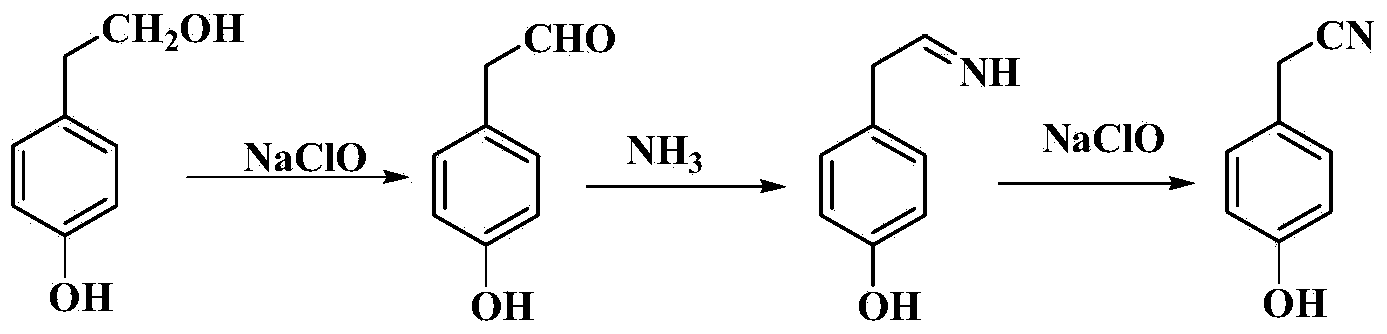
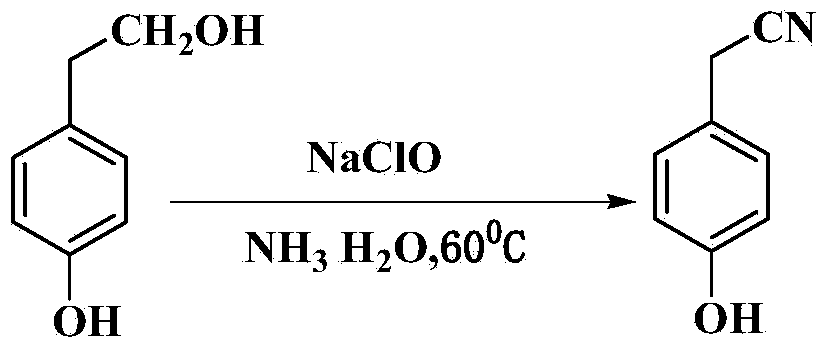Method for preparing para hydroxybenzene acetonitrile
A technology of hydroxyphenylacetonitrile and p-hydroxyphenylethanol, which is applied in the field of organic chemical synthesis, can solve the problem of high price, and achieve the effects of short route, mild reaction conditions and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Add 4.56 g (0.033 mol) of p-hydroxyphenethyl alcohol and 8.8 mL (0.132 mol) of ammonia water (28 wt%) into a 100 mL three-necked flask (equipped with a thermometer, magnetic stirring, reflux condenser and constant pressure dropping funnel), and the reaction mixture Heat to 50°C to dissolve p-hydroxyphenethyl alcohol, then slowly add 36.3mL (0.073mol) of 6.5wt% sodium hypochlorite aqueous solution dropwise, and control the reaction temperature at 50°C. Reaction adopts TLC detection reaction to carry out degree, developing agent sherwood oil / ethyl acetate (volume ratio 2:1), the R of p-hydroxyphenylacetonitrile f About = 0.6. After 20 hours of reaction, the reaction was completed, and the reaction solution was cooled to about 0°C to crystallize. After suction filtration and drying, 4.13 g of p-hydroxybenzonitrile was obtained, with a yield of 95.0% and a purity of 98.8% by gas chromatography.
[0025] m.p.: 69-71°C;
[0026] 1 HNMR (CDCl 3 , 400M), TMS): δ3.63 (s, 2H...
Embodiment 2
[0028] Add 9.12 grams (0.066mol) of p-hydroxyphenylethyl alcohol and 17.6mL (0.264mol) of ammonia water (28wt%) into a 250mL three-neck flask (equipped with a thermometer, magnetic stirring, reflux condenser and constant pressure dropping funnel), and the reaction mixture Heat to 60°C to dissolve p-hydroxyphenethyl alcohol. Then, 99.0 mL (0.198 mol) of 6.5 wt % sodium hypochlorite aqueous solution was slowly added dropwise, and the reaction temperature was controlled at 60 °C. The progress of the reaction was detected by TLC. After 10 hours of reaction, the reaction was completed, and the reaction solution was cooled to about 0°C to crystallize. After suction filtration and drying, 8.63 g of p-hydroxybenzonitrile was obtained with a yield of 98.2% and a purity of 99.0% by gas chromatography.
Embodiment 3
[0030] Add 9.12 grams (0.066mol) of p-hydroxyphenylethyl alcohol and 16.0mL (0.30mol) of ammonia water (35wt%) into a 250mL three-necked flask (equipped with a thermometer, magnetic stirring, reflux condenser and constant pressure dropping funnel), and the reaction mixture Heat to 60°C to dissolve p-hydroxyphenethyl alcohol. Then, 99.0 mL (0.198 mol) of 6.5 wt % sodium hypochlorite aqueous solution was slowly added dropwise, and the reaction temperature was controlled at 60 °C. The progress of the reaction was detected by TLC. After reacting for 9 hours, the reaction was completed, and the reaction solution was cooled to about 0°C to crystallize. After suction filtration and drying, 8.65 g of p-hydroxybenzonitrile was obtained with a yield of 98.4% and a purity of 99.2% by gas chromatography.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com