is constructed by a constitutive promoter, and the insect-resistant gene mCry1Ia2can be stably and efficiently expressed in monocotyledonous plants to be further used for producing the insect-resistant transgenic plants.
Owner:黑龙江大鹏农业有限公司 +1
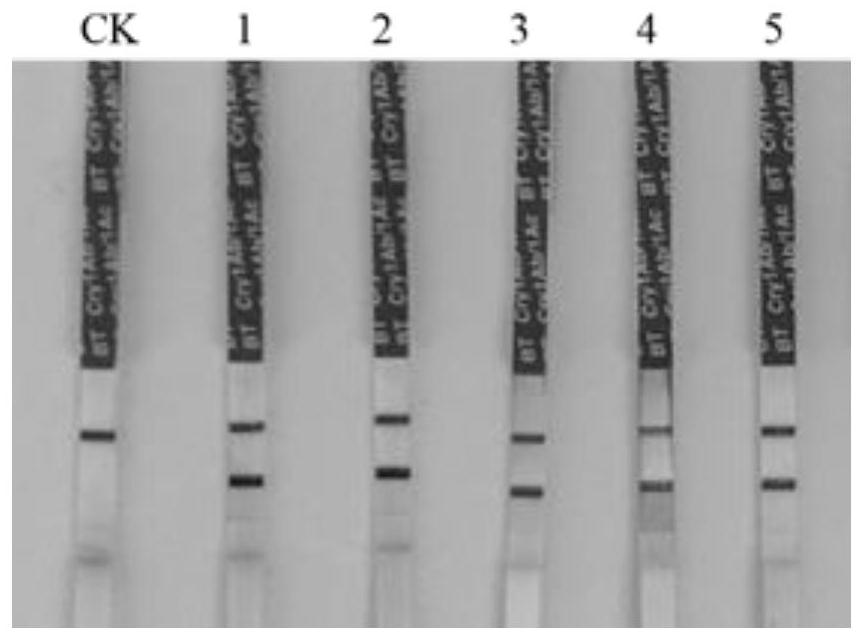
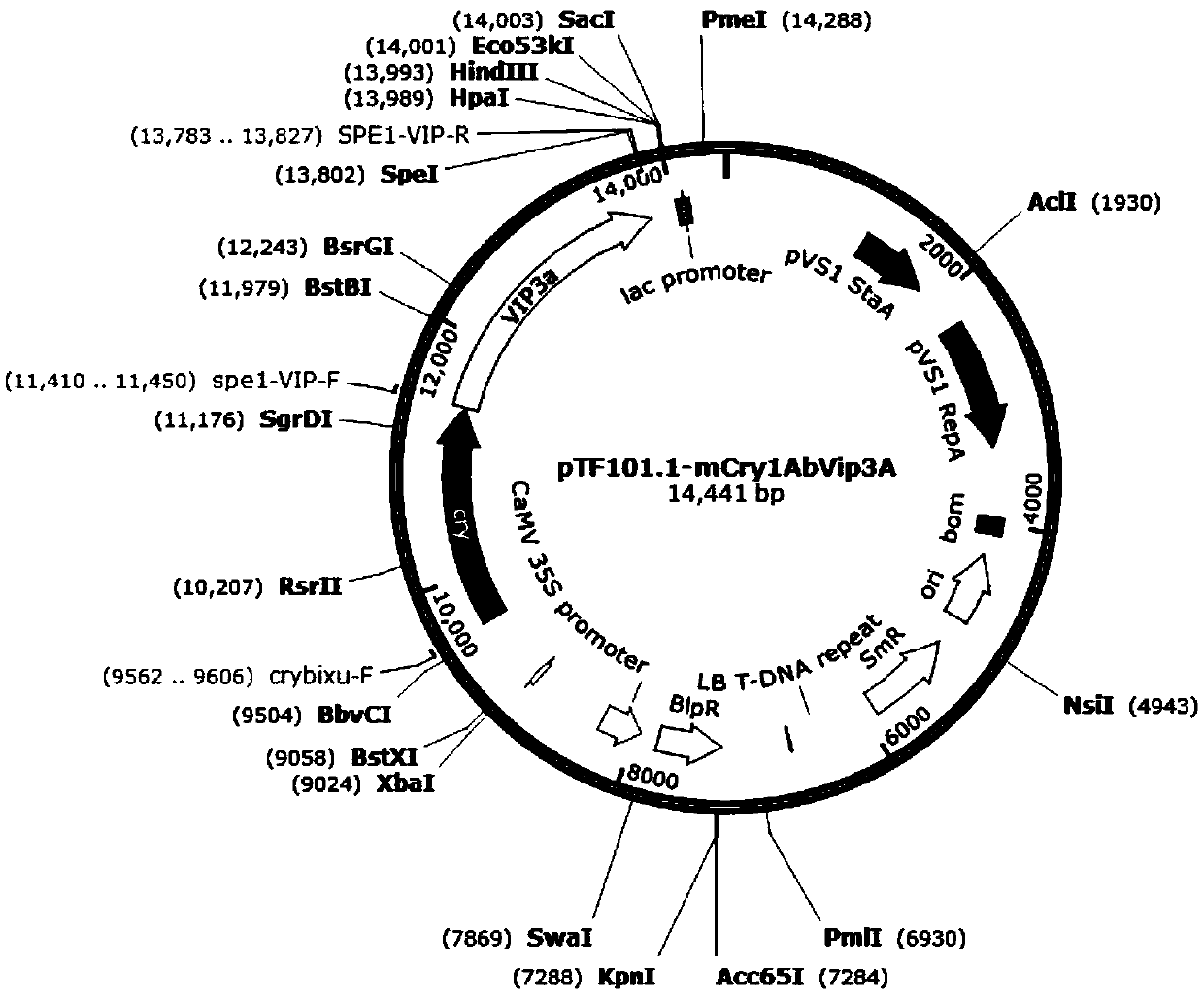
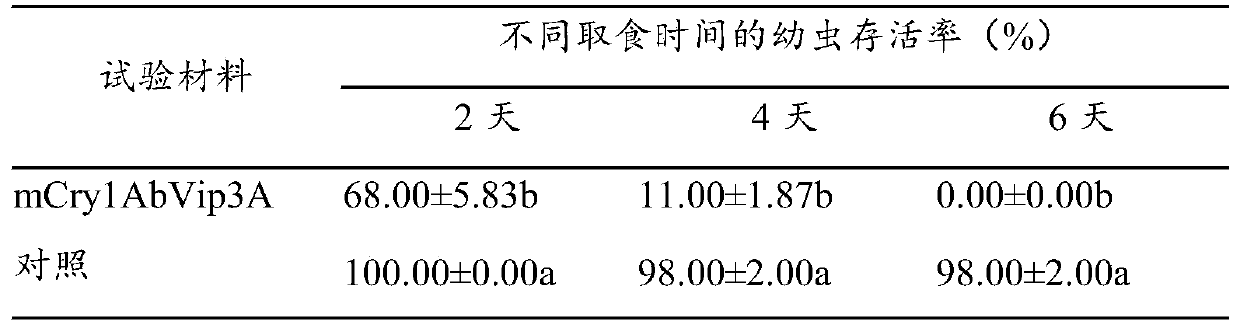
Insect-resistant fusion gene mCry1AbVip3A, and expression vector and application thereof
InactiveCN111440814APossesses anti-insect activityReduce usageBacteriaAntibody mimetics/scaffoldsBiotechnologyNucleotide
The invention discloses an insect-resistant fusion gene mCry1AbVip3A. The nucleotide sequence of the gene mCry1AbVip3A is as follows: (a) nucleotide as shown in SEQ ID NO:1; or (b) a nucleotide encoding an amino acid represented by SEQ ID NO:2; or (c) a nucleotide which can be hybridized with a complementary sequence of SEQ ID NO:1 under strict hybridization conditions, wherein the protein coded by the nucleotide has a function of an mCry1AbVip3A transcription factor. In-vitro toxicity determination tests prove that the modified and synthesized fusion gene toxin-producing protein has a remarkable insecticidal effect on ostrinia nubilalis and spodoptera frugiperda, can be stably and efficiently expressed in monocotyledons, and is further used for producing insect-resistant transgenic plants.
Owner:INST OF CROP SCI CHINESE ACAD OF AGRI SCI +1
Snake venom protein component of ahylysantinfarctase at southern Anhui Province, extraction method and uses thereof
InactiveCN101367871AAnti-tumorImprove immune system functionPeptide/protein ingredientsPeptide preparation methodsMolecular sieveAgkistrodon acutus
The present invention relates to an agkistrodon acutus venom protein component and an extraction method and applications thereof. The agkistrodon acutus venom protein component is composed of venom proteins, the molecular weights of which are respectively 25KDa, 30KDa and 50KDa. The extraction method includes the following steps: (a) anion chromatography; (b) cation chromatography; (c) molecular sieve chromatography. Compared with the prior art, the extraction method utilizes the anion chromatography, the cation chromatography and the molecular sieve chromatography to extract a new anti-coagulating active component from agkistrodon acutus venom, and the prepared component has an anti-tumor effect and can improve the immune system.
Owner:芮景
Construction of high-copy and high-expression recombinant plasmid and its application in exogenous gene expression
ActiveCN108823228BIncrease productionEfficient replicationFermentationVector-based foreign material introductionAmpicillinGenetic engineering
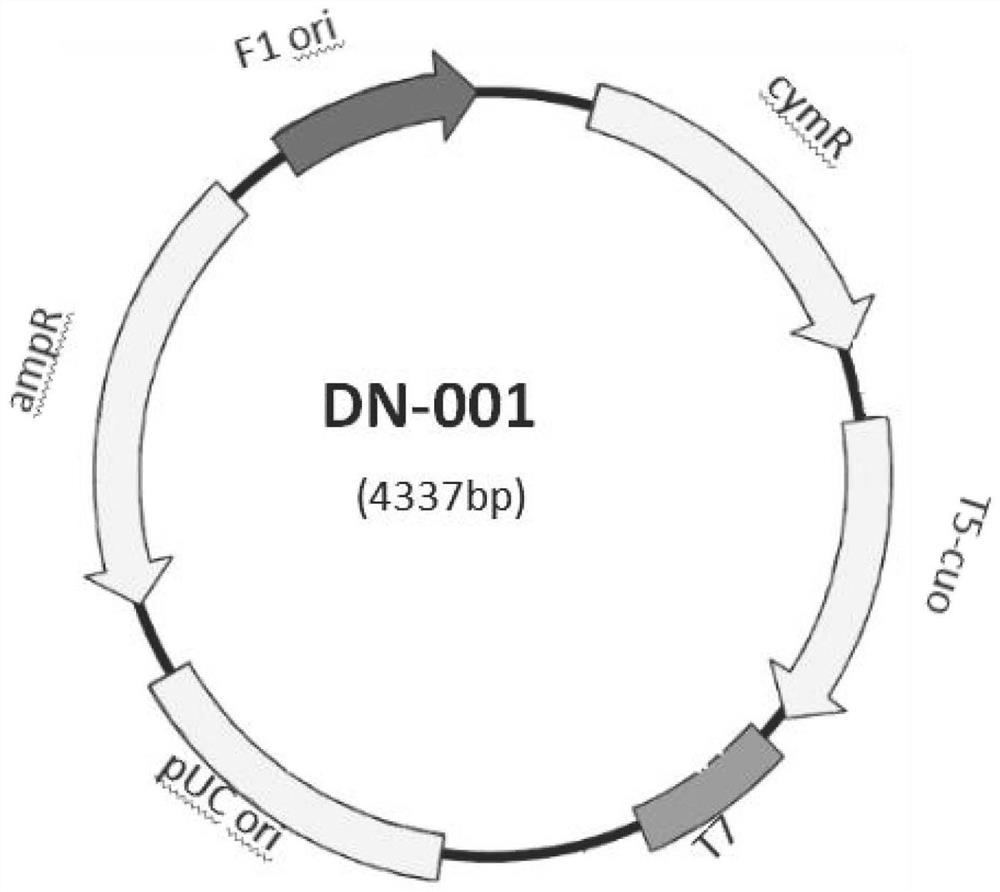
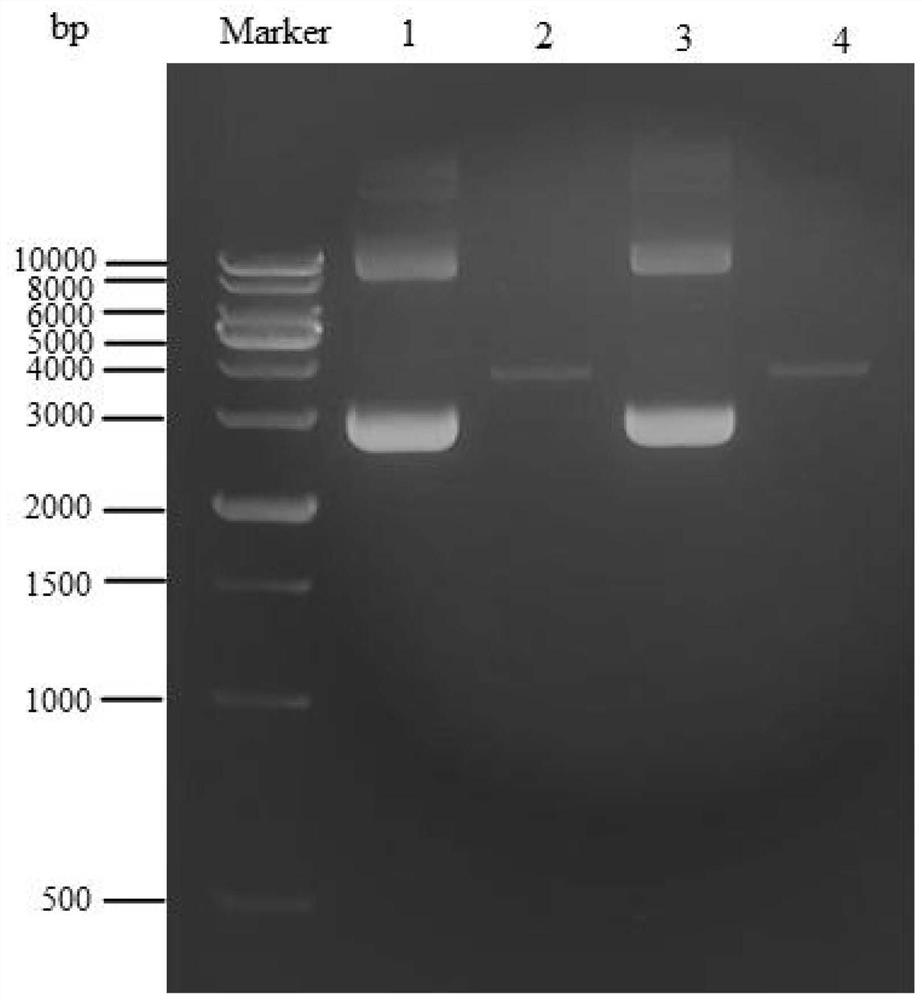
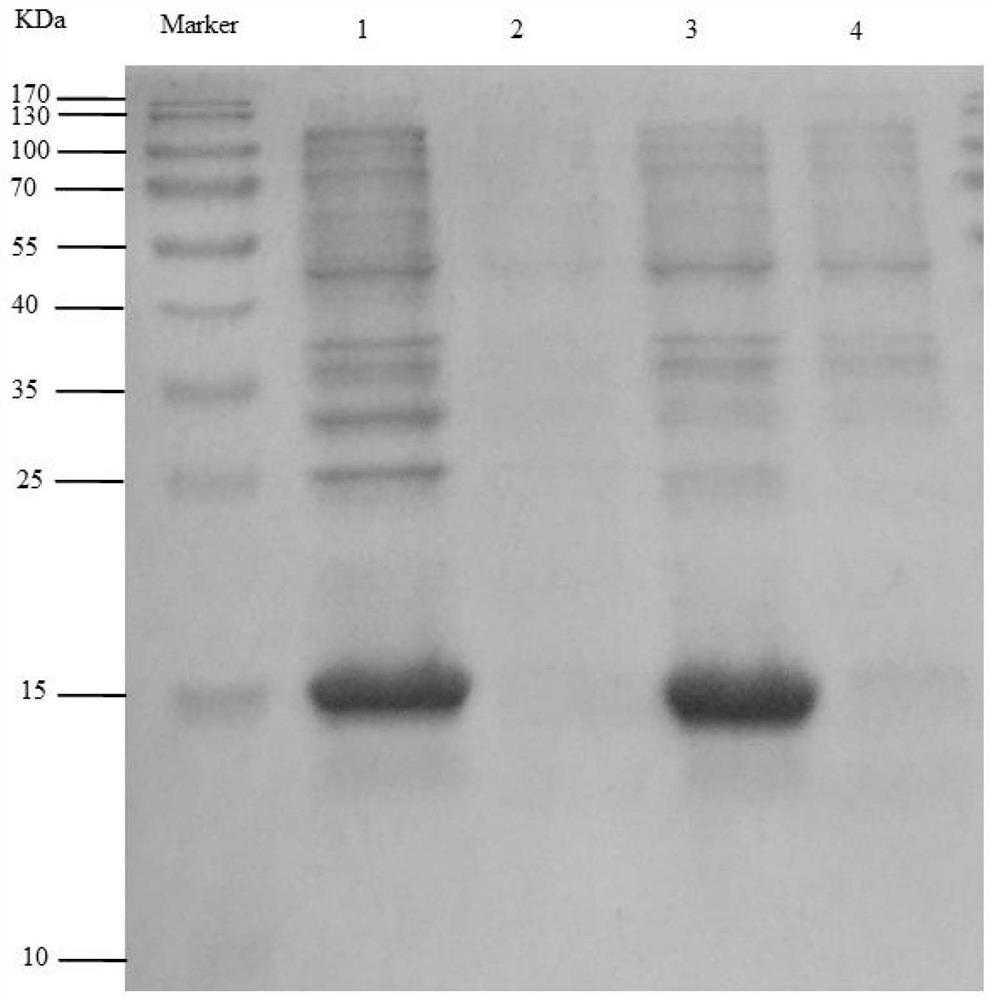
The invention discloses the construction of a high-copy and high-expression recombinant plasmid and its application in exogenous gene expression, and relates to the technical field of genetic engineering. In the present invention, the pcDNA3.1(-) plasmid is used as the backbone, and the cumate-cymR inducible expression regulatory unit is loaded between the pUC ori high-copy replicon and the Ampicillin resistance gene, and at the same time, the multiple cloning site downstream of the pET-28a T7 promoter Point, 6xHis coding sequence and T7 transcription termination sequence were inserted downstream of the cumate-cymR regulatory unit to construct a recombinant plasmid DN-001 with high replication and high expression. The recombinant plasmid DN‑001 of the present invention has the advantages of being the most economical, the most convenient, and high yield, making the recombination of gene expression vectors time-saving and labor-saving; it can be applied to the production of large doses of recombinant proteins; at the same time, it effectively suppresses leaky expression, making This vector is especially suitable for the expression of toxic proteins, which makes this vector have broad market prospects.
Owner:安徽朵能生物科技有限公司
Methods for developing virus protein specific capture agents, capture agents, and methods of using the capture agents
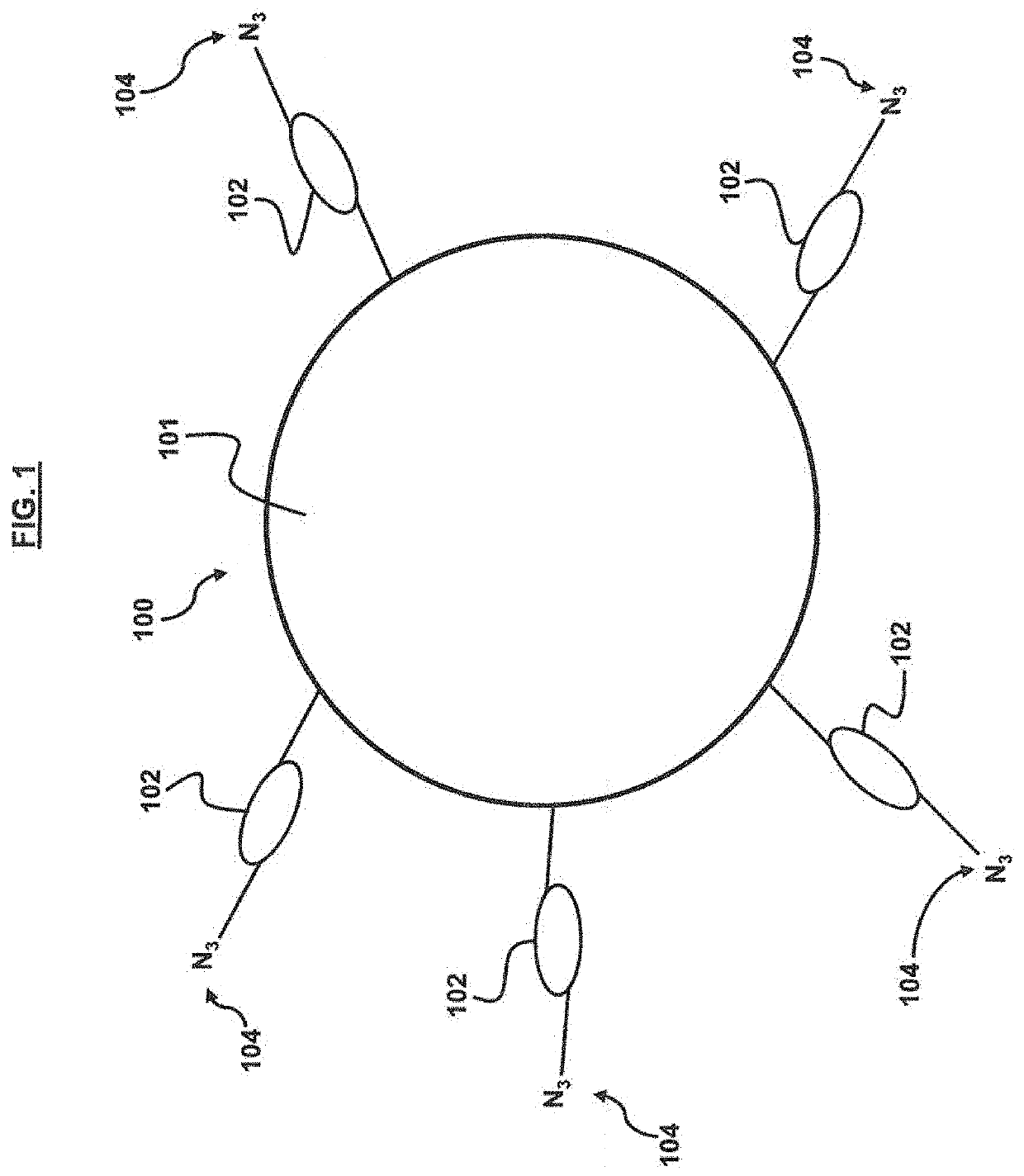
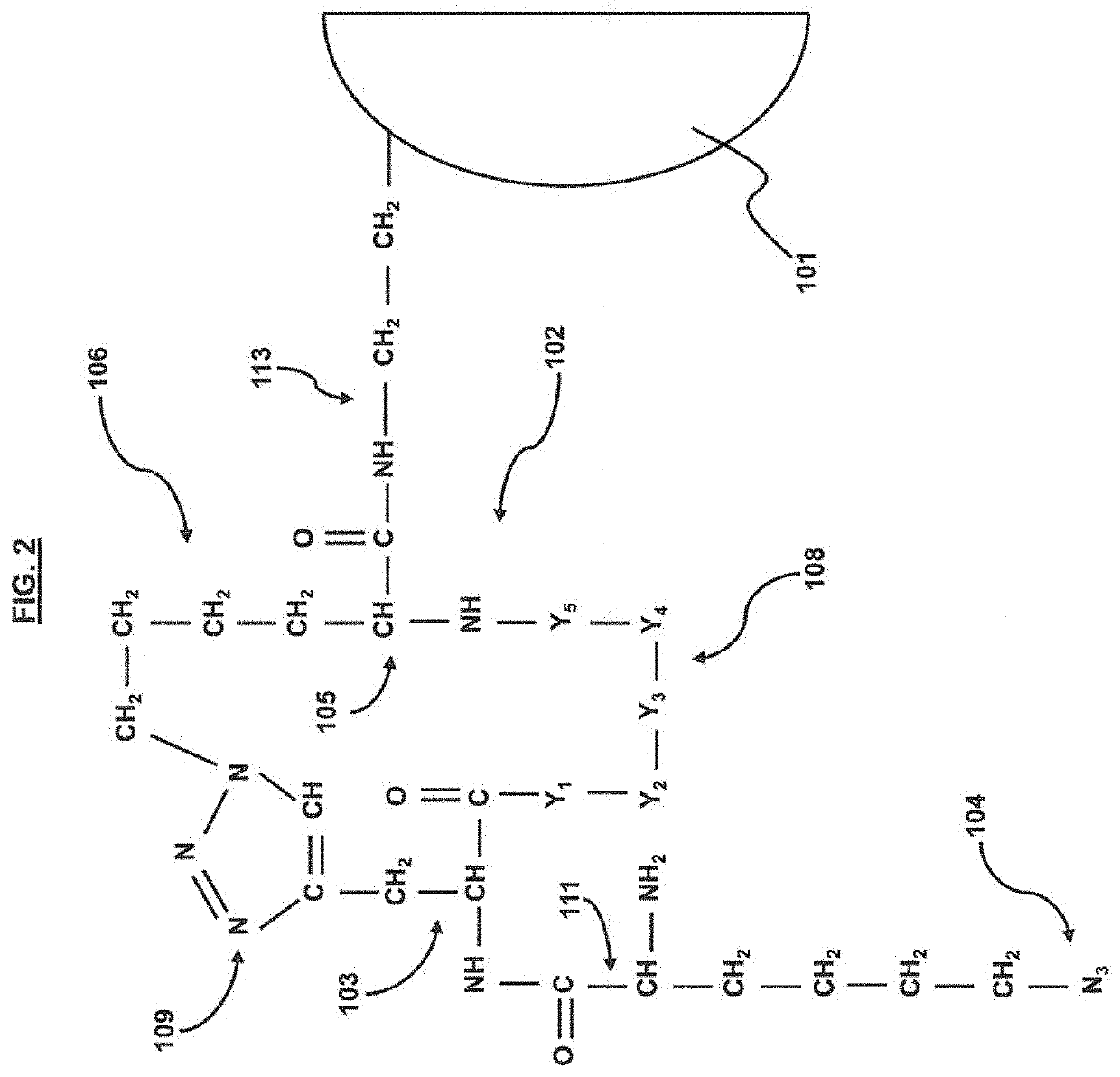
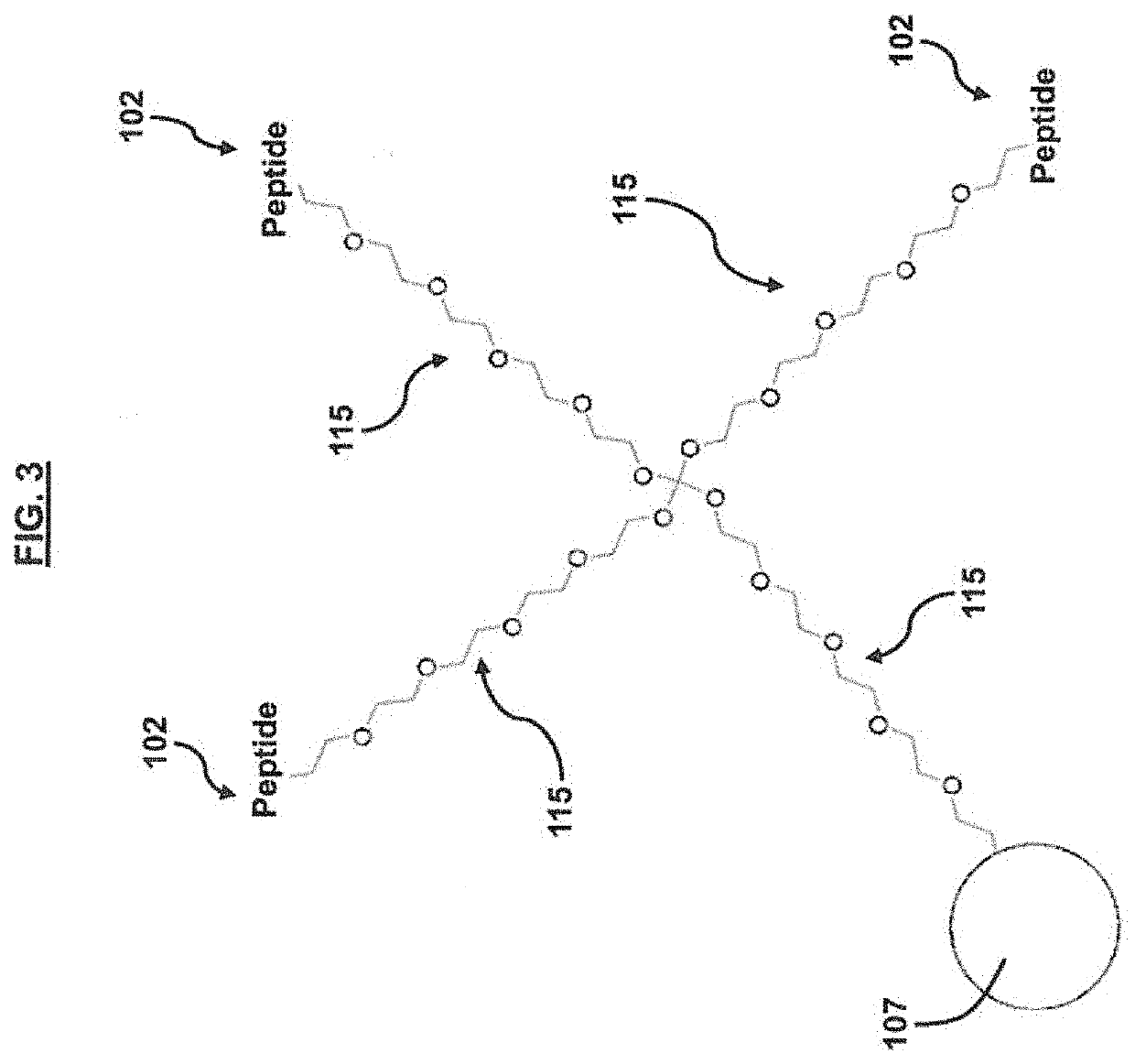
ActiveUS20200354713A1Improve targeting selectivityQuick filterPeptide librariesMicroorganism librariesCyclic peptideProtein target
A method for developing capture agents for target proteins employs a compound library to find cyclic peptide sequences that bind the target protein. The target protein is also reacted with a clickable group-provider reagent to provide the protein with clickable groups. The compounds in the library are provided with complementary clickable groups that bind the clickable group on the target protein when the peptide sequences bind the target protein. In some embodiments, the cyclic peptide sequences that bind the target protein are incorporated into constructs having one or more arms that can serve as capture agents or potential treatments against the pathogens from which the target protein is derived. Some embodiments provide pharmaceutical compositions for immunoassays, diagnostics, therapeutics or the like, that employ the constructs.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
Polypeptide extracted from stinging cell venom of fire medusa and application thereof
InactiveCN104926932AHas anti-nasopharyngeal carcinoma tumor activityHas anti-nasopharyngeal carcinoma activityPeptide/protein ingredientsPeptide preparation methodsNasopharyngeal carcinomaAmino acid
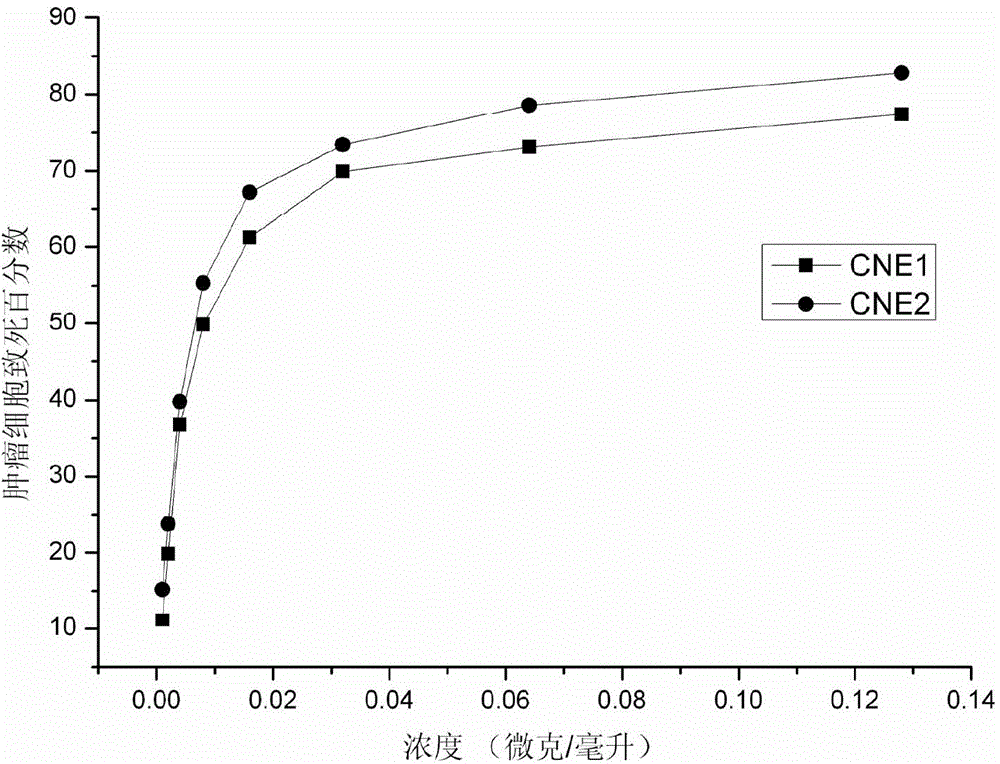
The invention discloses a polypeptide extracted from stinging cell venom of a fire medusa. The amino acid sequence of the polypeptide is showed in the SEQ ID No.1. The invention further discloses the extraction method for the polypeptide. The extraction method comprises the steps that venom protein is extracted from the stinging cell venom of the fire medusa, then a Histrap SP strong positive ion exchange column and a Histrap Q strong anion exchange column are used sequentially to separate the polypeptide in the venom protein. The invention further discloses the application of the polypeptide in preparation of anti-nasopharynx-cancer medicine. The polypeptide can restrain the growth of a nasopharynx cancer cell strain CNE-1 and the growth of a nasopharynx cancer cell strain CNE-2 and induce the nasopharynx cancer cell to die. The extraction method for the polypeptide extracted from the stinging cell venom of the fire medusa has the advantages that the operation is simple, the separation procedures are few, the extraction method is suitable for quick separation, the separation condition is mild, and the anti-tumor activity of the obtained protein can be reserved to the largest content.
Owner:GUANGXI BOTANICAL GARDEN OF MEDICINAL PLANTS
EB virus BALF4 protein epitope
ActiveCN113493495AStrong specificityHigh antibody titerViral antigen ingredientsVirus peptidesVirus ProteinTitin Antibody
The invention relates to the technical field of molecular biology and immunology, in particular to an EB virus BALF4 protein epitope. The invention provides an the EB virus BALF4 protein epitope. The EB virus BALF4 protein epitope is shown as SEQ ID NO: 1, provides a nucleic acid molecule for coding the epitope, and also provides an antigen protein, a specific antibody and application of the epitope. The epitope has good conservative property, sensitivity and specificity, and a prepared antibody is high in titer and can be applied to clinical diagnosis and molecular mechanism research.
Owner:RUIJIN HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
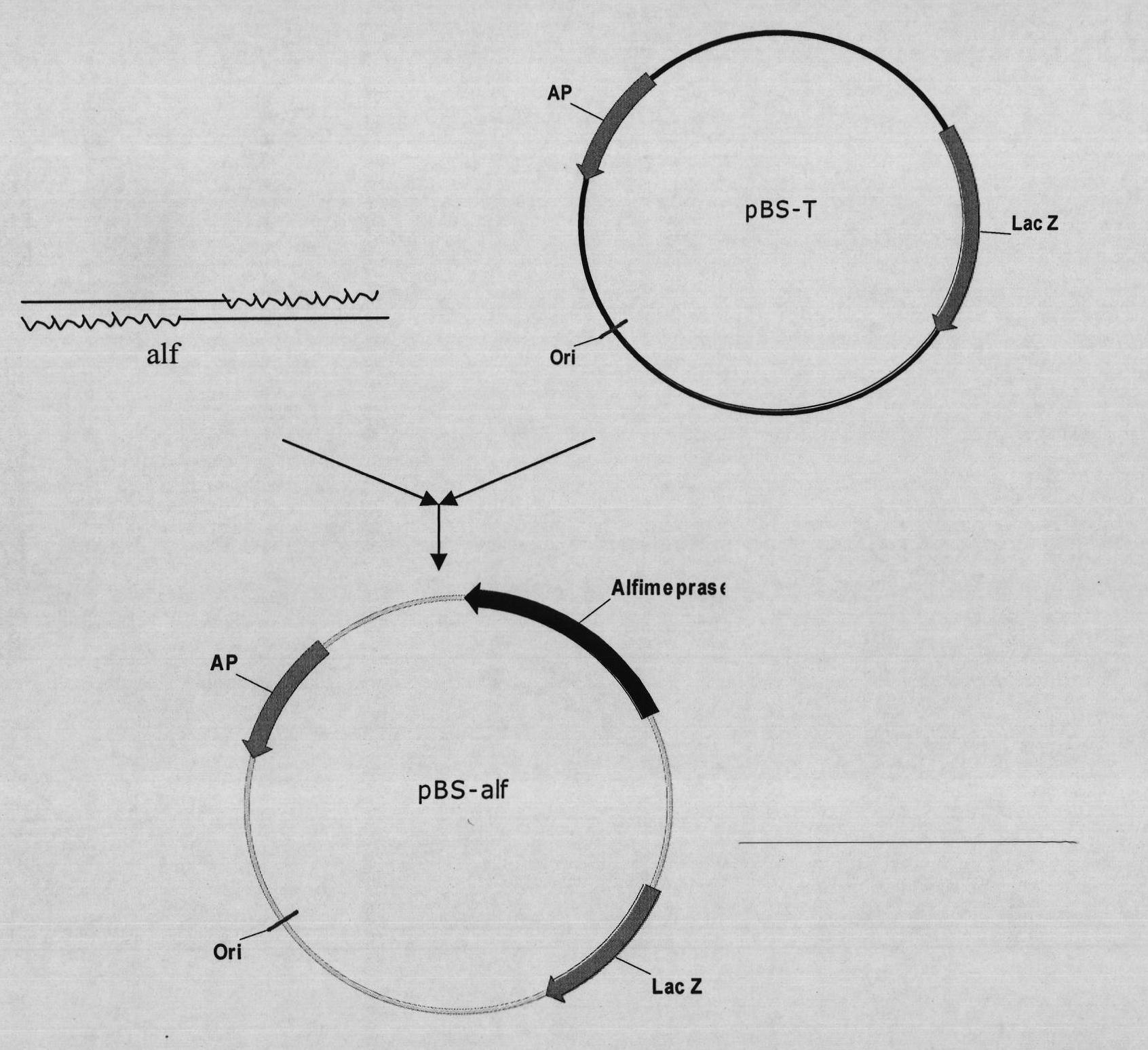
Mutant Alfimeprase engineering strain, and preparation method and applications thereof
The invention relates to a preparation method of a recombinant viper venom protein. A mutant Alfimeprase gene of artificially synthesized Agkistrodon contortrix is utilized, and the gene has the nucleotide sequence shown as SEQ ID No.2. The gene is linked to yeast expression plasmids pPIC9K to construct a high efficiency expression vector pPIC9K-His-Alf, a Pichia yeast strain GS115 is introduced, and the vector is named as FY-Alf. Induced expression is carried out with methanol, the expressed recombinant protein is purified and then subjected to activity verification by a fibrin plate assay, and the result shows that the recombinant protein Alfimeprase expressed by the yeast strain GS115 containing pPIC9K-His-Alf plasmids has high fibrinolytic activity.
Owner:ANHUI FENGYUAN PHARM CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com