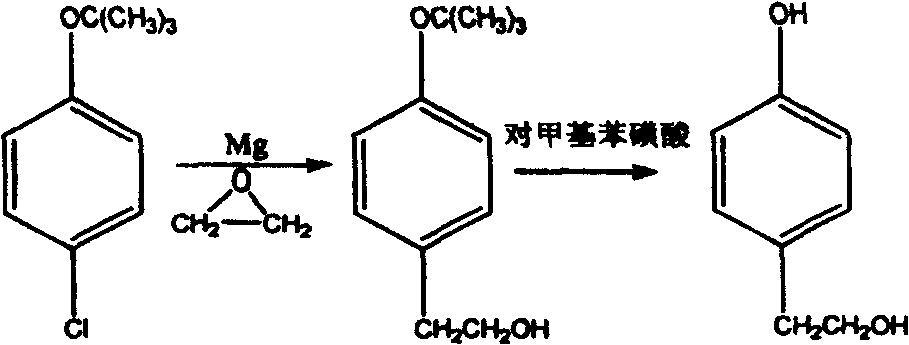
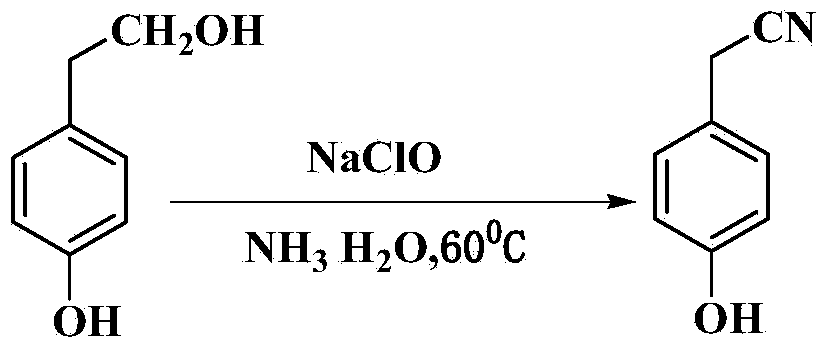Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
62 results about "P-hydroxyphenylethanol" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Glossy privet fruit total triterpenes extract and total phenols extract as well as preparation method thereof
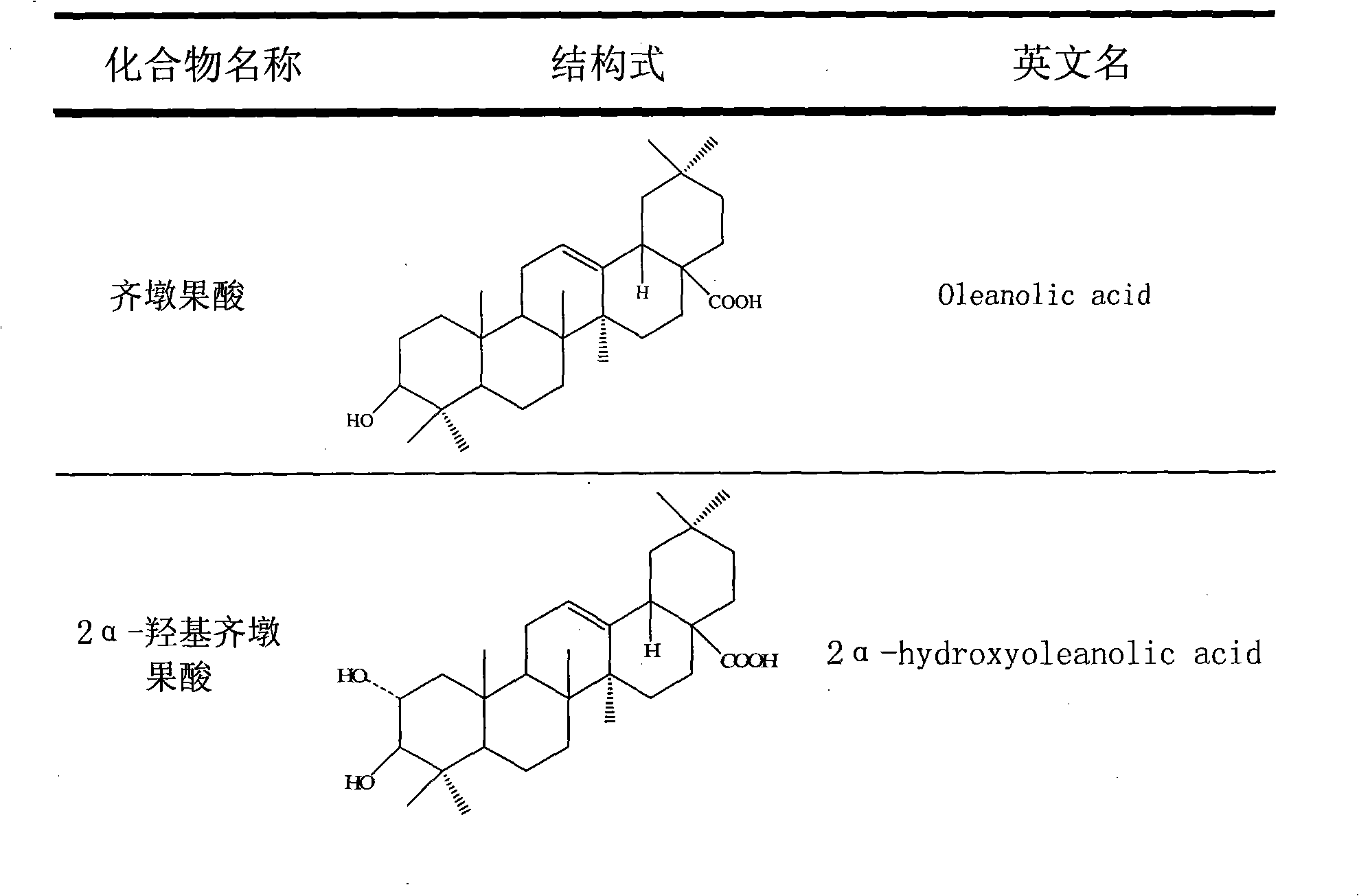
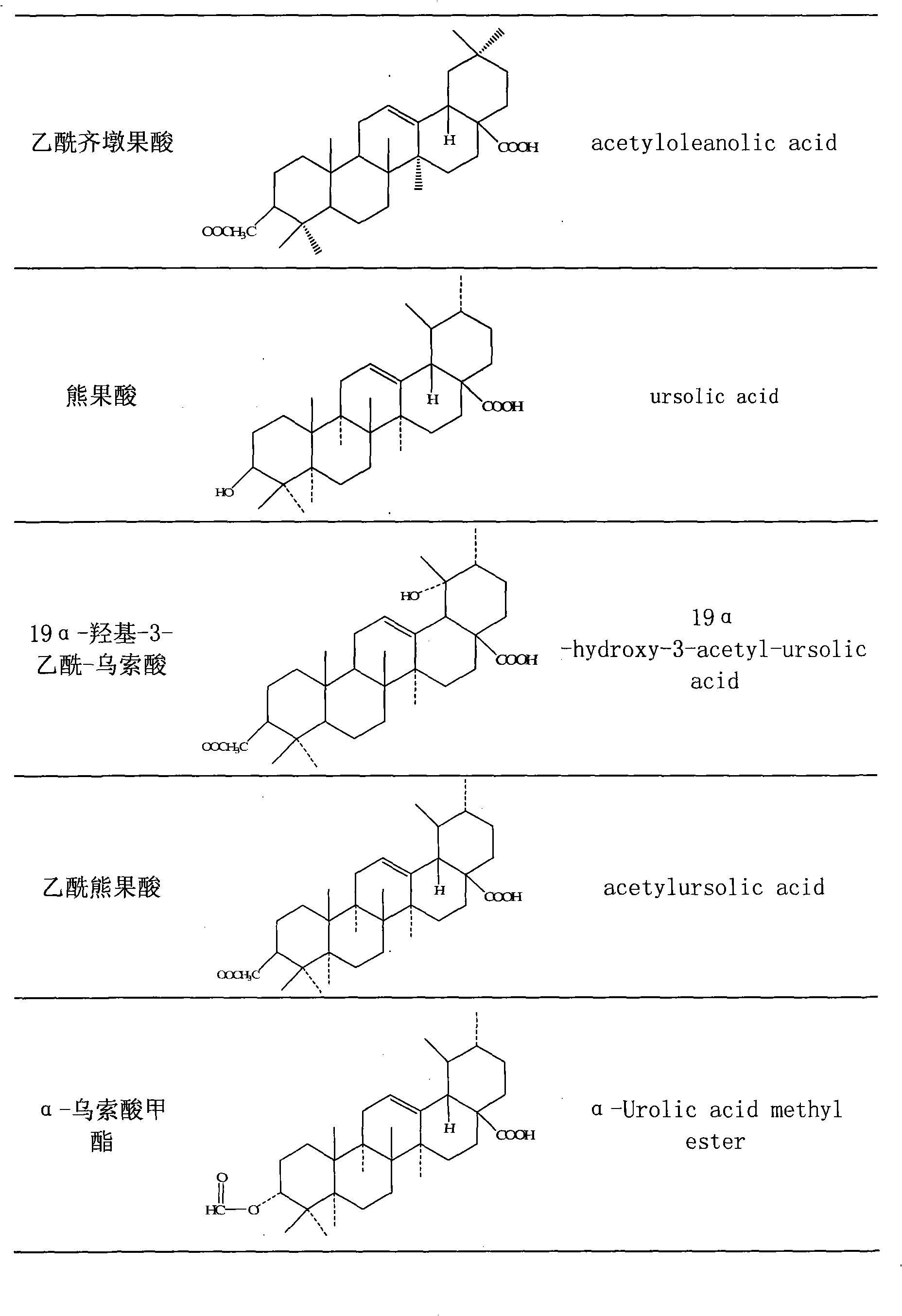
The invention discloses total triterpene extract and total phenol extract extracted from Chinese herbal medicine glossy privet fruit and a preparation method thereof. The total triterpene extract mainly contains oleanolic acid, ursolic acid, acetyl oleanolic acid, indicant compound and other derivatives with acetyl oleanolic acid as the mother nucleus. The total phenol extract mainly contains hydroxyl mandelic, 3, 4-dihydroxy phenylethyl alcohol, salidroside and indicant compound and other derivatives with salidroside as the mother nucleus. The glossy privet fruit total triterpene extract and total phenol extract can be prepared by any method of solvent distillation, solvent extraction, deposition, macroporous resin absorption, supercritical fluid extraction, column chromatography and liquid-liquid countercurrent distribution chromatography, or any combination of these methods. The total percentage of various triterpenoids of the prepared glossy privet fruit total triterpene extract is 5 to 100 percent (w / w); wherein, the content of oleanolic acid and ursolic acid accounts for 5 to 100 percent (w / w) of the total triterpene. The total percentage of various phenols of the prepared glossy privet fruit total phenol extract is 5 to 100 percent (w / w); wherein, the content of salidroside accounts for 5 to 100 percent (w / w) of the total phenol.
Owner:石任兵
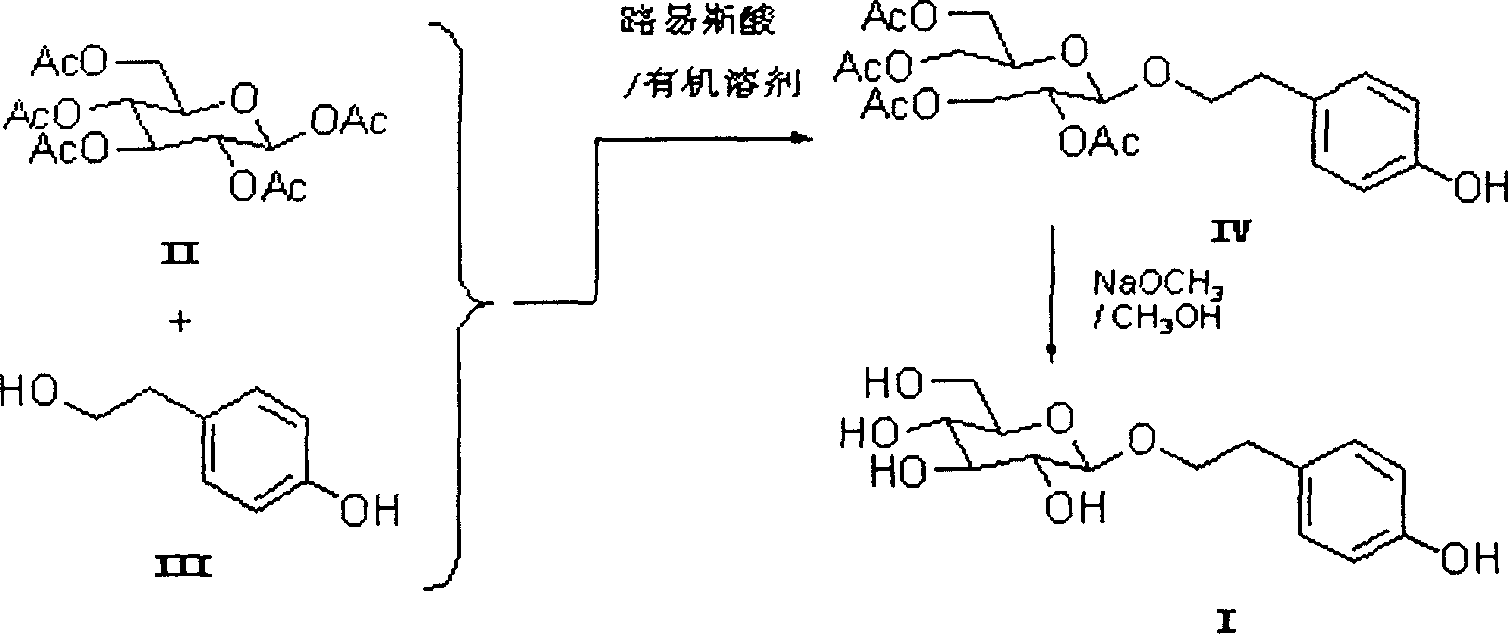
Method of chemical synthesizing hongjingtian glycoside
InactiveCN1911949AFew reaction stepsReduce manufacturing costSugar derivativesChemical synthesisPhenethyl alcohol
The chemical process of synthesizing rhodiola glycoside includes the following steps: the Lewis acid catalyzed glycosidation between pentacetyl-beta-D-glucose and p-hydroxy phenethyl alcohol inside organic solvent to produce tetracetyl rhodiola glycoside; and the subsequent deacetylation of tetracetyl rhodiola glycoside inside the methanol solution of NaOCH3 to obtain rhodiola glycoside. Compared with traditional synthesis process, the present invention has the advantages of wide material source, less reaction steps and low preparation cost. The process of the present invention is suitable for industrial production.
Owner:ZHEJIANG UNIV
Para-(2-methoxyl) ethylphenol synthesis method
ActiveCN1800128AThe reaction route is simpleThree wastes lessEther preparation from oxiranesTyrosolMethyl carbonate
The invention discloses a method for synthesizing phenetyl, which uses p-chlorophehol or p-bromophenol as starting reaction raw material. It first uses methyl, benzyl or tert-butyl to protect the phenolic hydroxyl, the parivis which is protected by the phenolic hydroxyl reacts with the magnesium in the ether, tetrahydrofuran, tert-butyl methyl ether, isopropyl ether and its mixing solution to obtain the Grignard reagent, the Grignard reagent directly reacted with the etox to obtain the tyrosol which is protected by the phenolic hydroxyl, the tyrosol reacts with the dimethyl sulfate, dimethyl carbonate, trimethyl orthoformate to obtain the tyrosol ether which is protected by phenolic hydroxyl, which obtains the product in acid or hydrogenation protection.
Owner:SHANDONG HANXING PHARM TECH CO LTD +1
Process of preparing beta-p-hydroxyphenyl ethanol
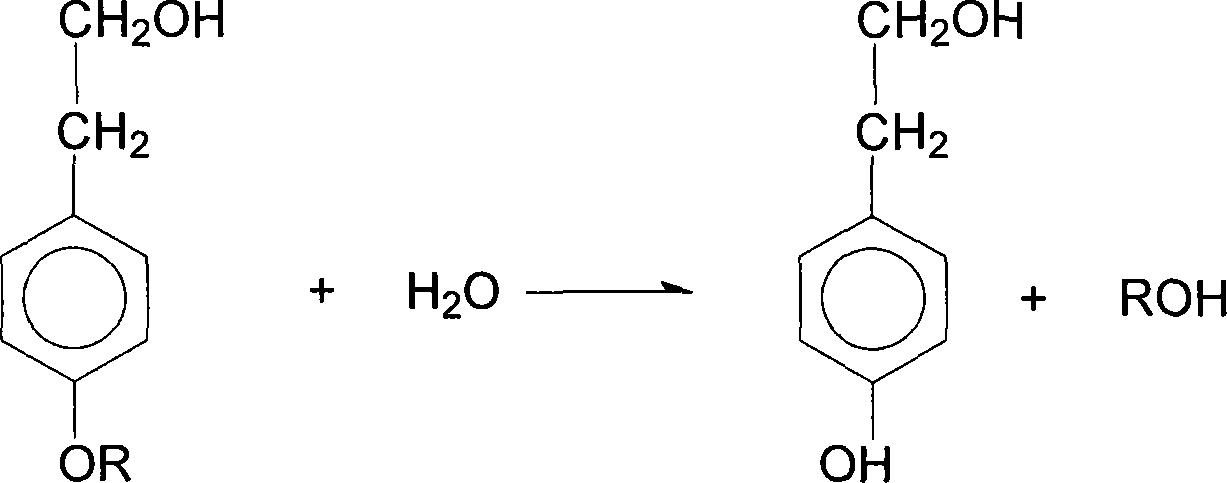
The present invention discloses process of synthesizing beta-p-hydroxyphenyl ethanol. The materials including p-alkoxyphenyl ethanol, inorganic acid, water and organic solvent in the molar ratio of 1 to 0.28-0.5 to 1.7-2.0 to 1-2 are first made to produce hydrolysis reaction at 30-60 deg.c for 2-4 hr; the hydrolysis product is cooled to 0-8 deg.c to separate out crystal; and the crystal is post-treated to obtain beta-p-hydroxyphenyl ethanol. The beta-p-hydroxyphenyl ethanol preparing process of the present invention has simple operation and low cost, and is suitable for industrial production.
Owner:ZHEJIANG APELOA MEDICAL TECH
Acne removing water
InactiveCN106265283AIncrease elasticityReasonable formulaCosmetic preparationsToilet preparationsDiethylene glycolHexylene glycol
The invention discloses acne removing water, and relates to the field of cosmetics. The acne removing water is prepared from A raw materials, B raw materials and C raw materials. The A raw materials are prepared from water, betaine salicylate and butanediol; the B raw materials are prepared from triethanolamine, hydrolyzed algin, chlorella extract, witchhazel extract and diglycerol; the C raw materials are prepared from p-hydroxyphenylethanol and hexylene glycol. The acne removing water has the advantages of being reasonable in formula, convenient to use, good in acne removing effect, fast in healing and the like.
Owner:广州君恒生物科技有限公司
Technique of synthesizing levorotatory betaxolol hydrochloride
InactiveCN101012175AEasy to separateEasy to purifyOrganic compound preparationAmino-hyroxy compound preparationState of artHalogen
The invention discloses a synthesizing technique of left-handed hydrochloric betamicin, which comprises the following steps: adopting p-hydroxyphenethylol and R-epoxy halogen propane as original raw material; alkylating; aminating; protecting; alkylating; removing protection; obtaining pure high-antimer left-handed betamicin and hydrochlorate.
Owner:刘宏民
The synthetic method of salidroside
InactiveCN102286036AGood chiral selectivityShort reaction pathSugar derivativesSugar derivatives preparationSalidrosidePtru catalyst
The invention discloses a chemical method for synthesizing salidroside. The synthesis steps are: tetraacetyl-β-D-glucose trichloroacetate or tetraacetyl-α-D-glucose trichloroacetate and p-hydroxybenzene Ethanol in anhydrous organic solvents, under the dehydration of molecular sieves and under the catalysis of Lewis acids such as tin tetrachloride, glycosides to generate tetraacetyl salidroside; In the solution, the acetyl group is removed to obtain salidroside. Compared with the traditional synthetic method, the method of the present invention has high reaction yield, good chiral selectivity, easy to obtain raw materials, few reaction steps, can use cheap Lewis acid catalyst, greatly reduces the cost, and is applicable to the production of salidroside mass production.
Owner:WUHAN SYNCHALLENGE UNIPHARM INC
Separation method suitable for chemical synthesis of salidroside for industrial production
ActiveCN104045669ALow costFew reaction stepsSugar derivativesSugar derivatives preparationSalidrosideChemical synthesis
The invention discloses a separation method suitable for chemical synthesis of salidroside for industrial production. The method comprises the following steps: carrying out a reaction on Beta-D-pent-acetyl glucose and hydroxyphenethyl alcohol under the catalytic action of a lewis acid; and carrying out deacetylation on a generated tetraacetyl salidroside product so as to prepare the salidroside. Thus, the salidroside product is synthesized in a separating manner via an extraction and recrystallization method while a simple and convenient synthesis method is adopted at the same time. Compared with the traditional chemical synthesis process, the method disclosed by the invention is low in cost, high in yield, short in reaction time, simple and simple, convenient and environmentally friendly in synthesis process, thereby being suitable for industrial production, and the raw materials are easily available.
Owner:LIAONING UNIVERSITY
Technical method for synthesizing beta p-hydroxy phenethyl alcohol
InactiveCN1915949AWide variety of sourcesShort synthetic routeOrganic chemistryOrganic compound preparationAcetic anhydridePhenethyl alcohol
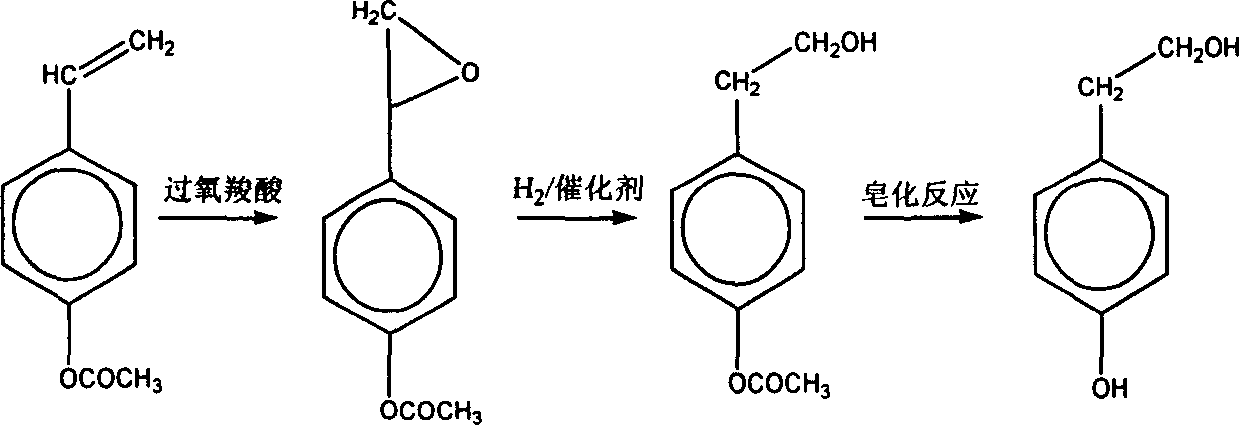
This invention discloses a method for synthesizing beta-p-hydroxyphenylethanol from phenol and acetic anhydride by esterification, halogen-alkylation and hydrolysis. Since the O atom on phenolic hydroxyl has a high electronegativity, it is easily subjected to alkylation reaction. To avoid the reaction, esterification is employed to preotect the O atom and phenyl acetate. Then 1,2-dihaloethylene is added on the benzene ring of phenyl acetate to obtain (4-beta-haloethyl)phenyl acetate. The protection groups are removed by hydrolysis to obtain beta-p-hydroxyphenylethanol. The method has such advantages as short synthesis route, mild reaction conditions, high efficiency, high yield (up to 93%), and abundant raw material resources.
Owner:SHENYANG LIGONG UNIV
Beta-receptor agonist substituted template molecularly imprinted polymer microsphere, preparation method and application thereof
InactiveCN106084115AImprove performanceGood inclusion and complexation abilityOther chemical processesMicroballoon preparationAcetic acidMicrosphere
The invention discloses a beta-receptor agonist substituted template molecularly imprinted polymer microsphere, a preparation method and application thereof. The method comprises the steps of: (1) subjecting a template molecule and methacrylic acid to prepolymerization, with the template molecule being p-hydroxyphenylethanol or p-hydroxyphenethylamine; (2) adding divinyl benzene and azodiisobutyronitrile into the prepolymerized system to carry out polymerization reaction so as to obtain a polymer; and (3) using a methanol solution of acetic acid to wash the polymer, thus obtaining the beta-receptor agonist substituted template molecularly imprinted polymer microsphere. The molecularly imprinted polymer microsphere provided by the invention has the template molecule matching with the steric configuration of the agonist and having holes with multiple action points, and can selectively recognize agonists, thus having better inclusion complexation ability and chemical stability. The invention tests the adsorption properties of the molecularly imprinted polymer microsphere to the beta-receptor agonist, also uses HPLC / MS / MS to conduct adsorption performance detection, and applies the molecularly imprinted polymer microsphere to determination of beta-receptor agonist in urine.
Owner:INST OF QUALITY STANDARD & TESTING TECH FOR AGRO PROD OF CAAS
Technique for synthesizing levorotatory betaxolol hydrochloride
InactiveCN101085742AEasy to separateEasy to purifyOrganic compound preparationAmino-hyroxy compound preparationState of artTyrosol
The invention discloses a method for synthesizing left-handed hydrochloric betaxolol. It employs tyrosol as raw material, and comprises benzyl protection, alkanisation, pulling off protection, alkanisation, aminating and getting high- enantiotropy product. The invention is characterized by simple operation, easy get raw material, low toxicity, safe and convenient operation, low pollution, high productivity and short period.
Owner:ZHENGZHOU UNIV
Method for synthesizing tyrosol
InactiveCN101225023ASolve the separation problemLow priceOrganic chemistryOrganic compound preparationTyrosolEthyl hydroxybenzoate
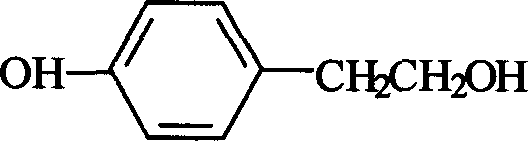
The invention relates to a synthesis method of 4-hydroxyphenethyl alcohol, which is characterized in that: ethyl-hydroxybenzoate is used as starting raw material and sodium borohydride (or potassium borohydride) is used as reducing agent and aluminium chloride or boron trifluoride diethyl etherate is used as catalyst for reduction reaction in proper solvent, thereby getting 4-hydroxyphenethyl alcohol. The synthesis method of 4-hydroxyphenethyl alcohol has the advantages of industrial production for the 4-hydroxyphenethyl alcohol, lower cost and feasible operation. The yield can reach 72%.
Owner:NANJING RALLY BIOCHEM
A biological sprout inhibitor and application thereof
InactiveCN1720802ASafe and non-toxic during production, processing and useNo side effectsBiocideAnimal repellantsTyrosolMicroorganism
The invention relates to a biological anti-sprouting agent and its use, wherein the anti-sprouting agent is prepared from biological strains through the steps of enlarged culture and fermentation, solvent extraction, concentration, separation and purification. The bacterial strain has a systematic name of Botrytis cinerea, its active constituents include natural tyrosol and phenethylol wherein the contents are 25-30% and 22-25%.
Owner:尹平
Method for preparing l-betaxolol hydrochloride
ActiveCN101665441AHigh chemical purityHigh optical purityOrganic compound preparationAmino-hyroxy compound preparationTosylhydrazoneBetaxolol
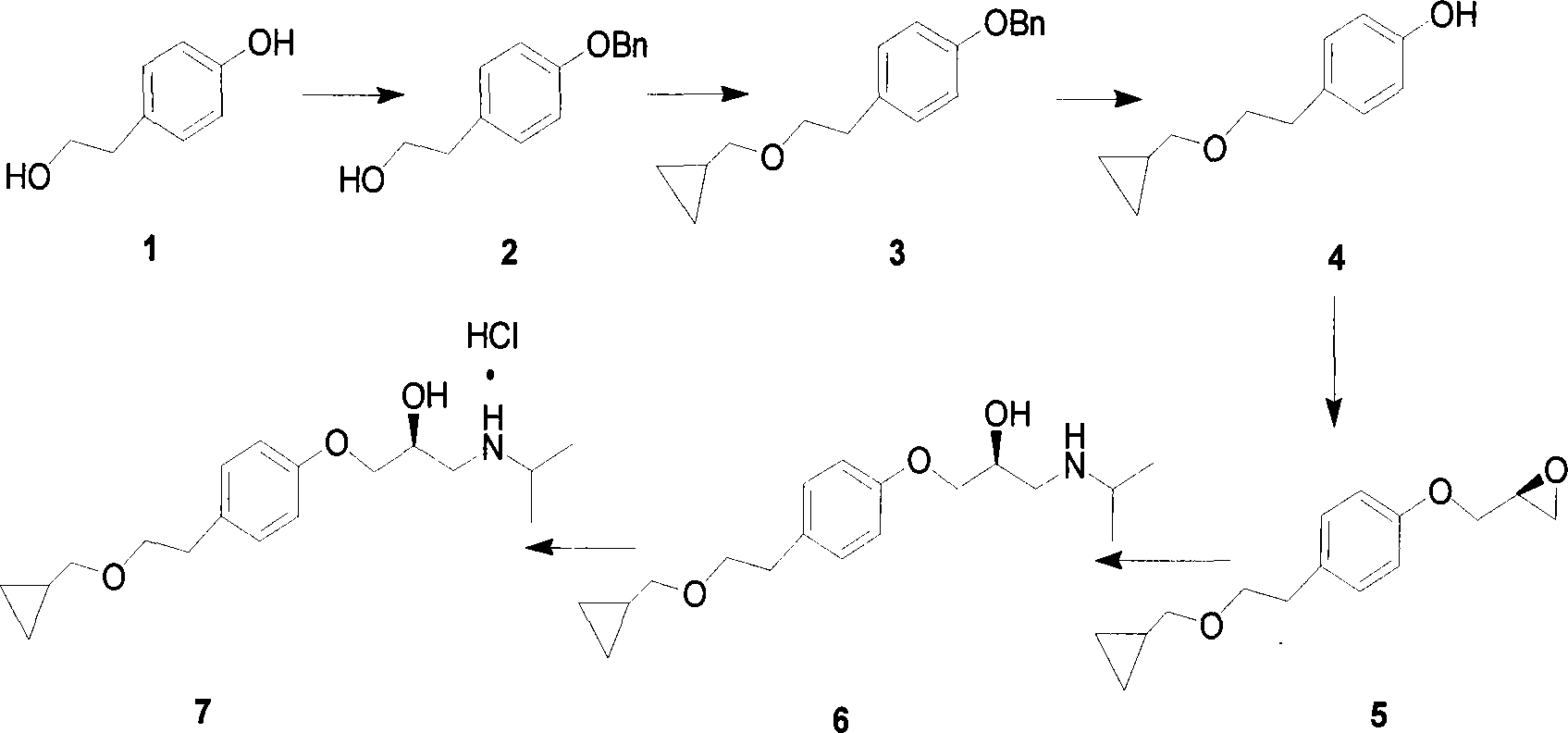
The invention provides a method for preparing l-betaxolol hydrochloride, which takes p-hydroxy phenylethanol as starting material and obtains the pure l-betaxolol hydrochloride by selectively esterfying alcoholichydroxyl, etherifying phenolichydroxyl, carrying out amination on isopropamide and finally etherifying cyclopropylmethanol to lead HCl to be salified. The invention also provides an important intermediate compound 2-(2S)-3-(4-p-toluene sulfonyloxy ethyl phenoxy)-1,2-propylene oxide which is used for preparing the l-betaxolol hydrochloride, and a preparation method thereof. The invention avoids using bromotoluene cyclopropane which is higher in price, unstable and very irritant; the final products are easy to separate from each other and purify; and the obtained product has higher chemical purity (99.0-100.0%) and optical purity (99.0-100.0%) as well as high yield (total yield is 62%), and is suitable for industrialized production.
Owner:GUANGZHOU BOJI MEDICINE SERVICES
Spot-fading essence
InactiveCN106214574AInhibit synthesisInhibit migrationCosmetic preparationsToilet preparationsBetaineTremella
The invention discloses spot-fading essence, and relates to the field of cosmetics. The formula of the spot-fading essence comprises a raw material group A, a raw material group B and a raw material group C. The raw material group A comprises water, tremella polysaccharide, diglycerol and butanediol. The raw material group B comprises poly (sodium glutamate), hydrolyzed algae extract, brown algae extract, pancratium zeylanicum extract, nicotinamide and glycine betaine, and the raw material group C comprises hydroxyphenethyl alcohol and hexanediol. The spot-fading essence is simple and reasonable in formula, practical in function and easy to absorb and has the advantages of capabilities of resisting ultraviolet rays, whitening skins, fading spots, preserving moisture, repairing injured cells and delaying senescence.
Owner:广州君恒生物科技有限公司
Moisturizing lotion
InactiveCN106214575AGood touchSimple recipeCosmetic preparationsToilet preparationsTremellaL glutamate
The invention discloses moisturizing lotion and relates to the field of cosmetics. The moisturizing lotion is prepared from raw materials including a raw material group A, a raw material group B and a raw material group C, wherein the raw material group A includes water, tremella polysaccharide, butanediol and sodium hyaluronate; the raw material group B includes sodium L-glutamate and diglycerol; the raw material group C includes hydroxyphenethyl alcohol and hexylene glycol. A preparation method of the moisturizing lotion includes the steps of 1), disinfection; 2), treatment of the raw material group A; 3), treatment of the raw material group C; 4), primary mixing; 5), mixed discharging. The moisturizing lotion has the advantages of being simple and reasonable in recipe, low in cost, practical in function, easy to absorb, good in moisture retention and moisturizing effect, long in maintaining time, and anti-ageing.
Owner:广州君恒生物科技有限公司
Preparation method of 4-hydroxyphenethyl alcohol
InactiveCN103804147AOrganic compound preparationEther preparation by ester reactionsAlcoholGrignard reaction
The invention relates to a preparation method of 4-hydroxyphenethyl alcohol. The preparation method is characterized in that: p-bromophenol is taken as a raw material, and is subjected to a series of reactions such as hydroxyl protection, Grignard reaction, and protecting group removing so as to obtain 4-hydroxyphenethyl alcohol. Product yield of the preparation method reaches about 90%.
Owner:徐州瑞赛科技实业有限公司
Technical method for synthesizing beta p-hydroxy phenethyl alcohol
InactiveCN100432035CWide variety of sourcesShort synthetic routeOrganic chemistryOrganic compound preparationAcetic anhydridePhenethyl alcohol
This invention discloses a method for synthesizing beta-p-hydroxyphenylethanol from phenol and acetic anhydride by esterification, halogen-alkylation and hydrolysis. Since the O atom on phenolic hydroxyl has a high electronegativity, it is easily subjected to alkylation reaction. To avoid the reaction, esterification is employed to preotect the O atom and phenyl acetate. Then 1,2-dihaloethylene is added on the benzene ring of phenyl acetate to obtain (4-beta-haloethyl)phenyl acetate. The protection groups are removed by hydrolysis to obtain beta-p-hydroxyphenylethanol. The method has such advantages as short synthesis route, mild reaction conditions, high efficiency, high yield (up to 93%), and abundant raw material resources.
Owner:SHENYANG LIGONG UNIV
Method for enzymatic synthesis of gadol glycoside through separation and coupling
InactiveCN1844405AIncrease concentrationHigh purityChemical recyclingFermentationEnzymatic synthesisTyrosol
The invention discloses a method of reaction and disconnect-linkage enzymatic synthesis gadol glycosides. The method use waste apple seed of candied fruits factory as cheap biocatalyst, use the glucose and tyrosol as substrate, enzymatic synthesis the precious nature health products gadol glycosides in organic dissolvent-water system, the reaction miscible liquids adsorbed by aluminum oxide or other adsorbent, thought selective adsorption of eluent to separating the substrate of tyrosol and gadol glycosides, simplifying the purification process of product yield, the non-reaction alcohol can utilize circulating, the method is a new method to batch process gadol glycosides and it has large application foreground.
Owner:EAST CHINA UNIV OF SCI & TECH +1
Hordenine synthesis method
InactiveCN103483209ARaw materials are cheap and easy to getRaw materials are easy to getOrganic compound preparationAmino-hyroxy compound preparationAlcoholSynthesis methods
The invention provides a hordenine synthesis method. The method is short in route, low in cost, high in yield, simple in operation and applicable to industrial production, raw materials are easy to get, and an obtained product is qualified. According to the method, 4-Hydroxyphenethyl alcohol is used as the raw materials and condensed with dimethylamine through chloro, and the qualified product-hordenine is obtained by two-step reaction with high yields. The hordenine synthesis method only includes the following steps: first, preparing 4-bromomethyl phenol, and second obtaining the hordenine through synthesis.
Owner:SHAANXI JIAHE PHYTOCHEM
Tobacco and vegetable sprouting inhibitor
InactiveCN1685817ANo side effectsNo pollution in the processBiocideAnimal repellantsNicotiana tabacumPhenethyl alcohol
A bud suppressing agent of vegetable and tobacco for improving their quality and increasing their yield is proportionally prepared from phenylethanol and / or p-hydroxyphenyl thanol through mixing and dissolving in water.
Owner:尹平
Synthetic method of p-acetoxystyrene
PendingCN111087303AThe synthesis process has less three wastesHigh yieldOrganic compound preparationCarboxylic acid esters preparationPolymer scienceOrganic solvent
The invention relates to a synthetic method of p-acetoxystyrene, in particular to a novel process for synthesizing p-acetoxystyrene serving as an intermediate of 248-nm photoresist monomer p-hydroxystyrene. The method comprises the following steps of: performing solid acid catalysis on p-hydroxyphenethyl alcohol serving as a raw material, and performing dehydrating in an organic solvent in the presence of a polymerization inhibitor to obtain a target product. According to the synthetic method of p-acetoxystyrene, the p-hydroxyphenethyl alcohol is taken as the raw material for solid acid catalysis to prepare the 248-nm photoresist monomer p-hydroxystyrene intermediate p-acetoxystyrene through one-pot boiling, and the new synthesis process has the advantages that the yield is high, a catalyst is easy to recycle and reuse, few three wastes are generated and the like.
Owner:ZHEJIANG UNIV OF TECH
Cultivation method and application for salidroside-containing plant
The invention provides a cultivation method for a salidroside-containing plant. The method comprises the following steps: spraying hydroxyphenethyl alcohol to growing plant leaves; harvesting the growing plant leaves to obtain the salidroside-containing plant. According to the method provided by the invention, the steps are simple; the synthetic precursor, namely the hydroxyphenethyl alcohol, of the salidroside-containing plant is directly supplied by outside, and the salidroside is synthesized in a plant body by taking uridine diphosphate glucose and glucosyltransferase which are contained in the plant as raw materials. The invention further provides the application of the salidroside-containing plant.
Owner:HUNAN UNIV OF CHINESE MEDICINE
Method for preparing para hydroxybenzene acetonitrile
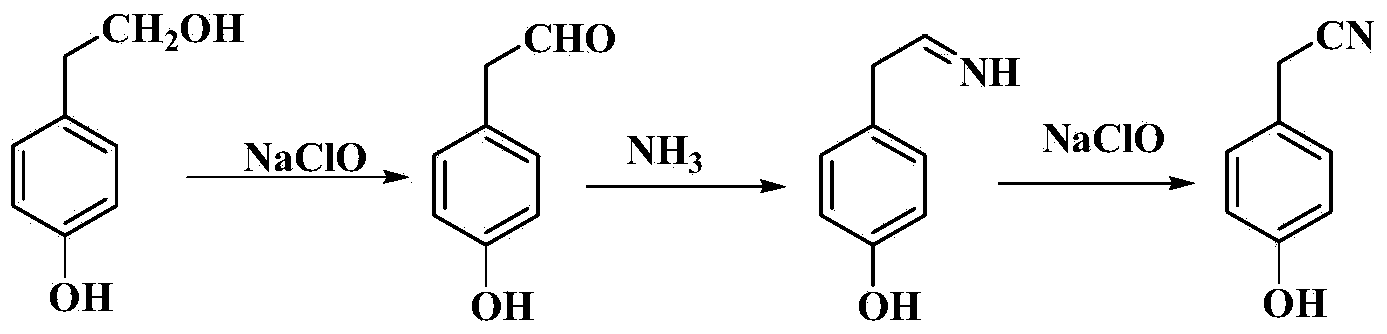
InactiveCN103450046AHigh yieldReduce manufacturing costCarboxylic acid nitrile preparationOrganic compound preparationChemical synthesisSal ammoniac
The invention belongs to the field of organic chemical synthesis, and relates to a method for preparing para hydroxybenzene acetonitrile. The method comprises the following step: carrying out oxidation, ammoniation and reoxidation reactions on para hydroxybenzene ethanol in the presence of ammonia water and hypochlorite by taking the para hydroxybenzene ethanol as a raw material and water as a reaction medium to obtain the para hydroxybenzene acetonitrile. The process route for oxidation, ammoniation and reoxidation on the para hydroxybenzene ethanol by taking the lower-price (relative to para hydroxybenzene acetamide) para hydroxybenzene ethanol as the raw material has the characteristics of mildness in reaction condition and high yield; meanwhile, the production cost of the para hydroxybenzene acetonitrile is greatly reduced; the technical route has the characteristics of short route, easiness and convenience for operation, mildness in reaction condition and easiness for industrialized production and is a very economic, simple and convenient method for preparing the para hydroxybenzene acetonitrile.
Owner:JIAXING UNIV
Preparation method of galactose type salidroside and derivative thereof
ActiveCN106543244AEfficient synthesisSimple and fast operationSugar derivativesFermentationSalidrosideAlcohol
The invention relates to a preparation method of galactose type salidroside and a derivative thereof. The method comprises the following steps that 1, a reaction system with the lactose concentration being 0.1-1 M, the p-hydroxyphenylethyl alcohol concentration being 0.05-0.5 M and the beta-galactosidase concentration being 0.004-4 mg / mL is prepared a phosphate buffer; 2, the reaction system reacts for 5-30 min under the condition that the temperature ranges from 30 DEG C to 50 DEG C, a reaction is terminated, solid-liquid separation is conducted, and supernatant is taken; 3, the supernatant is separated and dried, and the galactose type salidroside and the derivative thereof are prepared. According to the method, a substrate is cheap, reacting is rapid, operation is easy and convenient, time and labor are saved, the production cost is low, and the application prospect is wide.
Owner:SHANDONG UNIV
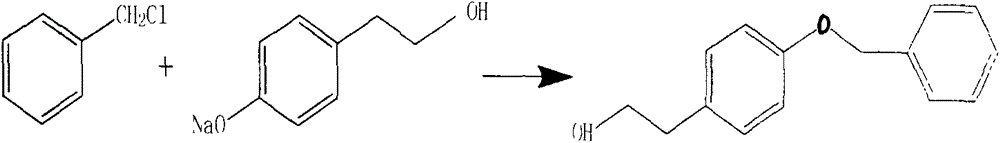
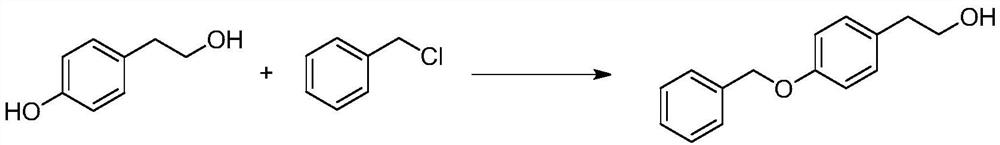
Preparation method of 2-(4-benzyloxyphenyl) ethanol
InactiveCN104086378AEasy to operateHigh yieldOrganic compound preparationEther preparation by ester reactionsDistillationBenzyl chloride
The invention discloses a preparation method of 2-(4-benzyloxyphenyl) ethanol. The preparation method comprises the following steps: stirring p-hydroxyphenylethyl alcohol and water, uniformly mixing, and dropwise adding a sodium hydroxide aqueous solution to perform salt forming reaction to generate 4-hydroxyphenylethyl alcohol sodium salt; then performing vacuum reduced pressure distillation, adding toluene to reflux with water, and then dropwise adding benzyl chloride to perform reaction to generate a 2-(4-benzyloxyphenyl) ethanol crude product; and then standing, filtering, crystallizing and drying to obtain a fine product namely 2-(4-benzyloxyphenyl) ethanol. The preparation method disclosed by the invention is simple and easy to operate, and is less in product by-product and high in product yield; preferably, the product yield can reach 85%; and the preparation method can be used for reducing the production cost and improving the production efficiency at the same time.
Owner:NANTONG HUAFENG CHEM
Synthesis method of salidroside
InactiveCN104725440AAvoid synthetic stepsSubsequent reaction selectivity improvedSugar derivativesSugar derivatives preparationSulfonyl chlorideSalidroside
The invention discloses a synthesis method of salidroside, which is implemented by taking p-hydroxyphenylethyl alcohol as a raw material through the steps of firstly, protecting hydroxyl by using methoxy-chloromethyl ether (MOMCl) or benzene sulfonyl chloride, benzoyl chloride and the like, and carrying out acylation and bromination on glucose so as to prepare tetra-acetyl-1-bromoglucose; then, reacting the protected p-hydroxyphenylethyl alcohol with the tetra-acetyl-1-bromoglucose in the presence of potassium superoxide or triethylamine and other organic alkalis, silver nitrate, silver carbonate and the like; and feeding hydrogen chloride gas into methanol so as to remove protecting groups on phenolic hydroxyl groups. According to the invention, by simplifying the synthesis process route, the reaction selectivity is improved, the production cost of salidroside is significantly reduced, and the increasing demands of people on salidroside are satisfied better.
Owner:CHANGSHA UNIVERSITY
Preparation method of 4-benzyloxy phenyl ethyl n-decanoate
PendingCN114213248AReduce usageReduce pollutionPreparation from carboxylic acid halidesOrganic compound preparationBenzyl chloridePhenyl group
The invention discloses a preparation method of 4-benzyloxy phenyl ethyl n-decanoate, which comprises the following steps: A, adding methanol, p-hydroxyphenethyl alcohol and equivalent benzyl chloride into a clean three-neck flask, uniformly stirring and mixing, then adding sodium hydroxide solid in three batches, heating to reflux after each time of addition, and reacting for 0.5 hour, so as to obtain 4-benzyloxy phenyl ethyl n-decanoate; b, after the reaction is finished, directly distilling out the solvent, then after the solvent is completely distilled, directly dropwise adding n-decanoyl chloride with the same equivalent amount, and controlling the temperature to be lower than 20 DEG C, C, after the reaction is finished, adding a large amount of water, separating out a large amount of product at the moment, uniformly stirring, and centrifuging to obtain a crude product; and D, adding ethanol into the crude product, heating and dissolving, cooling and separating out, and centrifuging again to obtain a refined product 4-benzyloxy phenyl ethyl n-decanoate. In the reaction stage, the usage amount of solvents and reagents is reduced, no acid-binding agent is used, and therefore pollution to the environment is reduced, no by-product exists in the reaction process, and the production process is more environmentally friendly.
Owner:郑州睿嘉纳米新材料科技有限公司
Method for producing p-hydroxyphenylethanol and use of penicillium chrysogenum
ActiveCN110195084ALarge industrial productionSimple processOrganic chemistryOrganic compound preparationP-hydroxyphenylethanolChemistry
The invention discloses a use of penicillium chrysogenum DXY-1 in the production of p-hydroxyphenylethanol. The invention also discloses a method for producing p-hydroxyphenylethanol, comprising the step of using penicillium chrysogenum DXY-1. The invention adopts the production of p-hydroxyphenylethanol by penicillium chrysogenum for the first time, the process is simple, and the use has great significance for expanding the industrial production of p-hydroxyphenylethanol.
Owner:FUJIAN NORMAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com