Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
33 results about "Methylomonas methanolica" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Method for simultaneous determination of six active components in Niuhuang Ninggong tablet
InactiveCN104897787ASimple methodAccurate methodComponent separationPhosphoric acidColumn temperature
The invention discloses a method for simultaneous determination of six active components consisting of chrysophanol, emodin, liquiritin, forsythin, baicalin and berberine hydrochloride in a Niuhuang Ninggong tablet through HPLC. Chromatographic conditions employed in the invention are as follows: a chromatographic column is TC-C18 (4.6 mm * 250 mm, 5 [mu]m); detection wavelength is 280 nm; a mobile phase is methanol-0.05% phosphoric acid; gradient elution comprises three parts, i.e., elution with methanol with a concentration varying in a range of 10 to 80% in the time period from 0 min to 35 min, then elution with methanol with a concentration of 80% in the time period from 35 to 50 min, and finally elution with methanol with a concentration varying in a range of 80 to 10% in the time period from 50 to 60 min; flow velocity is 1.0 mL / min; column temperature is 25 DEG C; and sample size is 10 [mu]L. Under the above-mentioned chromatographic conditions, chromatographic peaks are perfectly separated, and concentrations and peak areas of chrysophanol, emodin, liquiritin, forsythin, baicalin and berberine hydrochloride show good linear relation. The method is simple, rapid and accurate, has good repeatability and can provide quality bases for comprehensive evaluation and control of the Niuhuang Ninggong tablet.
Owner:JILIN NORMAL UNIV
Process for producing methyl carbamate by low pressure solvation homogeneous phase reaction
InactiveCN1693302ASignificant environmental benefitsReaction raw materials are readily availableCarbamic acid derivatives preparationOrganic compound preparationUreaPhase reaction
A process for preparing methyl aminoformate without environmental pollution includes such steps as proportionally mixing urea with methanol, reacting while supplementing the solution of urea-methanol for balancing the released ammonia-methanol vapor, purifying to obtain target product, rectifying said released vapor to obtain NH3 gas and methanol, cooling methol for recovering it, and using NH3 gas to prepare industrial ammonia water.
Owner:山东康瑞集团有限公司 +1
Sesquiterpene dimers in vernonia anthelmintica, and preparation method and application
The invention relates to sesquiterpene dimmers in vernonia anthelmintica, and a preparation method and application. The dimers comprises vernodalin dimer I, vernodalin dimer J and vernodalin dimer K. The preparation method comprises crushing vernonia anthelmintica seeds, percolating and degreasing by petroleum ether, so as to obtain total extractives, then performing gradient elution respectively by using petroleum ether / ethyl acetate, chloroform / methanol and methanol / water, and then performing repeated purification by using semi-preparative high performance liquid chromatograph. Three new sesquiterpene dimers, vernodalin dimer I, J and K are proved to be separated from vernonia anthelmintica seed through analysis on spectrum and mass spectrum data. In-vitro anti-tumor activity research shows that the provided compounds possess relatively obvious cytotoxic activity on human lung cancer cell A-549, human colon cancer cell HCT-15 and human prostate cancer cell PC-3, are applicable to low-toxicity anti-tumor medicines, and are new lead compounds for developing anti-tumor medicines.
Owner:XINJIANG TECHN INST OF PHYSICS & CHEM CHINESE ACAD OF SCI
Method for semi-continuously synthesizing trimethyl borate-methanol azeotrope
ActiveCN111233912ASolve the problem that it is difficult to discharge the inhibitory reactionImprove product qualityGroup 3/13 element organic compoundsPhysical chemistryBoronic acid
The invention discloses a method for semi-continuously synthesizing trimethyl borate-methanol azeotrope. The method comprises the following steps: mixing and heating boric acid and a transforming agent in a premixing tank, introducing a mixture into a stirred and heated reaction kettle, condensing gas generated in the reaction kettle, and separating; flowing a product into a mixing tank from the reaction kettle to be mixed with methanol, introducing the mixed material into a first rectifying tower, and obtaining the trimethyl borate-methanol azeotrope after tower top gas of the first rectifying tower is condensed; introducing bottom products of the first rectifying tower into a second rectifying tower; condensing tower top gas of the second rectifying tower to obtain methanol, recycling the methanol, and cooling and recycling a bottom product of the second rectifying tower. The molecular formula of the transforming agent is CxH2x+2+zOyNz, O exists in the form of hydroxyl, x is a natural number ranging from 2 to 8, y is a natural number ranging from 1 to 3, and z is a natural number not larger than 1. The method solves the problem that water generated in a traditional trimethyl borate preparation process is not easy to discharge, and digitization and automation of the process are easy to realize.
Owner:DALIAN UNIV OF TECH
Equipment and method for recycling dimethyl-4-pentenoic acid methyl ester rectification residual liquid
PendingCN112479872AImprove quality scoreSimple recycling processOrganic compound preparationPreparation by ester-hydroxy reactionSodium methoxideTrans esterification
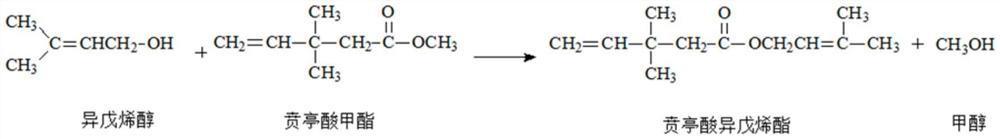
The invention relates to equipment and a method for recycling dimethyl-4-pentenoic acid methyl ester rectification residual liquid, and belongs to the technical field of 3,3-dimethyl-4-pentenoic acidmethyl ester synthesis. A condensation reaction kettle and a metering tank in the recovery equipment are connected with an inlet of an ester exchange reaction kettle through pipelines, and an outlet of the ester exchange reaction kettle is connected with an inlet of a vacuum degree adjusting tank through a condensing device; an outlet of the vacuum degree adjusting tank is connected with three branch pipes, the three branch pipes are respectively connected with the condensing device, the methanol recovery tank and the crude product tank, and an outlet of the crude product tank is connected with a feeding hole of the condensation reaction kettle and the rectification kettle. The method has the beneficial effects that the 3, 3-dimethyl-4-pentenoic acid methyl ester rectification residual liquid 3, 3-dimethyl-4-pentenoic acid isopentene ester is subjected to ester exchange reaction with methanol and sodium methoxide; by adjusting the temperature of the transesterification reaction, the vacuum degree after condensation and the reaction time, methanol, isopentenol anddimethyl-4-pentenoic acid methyl ester with high mass fraction can be collected, the recycling process is simple, and therecycling cost is reduced.
Owner:安徽鑫泰新材料有限公司
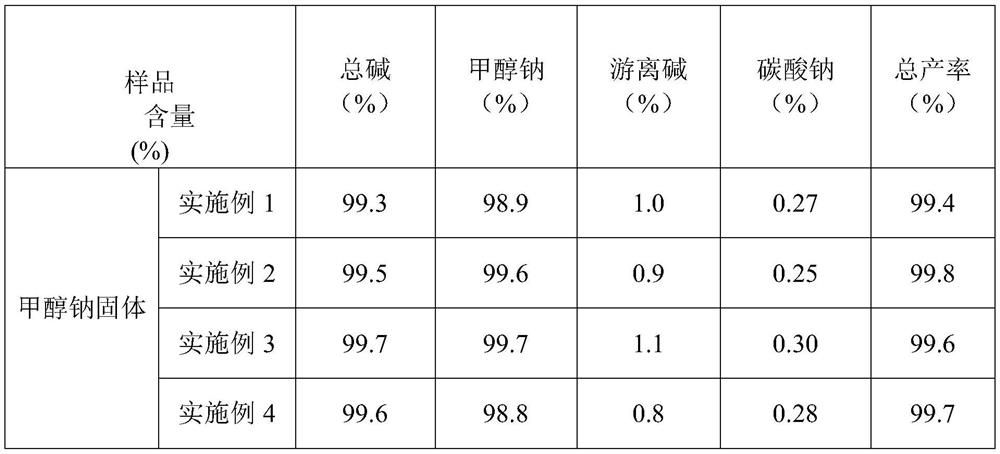
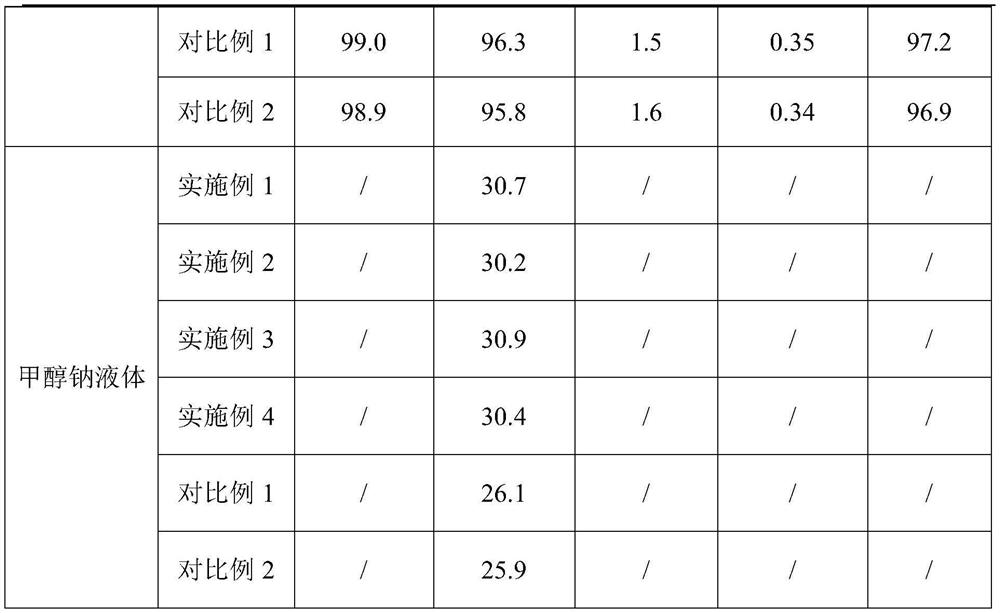
Production process of medicinal raw material sodium methoxide
ActiveCN112250543AWell mixedAdequate responseHydrogen separationPreparation of metal alcoholatesSodium methoxidePhysical chemistry
The invention relates to a production process of a medicinal raw material sodium methoxide, which comprises the following steps: proportionally adding metal sodium into a reaction kettle, slowly adding condensed methanol liquid into the reaction kettle while stirring, and continuously introducing inert gas into the reaction kettle; reacting metallic sodium with methanol liquid for 2-3 hours undernormal pressure, and thoroughly reacting to obtain a sodium methoxide mixed solution; performing heat preservation and filtration on the sodium methoxide mixed solution obtained in the step S2, obtaining filter residues and a target solution, enabling the filter residues to enter the next procedure, obtaining sodium methoxide after the target solution is subjected to negative-pressure distillationand drying, and condensing exhausted methanol gas for entering the reaction kettle again for continuous reaction. According to the method, the raw material sodium metal is prepared into molten or nanoscale powder, so that the sodium metal and methanol are fully mixed, the reaction between the sodium metal and methanol is more sufficient, the reaction speed is increased, and the reaction efficiency is improved.
Owner:安徽金邦医药化工有限公司
Method for preparing 2-(2-chloro-1-ethidene)hydrazide methyl formate
The invention provides a method for preparing a method for preparing an aprepitant intermediate 2-(2-chloro-1-ethidene)hydrazide methyl formate. The method comprises the following steps: carrying out a condensation reaction between chloroacetonitrile and methyl hydrazinocarboxylate in a methanol / sodium methylate reaction solution by virtue of catalysis of glacial acetic acid, and performing after-treatment purification on the reaction solution by adopting acetone. The operation is simple, the purity of the obtained product is over 99%, and the method is high in yield and suitable for industrial production.
Owner:深圳万乐药业有限公司
A method for simultaneous determination of six active ingredients in Niuhuang Ninggong Tablets
InactiveCN104897787BSimple methodAccurate methodComponent separationPhosphoric acidColumn temperature
The invention discloses a method for simultaneous determination of six active components consisting of chrysophanol, emodin, liquiritin, forsythin, baicalin and berberine hydrochloride in a Niuhuang Ninggong tablet through HPLC. Chromatographic conditions employed in the invention are as follows: a chromatographic column is TC-C18 (4.6 mm * 250 mm, 5 [mu]m); detection wavelength is 280 nm; a mobile phase is methanol-0.05% phosphoric acid; gradient elution comprises three parts, i.e., elution with methanol with a concentration varying in a range of 10 to 80% in the time period from 0 min to 35 min, then elution with methanol with a concentration of 80% in the time period from 35 to 50 min, and finally elution with methanol with a concentration varying in a range of 80 to 10% in the time period from 50 to 60 min; flow velocity is 1.0 mL / min; column temperature is 25 DEG C; and sample size is 10 [mu]L. Under the above-mentioned chromatographic conditions, chromatographic peaks are perfectly separated, and concentrations and peak areas of chrysophanol, emodin, liquiritin, forsythin, baicalin and berberine hydrochloride show good linear relation. The method is simple, rapid and accurate, has good repeatability and can provide quality bases for comprehensive evaluation and control of the Niuhuang Ninggong tablet.
Owner:JILIN NORMAL UNIV
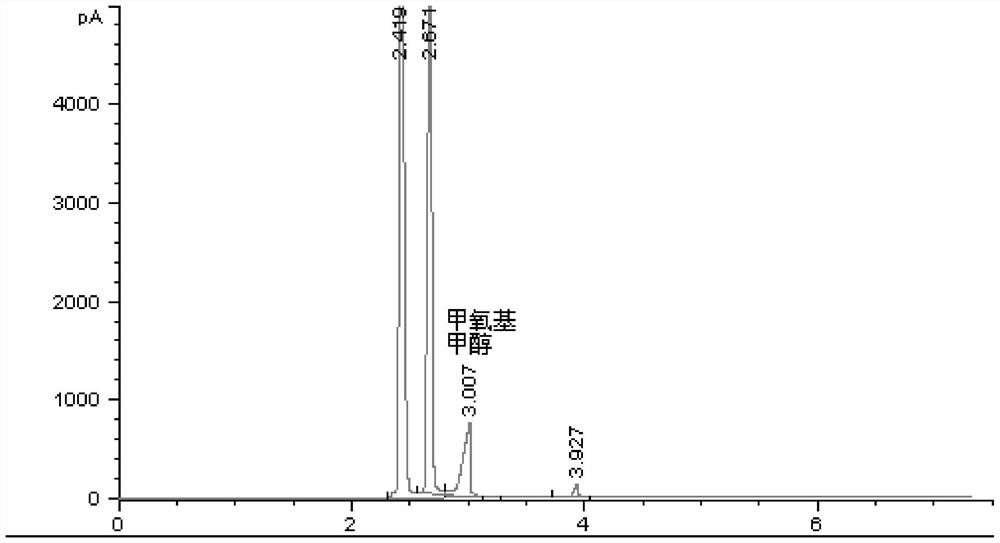
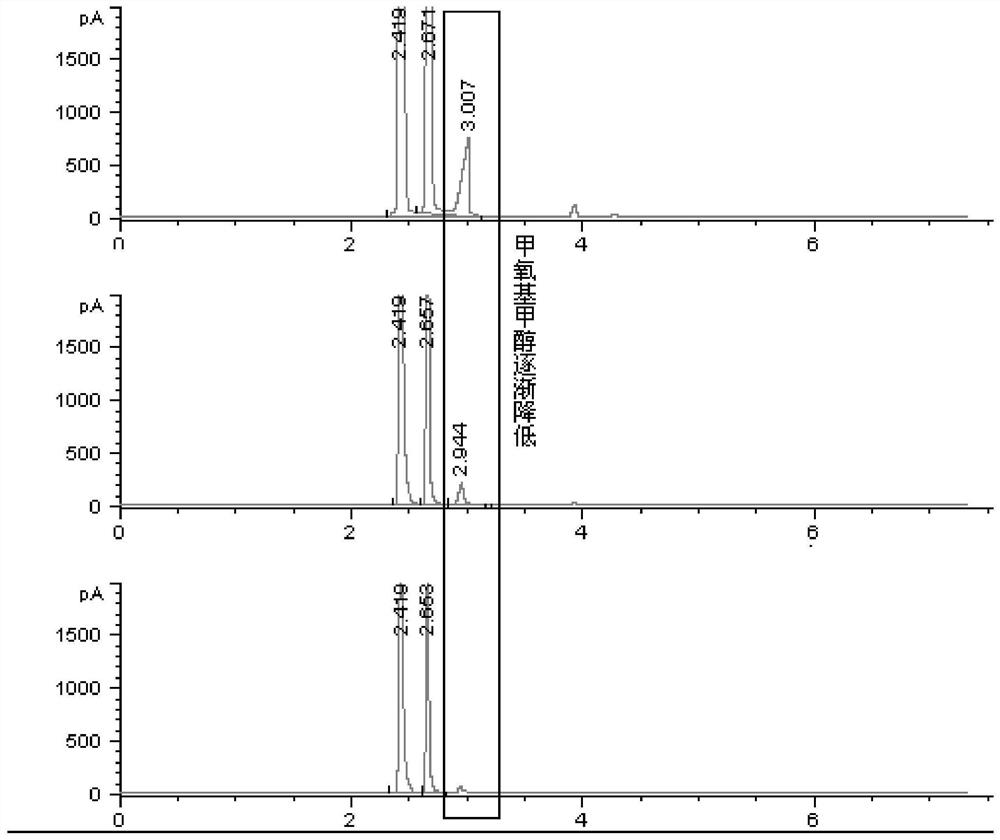
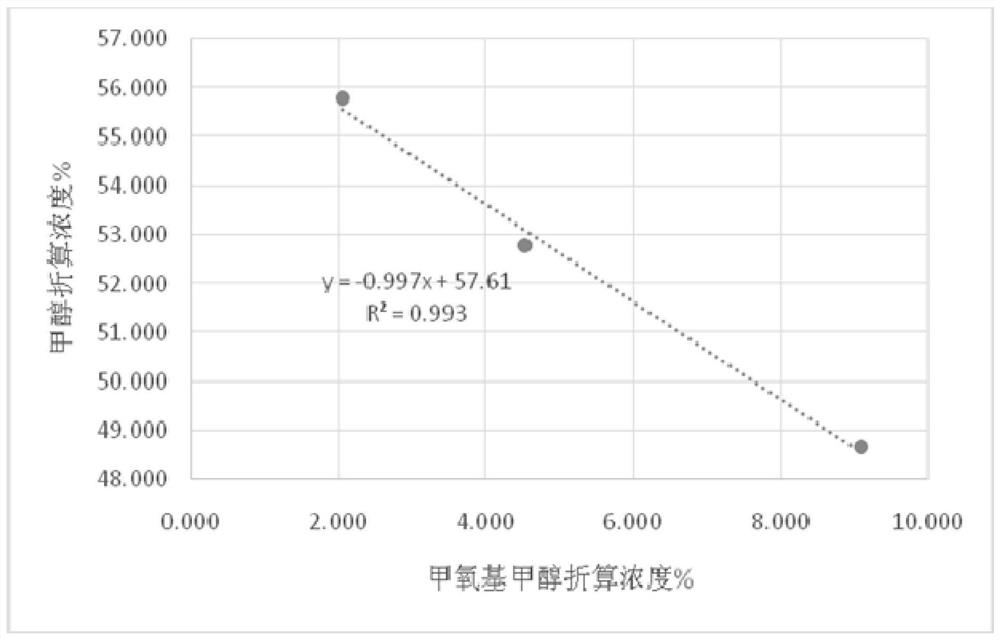
A method and application of measuring methanol relative correction factor in methoxymethanol without standard sample
ActiveCN111579663BAccurate determination of methanol contentThe result is accurateComponent separationMethylomonas methanolicaStandard samples
The invention discloses a method for measuring the relative correction factor of methanol in methoxymethanol without a standard sample. Methoxymethanol is a product of equimolar condensation of methanol and formaldehyde. The formaldehyde in methoxymethanol is used to react with the reagent to release methanol in methoxymethanol. The ratio of the reduction is the correction factor for methanol in methoxymethanol relative to free methanol. During the analysis process, adding a reference reagent is convenient for area normalization determination. After the response standard of the reference reagent is finally unified, the content of methanol and methoxymethanol after calibration under different reaction reagent additions is plotted, and linear fitting is performed. Then the relative correction factor for methanol in methoxymethanol was obtained. Using this method, the methanol content in methoxymethanol can be accurately determined without a standard sample. This method has the advantages of simple operation and accurate results.
Owner:SOUTHWEST RES & DESIGN INST OF CHEM IND
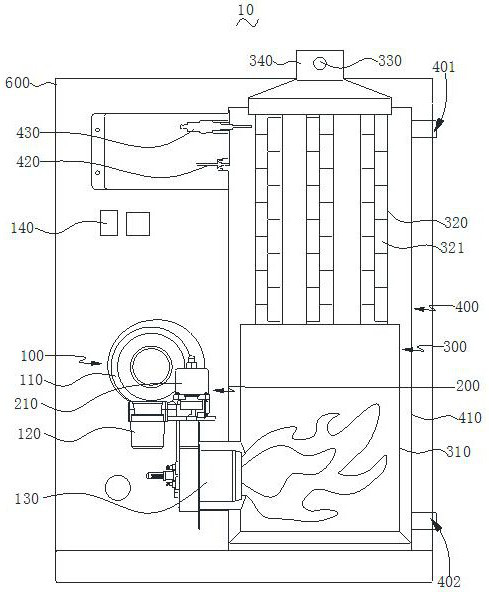
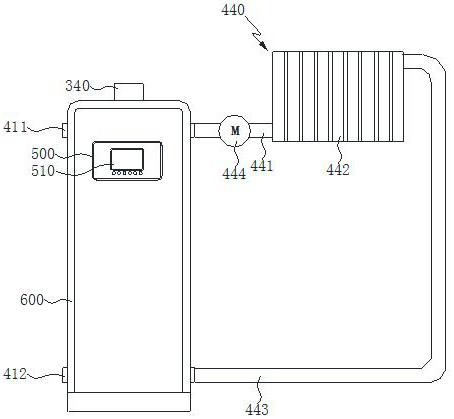
Energy-saving and environment-friendly closed-loop control methanol heating stove
The invention provides an energy-saving and environment-friendly closed-loop control methanol heating stove. In the working process, a controller regulates and controls a frequency conversion fan and a throttle valve to work in a coordinated mode according to the external air pressure value detected by an atmospheric pressure sensor, and the air pressure and the air volume matched with the external air pressure value are output. The controller controls a combustor to do ignition work, the controller controls a variable-frequency electromagnetic pump to eject methyl alcohol to an igniter of the combustor, the methyl alcohol is ignited after passing through the igniter of the combustor and generates flames, and the flames enter a combustion chamber under the action of wind power. And when a flame detection sensor detects that after the methyl alcohol is ignited, the controller controls the igniter of the combustor to stop working. And the temperature of the combustion chamber and the temperature of a smoke exhaust pipe rise, and refrigerant water in a heat exchange water tank is heated. The controller regulates and controls the ignition mechanism and the oil injection mechanism to work coordinately according to the oxygen concentration, detected by a wide-range oxygen sensor, in tail smoke exhausted by the smoke exhaust pipe, and therefore it is guaranteed that high combustion efficiency of the methyl alcohol can be maintained.
Owner:广东瀚宇新能源装备有限公司
Method for recycling cyanoacetamide in mother liquor
ActiveCN103113260BHigh recovery rateReduce processing costsCarboxylic acid nitrile purification/separationPhysical chemistryToluene
The invention discloses a method for recycling cyanoacetamide in mother liquor. The method comprises the following steps of: adding primary mother liquor into a reaction kettle, and stirring; slowly rising the temperature of the reaction kettle, evaporating methanol, starting to discharge methanol when the kettle temperature achieves about 65 DEG C, cooling the methanol by using a condenser and collecting the methanol; and finishing the evaporation when the temperature inside the reaction kettle achieves 90-92 DEG C; decreasing the temperature of the reaction kettle to about 50+ / -10 DEG C, subsequently adding water, toluene or heptane and the like into the reaction kettle, decreasing the temperature to 10-20 DEG C, filtering, centrifuging and drying so as to obtain a cyanoacetamide product. The method has the advantages of high recycling rate, emission reduction and pollution reduction.
Owner:NANTONG NABAIYUAN CHEM
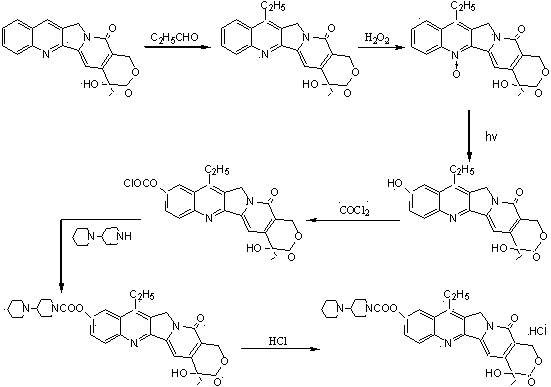
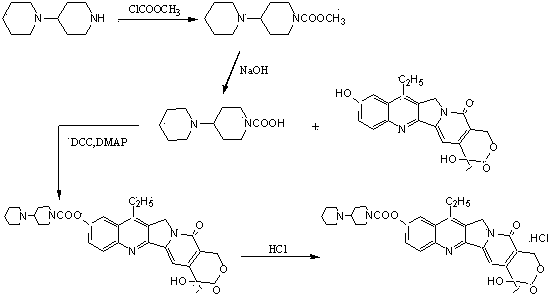
The preparation method of irinotecan hydrochloride
ActiveCN102627653BReduce transportationEasy to transportOrganic chemistryEthyl groupMethylomonas methanolica
The invention relates to a preparation method of irinotecan hydrochloride. The preparation method comprises the following steps: 1, reacting 4-piperidylpiperidine with dimethyl carbonate in a dipolar aprotic solvent to generate 4-piperidylpiperidine dimethyl carbonate; 2, reacting 4-piperidylpiperidine dimethyl carbonate with 7-ethyl-10-hydroxycamptothecin in a nonpolar solvent to generate irinotecan monomers; and 3, adding water to dissolve the irinotecan monomers, adding a hydrochloric acid solution to adjust the pH value of the obtained solution to 3-4, adding acetone, crystallizing, filtering, and carrying out vacuum drying to obtain finished irinotecan hydrochloride. The preparation method which introduces carbonyl groups to the active substance dimethyl carbonate during preparation of irinotecan hydrochloride has the characteristics of safe use, convenience, small pollution, easy transportation and the like in production; the preparation method which allows use of phosgene substances to be completely avoided and pollution to the environment to be correspondingly migrated is green; and an acid binding agent is not needed by the method, and methanol which is the only byproduct is a volatile substance with a low boiling point, so post-processing is simple.
Owner:NANJING CHENGONG PHARM CO LTD
Sesquiterpene dimer compounds in insect-repelling vernonia and its preparation method and use
Owner:XINJIANG TECHN INST OF PHYSICS & CHEM CHINESE ACAD OF SCI
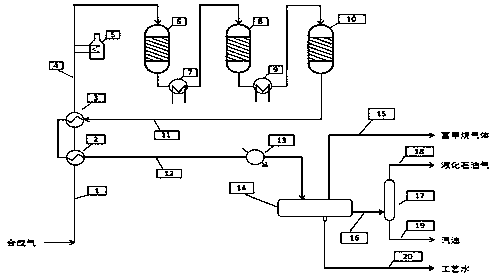
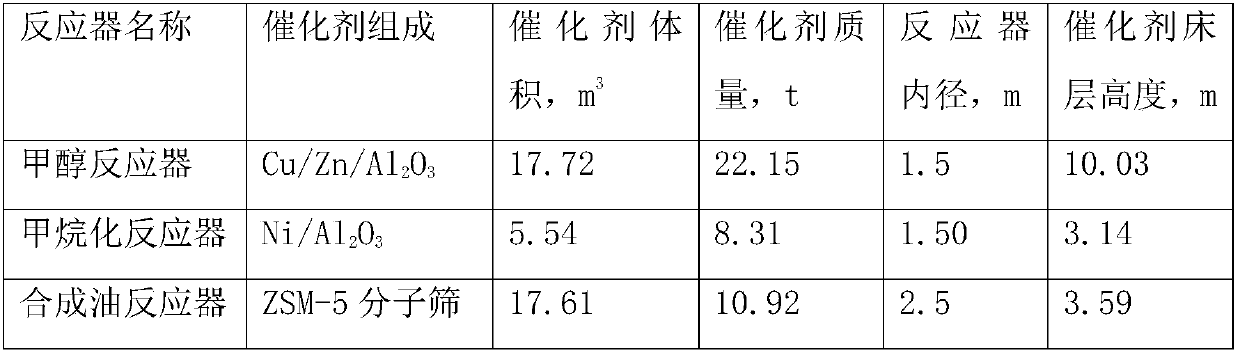
A method for producing methane-rich gas, liquefied petroleum gas and gasoline from synthesis gas
ActiveCN106085491BAvoid loopsReduce energy consumptionGaseous fuelsLiquid hydrocarbon mixture productionSyngasPtru catalyst
Owner:SHANXI FEISHI TECH
Method for recycling cyanoacetamide in mother liquor
ActiveCN103113260AHigh recovery rateReduce processing costsCarboxylic acid nitrile purification/separationPhysical chemistryToluene
The invention discloses a method for recycling cyanoacetamide in mother liquor. The method comprises the following steps of: adding primary mother liquor into a reaction kettle, and stirring; slowly rising the temperature of the reaction kettle, evaporating methanol, starting to discharge methanol when the kettle temperature achieves about 65 DEG C, cooling the methanol by using a condenser and collecting the methanol; and finishing the evaporation when the temperature inside the reaction kettle achieves 90-92 DEG C; decreasing the temperature of the reaction kettle to about 50+ / -10 DEG C, subsequently adding water, toluene or heptane and the like into the reaction kettle, decreasing the temperature to 10-20 DEG C, filtering, centrifuging and drying so as to obtain a cyanoacetamide product. The method has the advantages of high recycling rate, emission reduction and pollution reduction.
Owner:NANTONG NABAIYUAN CHEM
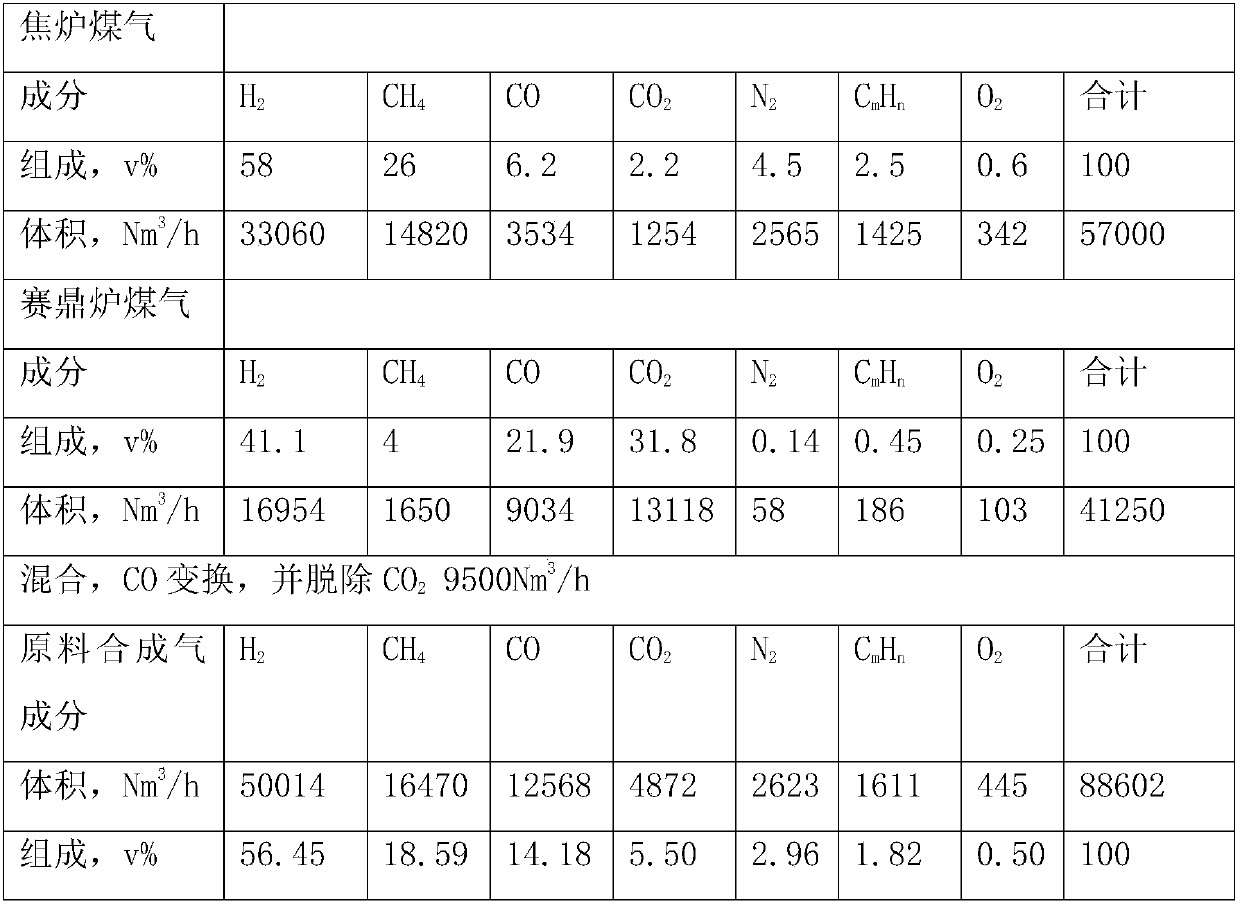
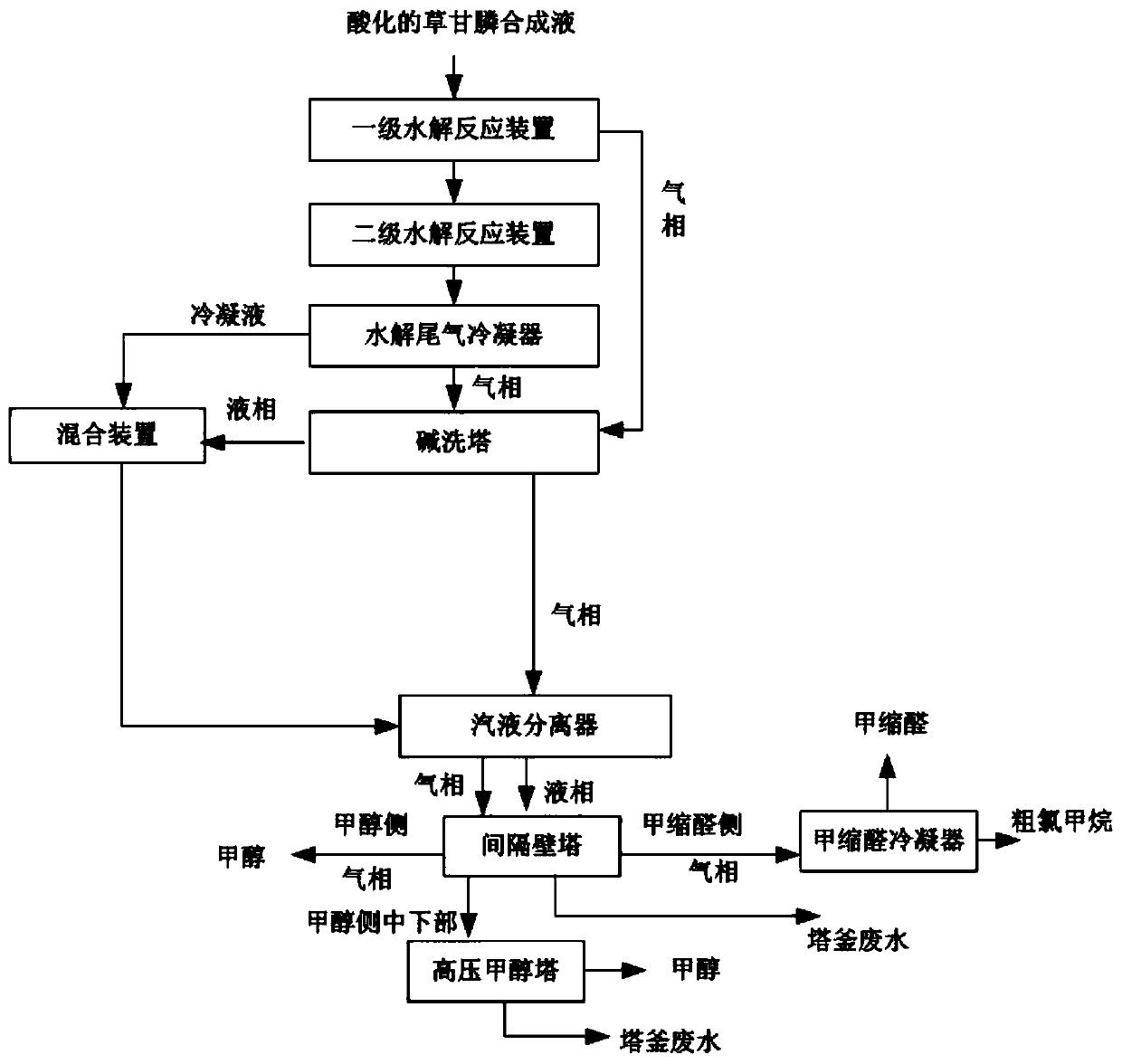
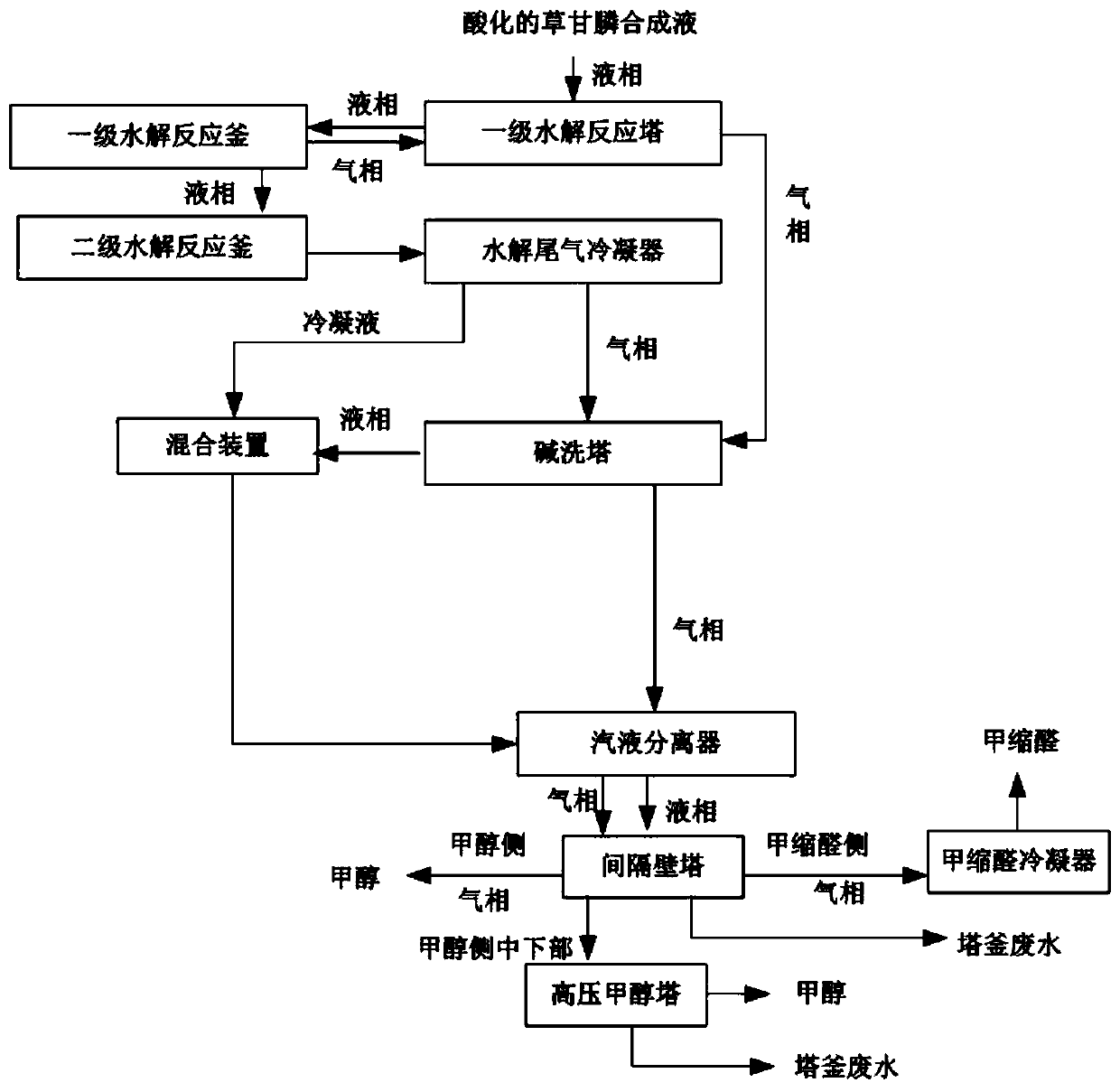
Method and system for recovering glyphosate synthesis liquid hydrolysis tail gas
PendingCN111214944AHigh recovery rateReduce consumptionDispersed particle separationVapor condensationGlyphosatePhysical chemistry
The invention relates to the technical field of glyphosate production, in particular to a method and system for recovering glyphosate synthesis liquid hydrolysis tail gas. The recovery method comprises the following steps of: carrying out primary hydrolysis reaction and secondary hydrolysis reaction on acidified glyphosate synthesis liquid, and condensing the gas subjected to secondary hydrolysisreaction to obtain condensate liquid and non-condensable gas; mixing the gas generated by the primary hydrolysis reaction, the non-condensable gas and alkali liquor, and carrying out neutralization reaction; mixing the condensate liquid with the solution subjected to the neutralization reaction, adjusting the pH value to 7.5-12, carrying out gas-liquid separation on the obtained solution and the gas obtained by the neutralization reaction, and enabling the solution and the gas obtained by the gas-liquid separation to respectively enter a dividing-wall tower; discharging methylal gas from the top of the methylal side tower, and performing condensation to obtain methylal and chloromethane crude products; discharging methanol from the top of the methanol side tower; and rectifying the mixturedischarged from the tower side of the methanol side tower to obtain methanol. The recovery method is simple in process, can effectively recover byproducts, and is low in steam consumption and low incost.
Owner:ZHEJIANG XINAN CHEM INDAL GROUP
Glucosamine donor and application thereof
PendingCN111925403AHigh synthesis efficiencyMild conditions for deprotectionSugar derivativesSugar derivatives preparationSodium methoxideLeaving group
The invention discloses a glucosamine donor and application thereof. According to the glucosamine donor, N, N-dimethylformamide is introduced into a C-2 position nitrogen atom for performing N, N-diacetyl protection; an electron-rich activated (armed) protecting group is introduced to at least one position of glucosamine C-3, C-4 and C-6, wherein the N, N-diacetyl has an ortho-group participationeffect, can generate a single beta-selective glycosylation product, and can be removed from acyl groups at other positions by a one-kettle method under mild conditions such as methanol / sodium methoxide and the like so that the synthesis efficiency is improved. The iso-head leaving group of the glucosamine donor can be halogen, glucosinolate, imine ester, pentene alkoxy, hydroxyl and the like, andcan react with different receptors to construct a beta-glucosidic bond so that the reaction efficiency is high.
Owner:SHANDONG UNIV
Synthesis method of p-hydroxyphenylethanol
ActiveCN111393264ASimple post-processingSimple and mild reaction conditionsOrganic compound preparationEther preparation by ester reactionsSodium methoxideChlorobenzene
The invention relates to the field of organic synthesis, and discloses a synthesis method of p-hydroxyphenylethanol, which comprises the following steps: (1) mixing methanol, sodium methoxide and a catalyst, and performing stirring; (2) adding 4-chlorophenethyl alcohol, and carrying out a heating reaction; (3) cooling to room temperature, carrying out filtering, and drying by distillation to obtain a 4-methoxyphenethyl alcohol crude product; (4) adding the crude product into a solvent, and performing stirring; (5) dropwise adding hydrobromic acid, carrying out heating reflux, and carrying outa heat preservation reaction until the reaction is finished; (6) cooling to 55-65 DEG C, slowly adding an alkaline solution, and adjusting the pH value to 6-7; and (7) cooling to 8-12 DEG C, stirringfor 0.5-1.5h, filtering the material to obtain a wet filter cake, adding a solvent, heating for dissolution, separating water, and carrying out recrystallizing, filtering, and spin-drying to obtain the final product. The product purity can reach 99% or more, the yield can reach 90% or more, and the whole synthesis process has the advantages of simple and mild reaction conditions, simple post-treatment, and low cost.
Owner:杭州盛弗泰新材料科技有限公司
The preparation method of 2-(2-chloro-1-ethylene) hydrazide methyl formate
The invention provides a method for preparing a method for preparing an aprepitant intermediate 2-(2-chloro-1-ethidene)hydrazide methyl formate. The method comprises the following steps: carrying out a condensation reaction between chloroacetonitrile and methyl hydrazinocarboxylate in a methanol / sodium methylate reaction solution by virtue of catalysis of glacial acetic acid, and performing after-treatment purification on the reaction solution by adopting acetone. The operation is simple, the purity of the obtained product is over 99%, and the method is high in yield and suitable for industrial production.
Owner:深圳万乐药业有限公司
A kind of evaporation system and production process of dimethylformamide
ActiveCN111450553BGuaranteed uptimeThe effect of inhibiting the reverse reactionEvaporator accessoriesEvaporator regulation/controlSodium methoxideProcess engineering
The invention specifically relates to an evaporation system and a production process of dimethylformamide. Using carbon monoxide and dimethylamine as raw materials to prepare dimethylformamide under the catalysis of methanol / sodium methoxide has the advantages of good product quality and low cost, but sodium methoxide dissolves in water and is prone to crystallization, and it will catalyze DMF in the evaporation process A reverse reaction occurs to form dimethylamine. The present invention provides a kind of DMF evaporation system and production process applicable to the above-mentioned production mode, and the described production process comprises the following steps: after the crude DMF of the DMF synthesis tower enters the separator for preliminary concentration, it is divided into two routes and the filter is forced Circulation, one way is to add water to the inlet of the second circulation pump to ensure the normal operation of the evaporation circulation pump by adding water, and the other is to inhibit the reverse reaction of DMF. The first circulating pump in the other path plays the role of filtering and discharging the salt mud in the system. The production process can effectively improve the DMF operation cycle and increase the output.
Owner:LIAOCHENG YANSHAN NEW MATERIAL TECH
A kind of hollow iron oxide material and preparation method thereof
ActiveCN107892333BReduce consumptionOvercoming the shortcoming of multi-step verbosityFerric oxidesHydrofluoric acidIron salts
The invention belongs to the technical field of material synthesis, and specifically relates to a hollow iron oxide material and a preparation method thereof. An iron salt and an organic ligand are used as raw materials, methanol, a mixed solution of methanol and water, N,N-2-methylformamide, or a mixed solution of N,N-2-methylformamide and water is used as a reaction solvent, nitric acid is usedas an oxidant, hydrofluoric acid is used as an etching agent, a reaction is performed for 12-72 h, and petal-like hollow iron oxide is obtained. The preparation method provided by the invention has the advantages of controllable etching conditions, simple process, short flow, uniform control of the product morphology and the like, and provides a promising synthesis idea for exploring synthesis ofhollow etching iron oxide.
Owner:CENT SOUTH UNIV
A kind of synthetic method of p-hydroxyphenethyl alcohol
ActiveCN111393264BSimple post-processingThe reaction conditions of the synthesis process are simple and mildOrganic compound preparationEther preparation by ester reactionsSodium methoxideChlorobenzene
The invention relates to the field of organic synthesis, and discloses a method for synthesizing p-hydroxyphenethyl alcohol. Evaporate to dryness to obtain the 4-methoxyphenethyl alcohol crude product; 4) add the crude product to the solvent and stir; 5) dropwise add hydrobromic acid, heat up and reflux, and keep the temperature for the reaction until the reaction is complete; 6) be cooled to 55-65 ° C , slowly add alkaline solution, adjust the pH value to 6-7; 7) cool down to 8-12 ° C, stir for 0.5-1.5h, filter the material to obtain a wet filter cake, add a solvent, heat up to dissolve, separate water, and recrystallize , filtered, and spin-dried to obtain the final product. The purity of the product of the method of the invention can reach more than 99%, the yield can reach more than 90%, and the reaction conditions of the whole synthesis process are simple and mild, the post-processing is simple, and the cost is low.
Owner:杭州盛弗泰新材料科技有限公司
Furanone preparation system and preparation method
PendingCN114588861AEmission reductionSuitable for industrial productionProcess control/regulationSequential/parallel process reactionsSodium methoxideFuran
According to the furanone preparation system and method, three raw material storage tanks, namely the ethyl lactate storage tank, the ethyl chloroacetate storage tank and the sodium methoxide storage tank, are arranged in a first reaction unit, so that the added raw materials are cheap and are suitable for industrial production, the danger coefficient of the reaction is reduced, methanol is generated after the sodium methoxide reaction, the methanol is neutral, and the furanone yield is improved. The outlet of the ethyl lactate storage tank is provided with a third valve, and the outlet of the ethyl chloroacetate storage tank is provided with a fourth valve, so that when raw materials are added into the first reaction kettle, a solvent is firstly added, then sodium methoxide is added, and finally ethyl chloroacetate is added; the adding sequence of ethyl lactate and ethyl chloroacetate is controlled by controlling the opening and closing of the third valve and the fourth valve, so that the situation that the yield of the main product alpha-methyl diethyl diglycolate is reduced due to the condensation reaction of ethyl chloroacetate in the presence of sodium methoxide is avoided.
Owner:宁夏万香源生物科技有限公司
A kind of synthetic gas one-step method for producing methyl acetate
ActiveCN108774130BHigh selectivityHighly selective preparationPreparation by carbon monoxide or formate reactionPtru catalystCombinatorial chemistry
A method for producing methyl acetate from synthesis gas by one-step method relates to a method for synthesizing the methyl acetate. The synthesis gas or the synthesis gas containing CO2 is used as areaction raw material, and the continuous relay reaction is carried out on a multifunctional catalyst to realize the preparation of methyl acetate by one-step method with high selectivity. In the process, the synthesis gas or the synthesis gas containing CO2 is first converted into methanol, then the methanol is dehydrated to form dimethyl ether, and further the dimethyl ether is carbonylated to obtain the methyl acetate. The one-step route significantly simplifies the reaction steps, improves catalytic efficiency, and significantly reduces costs. The used catalyst is a multifunctional composite catalyst, and the synthesis gas is converted into the methyl acetate in one step with high efficiency by designing and functionally coupling components. The target product has high selectivity, themethyl acetate selectivity is up to 90% or more, and the stability is high. By introducing a dehydration catalyst, the dehydration step and the dimethyl ether carbonylation step are separated to eliminate the interference of water to a reaction system. The preparation process is simple and controllable.
Owner:XIAMEN UNIV
A method for non-methanol-induced production of antimicrobial peptides
ActiveCN107058432BEfficient inductionEfficient expressionFungiMicroorganism based processesBiotechnologyAntimicrobial drug
The invention discloses a method for producing antibacterial peptide through non-methanol induction. The invention relates to the technical field of the bioengineering and bio-pharmaceuticals, the bad expression effect of the antibacterial peptide in the pichia pastoris is solved, the most important is the methanol resides in the antibacterial peptide product expressed by use of the methanol induction, the toxicity of the methanol enables the antibacterial peptide product to be not applied in the fields of the food, the fodder and the medicines. The technical scheme provided by the invention is to add the inductor choline chloride into an inoculated culture medium so that the final concentration of the choline chloride in the culture medium is 1%-5%w / v. The method disclosed by the invention has the advantages that the PisL9K22WK expression level is high, the production cost is low, the product is safe and free from poisonous residue; the engineering saccharomycetes and the produced pisL9K22WK product can be applied to the antibacterial medicines or the animal or aquiculture fodder additives.
Owner:深圳市微宇生物科技有限公司
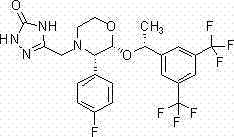
Preparation method of 5-[(3, 4, 5-trimethoxyphenyl)-methyl]-2, 4-pyrimidine diamine
The invention provides a preparation method of 5-[(3, 4, 5-trimethoxyphenyl) methyl]-2, 4-pyrimidine diamine, and the method comprises the following steps: performing stirring and dissolving, performing heating and refluxing, performing stirring and performing suction filtration. In the stirring and dissolving step, acrylonitrile, aniline, methanol and sodium methoxide are added into a four-neck flask with a mechanical stirrer and stirred to be dissolved; the heating reflux comprises the following steps: adding a 3, 4, 5-trimethoxybenzaldehyde crude product at 50-60 DEG C within 20-30 minutes,heating to reflux and react for 2-3 hours, and carrying out TLC tracking until spots of the TMB raw material disappear. The invention has the beneficial effects that the cost is saved, the generationof solid wastes is reduced, the safety of the production process is improved, the equipment cost is reduced, and the safety risk is avoided; the yield can be increased to 93%; the method can be directly used for the next synthesis without refining, and has no influence on the purity of trimethoprim, thereby lowering the production cost and the like.
Owner:SHOUGUANG FUKANG PHARMA +1
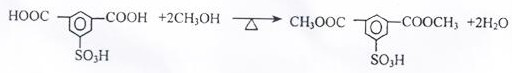
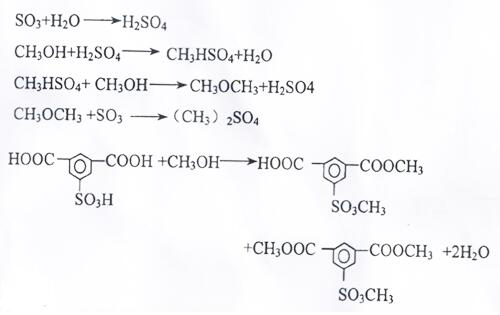
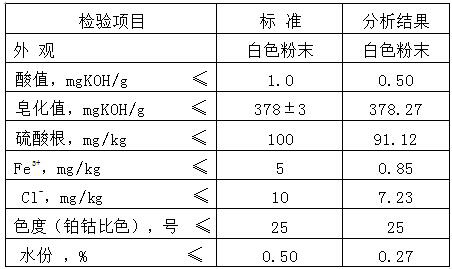
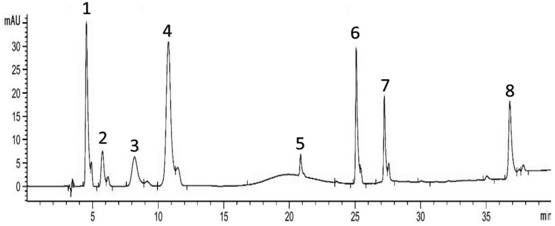
A production method for improving the yield and purity of dimethyl isophthalate-5-sulfonic acid for synthesizing three monomers
ActiveCN109608366BReduce unit consumptionImprove securitySulfonic acid preparationPtru catalystMethylomonas methanolica
The invention provides a kind of production method that improves three monomer synthesis dimethyl isophthalate-5-sulfonic acid yield and purity, described production method, comprises N 2 Gas purging, adding sulfonated material, adding part of methanol, temperature rise and pressure reaction, adding remaining methanol and catalyst, step temperature rise reaction. The invention reduces the unit consumption of methanol and improves the economic benefits of the product; equivalent to 413-439Kg of methanol consumed per ton of three monomers; the feed amount of methanol is 20% less than that of the existing process; the unit consumption of methanol per ton of three monomers is lower than that of the existing process more than 30%; the purity of dimethyl isophthalate-5-sulfonic acid exceeds 96%, reaching 96.85-97.05%, and the yield exceeds 97%, reaching 97.18-97.42%; the subsequent production of the three-monomer product The rate exceeds 84%; the reaction time is shortened; the esterification reaction operation cycle is shortened by 2 hours compared with the existing production process.
Owner:SHANDONG FIRST MEDICAL UNIV & SHANDONG ACADEMY OF MEDICAL SCI
A HPLC method for the simultaneous determination of eight active components in Codonopsis pilosula
ActiveCN109470792BSimple and fast operationImprove accuracyComponent separationMedicinal herbsO-Phosphoric Acid
The invention discloses an HPLC method for simultaneous determination of eight active components in Codonopsis pilosula. , Atractylodes Lactone III and 5-Hydroxymethylfurfural, its assay steps are as follows: (1) preparation of test solution; (2) chromatographic condition: C18 chromatographic column, mobile phase consists of methanol-0.01% phosphoric acid aqueous solution, gradient Elution program: 0‑10min, 10% methanol; 10‑25min, 10%‑65% methanol; 25‑40min, 65%‑80% methanol. Wavelength: 0‑5min, 260nm; 5‑6.5min, 210nm; 6.5‑36.5min, 260nm; 36.5‑38min, 220nm; 38‑40min, 260nm. Flow rate 1 mL / min. Column temperature: 30°C. The injection volume was 10 μL. (3) Establishment of standard curve for the determination of the content of eight active ingredients; (4) Determination of the content of active ingredients in the sample. The method is easy to operate, accurate, fast and efficient, can simultaneously measure the contents of eight active components in Codonopsis pilosula medicinal materials, and has great practical value.
Owner:CHONGQING MEDICAL UNIVERSITY
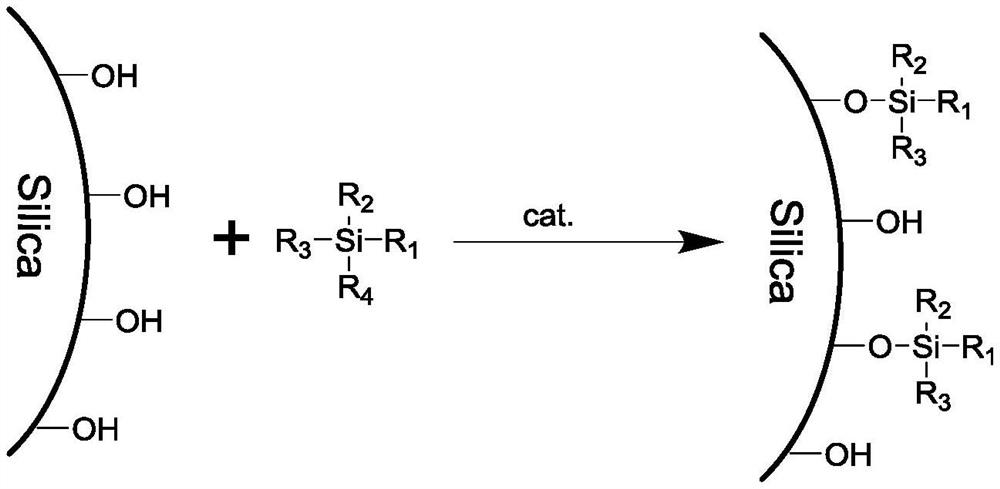
Pure water-resistant chromatographic stationary phase as well as preparation method and application thereof
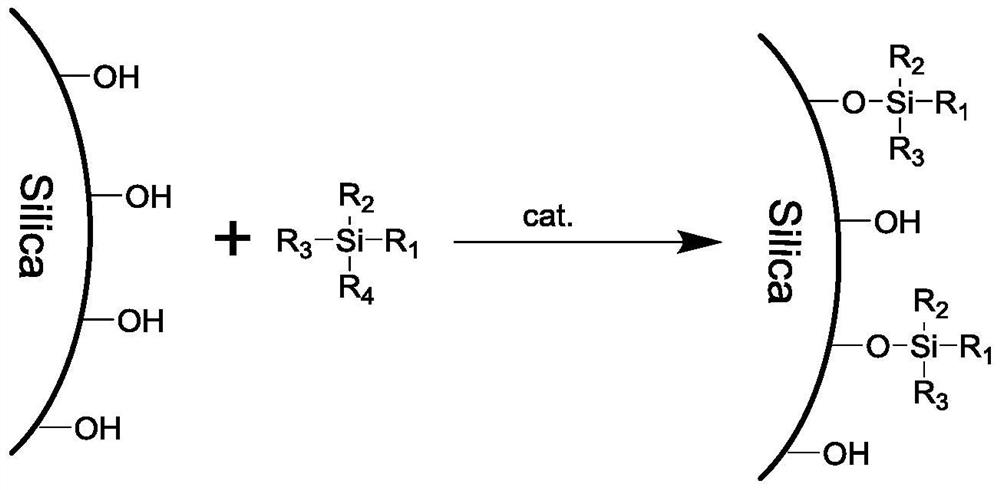
InactiveCN113694907ARealization of resistance to pure water phaseGood conditionComponent separationOther chemical processesStationary phasePtru catalyst
The invention discloses a pure water-resistant chromatographic stationary phase as well as a preparation method thereof. According to the stationary phase, silica gel is taken as a matrix and is bonded with alkyl C1-C30, and the bonding density is 1.0-2.5 micromole / m < 2 >. The preparation method of the stationary phase comprises the following steps of: dispersing silica gel in an organic solvent; adding an inorganic weak base or an organic base as a catalyst; adding silane according to the feeding amount of 0.2-1.0 g per gram of silica gel; performing a reaction at 50-150 DEG C for 3-24 hours; filtering reaction liquid; cleaning a filter cake with a reaction solvent, methanol, 30-70% methanol / water (v / v) and methanol in sequence; and drying an obtained solid at 50-80 DEG C under a vacuum condition for 6-24 hours to obtain the stationary phase product. By optimizing the surface bonding density of the stationary phase, the state of the surface bonding phase of the stationary phase is improved, and the pure water-resistant chromatographic stationary phase is obtained. According to the stationary phase and the preparation method thereof, the reaction conditions are mild, the steps are simple and feasible, and repeated and large-scale preparation is easy.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
A method for separating and purifying epothilone d based on molecular imprinting
Owner:NANJING TECH UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com





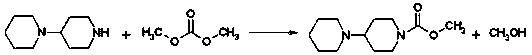


![Preparation method of 5-[(3, 4, 5-trimethoxyphenyl)-methyl]-2, 4-pyrimidine diamine Preparation method of 5-[(3, 4, 5-trimethoxyphenyl)-methyl]-2, 4-pyrimidine diamine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/81dd1c0d-98bd-4f38-af5b-bfcfb72e8ee3/162162DEST_PATH_IMAGE001.png)
![Preparation method of 5-[(3, 4, 5-trimethoxyphenyl)-methyl]-2, 4-pyrimidine diamine Preparation method of 5-[(3, 4, 5-trimethoxyphenyl)-methyl]-2, 4-pyrimidine diamine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/81dd1c0d-98bd-4f38-af5b-bfcfb72e8ee3/348424DEST_PATH_IMAGE002.png)