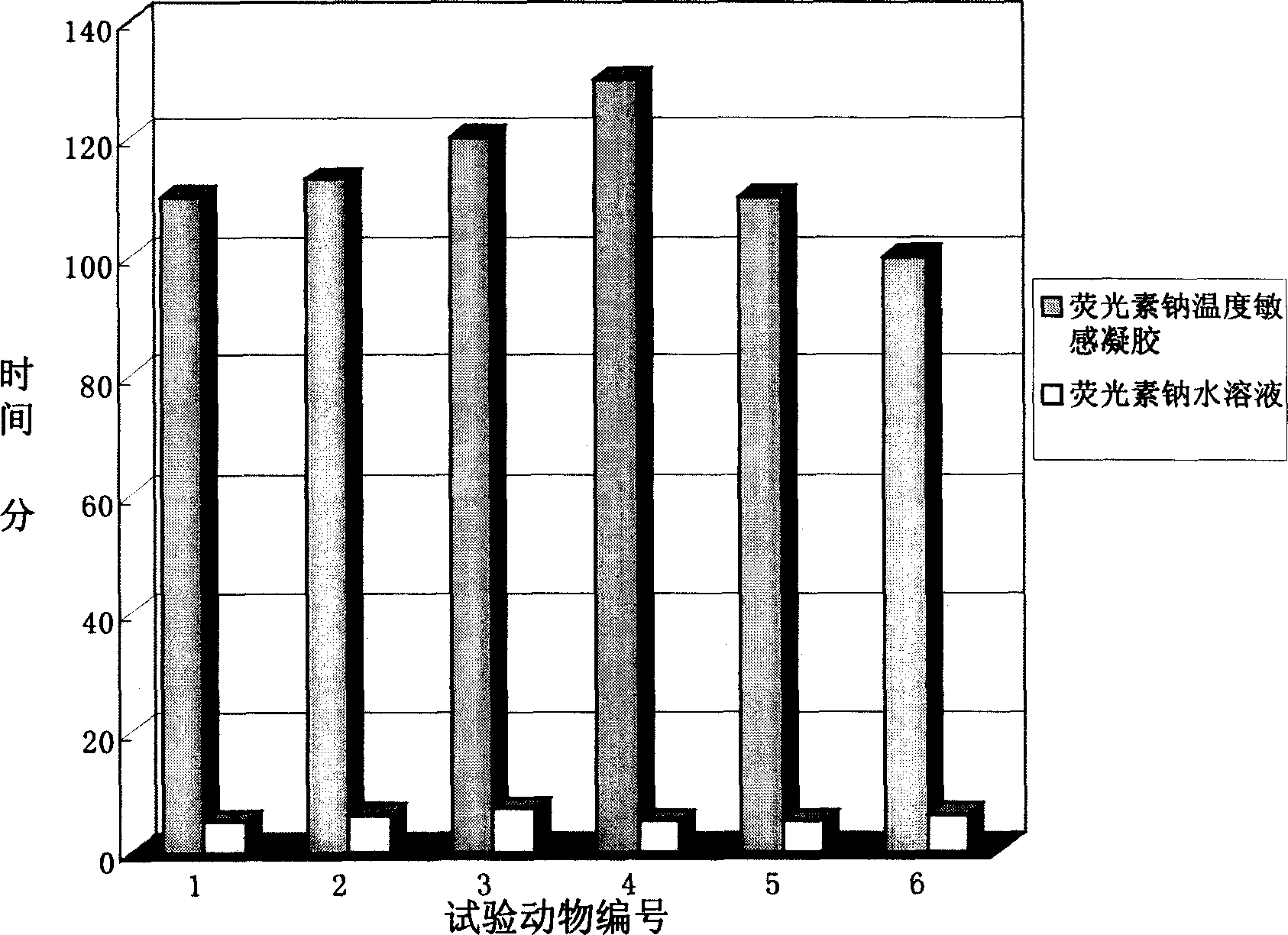
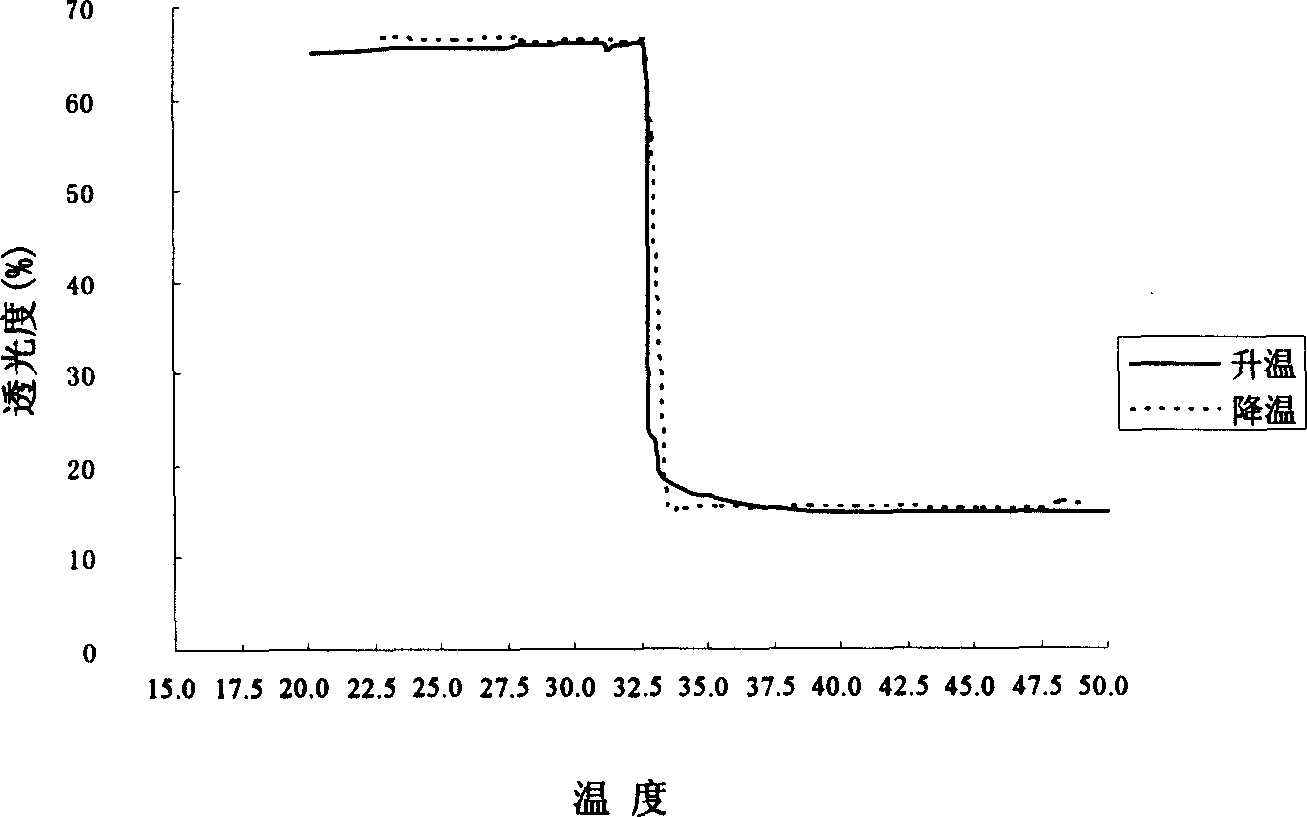
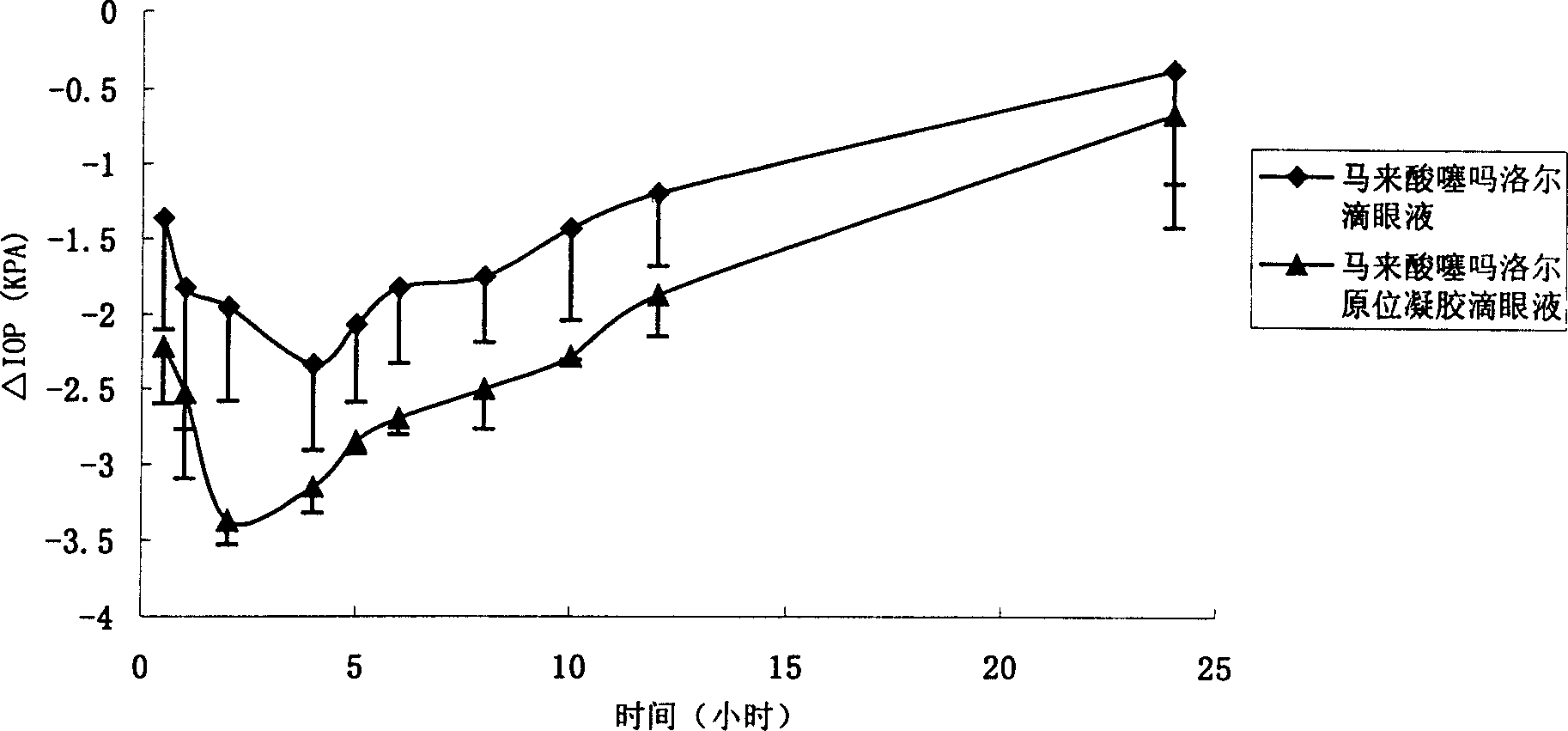
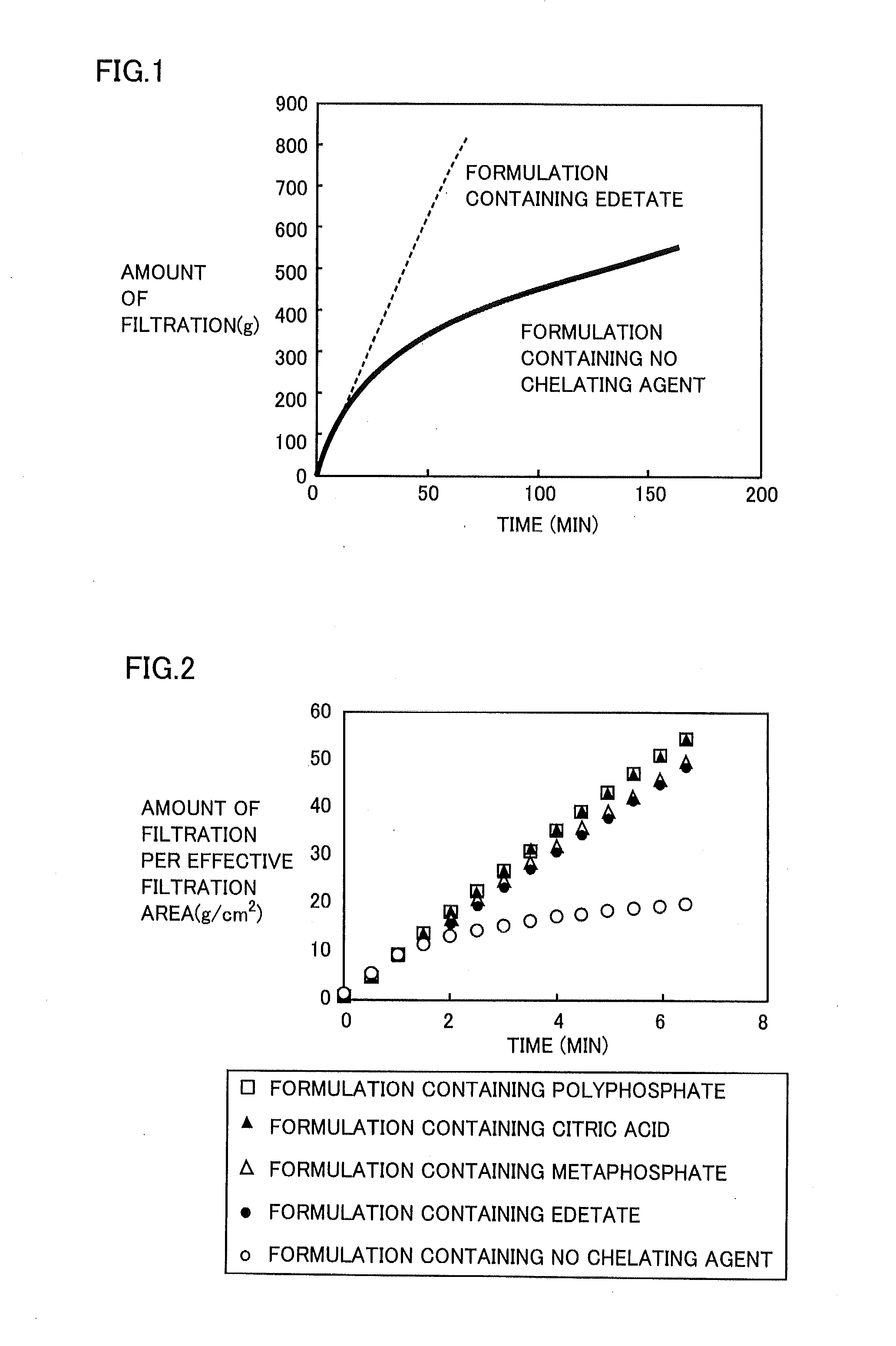
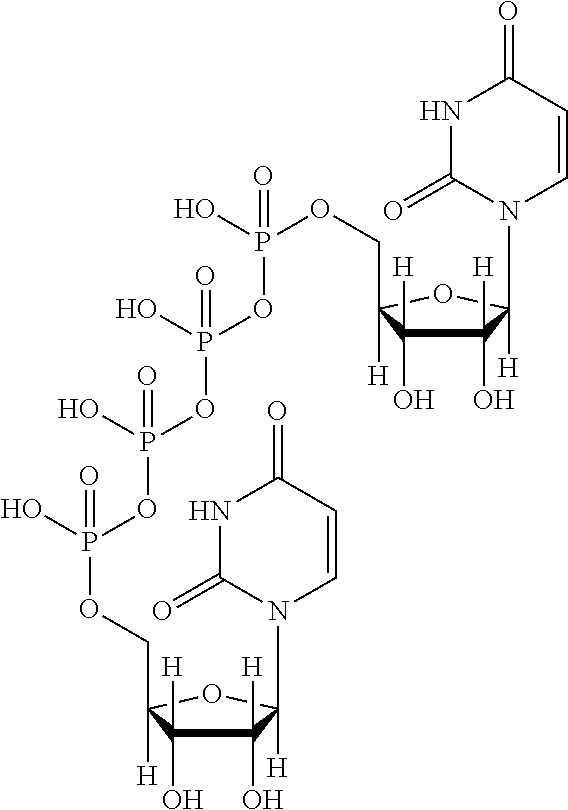
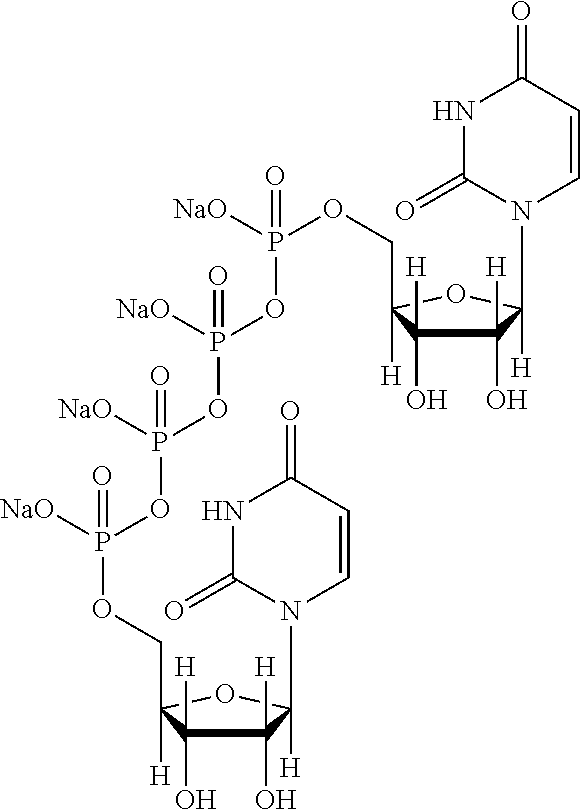
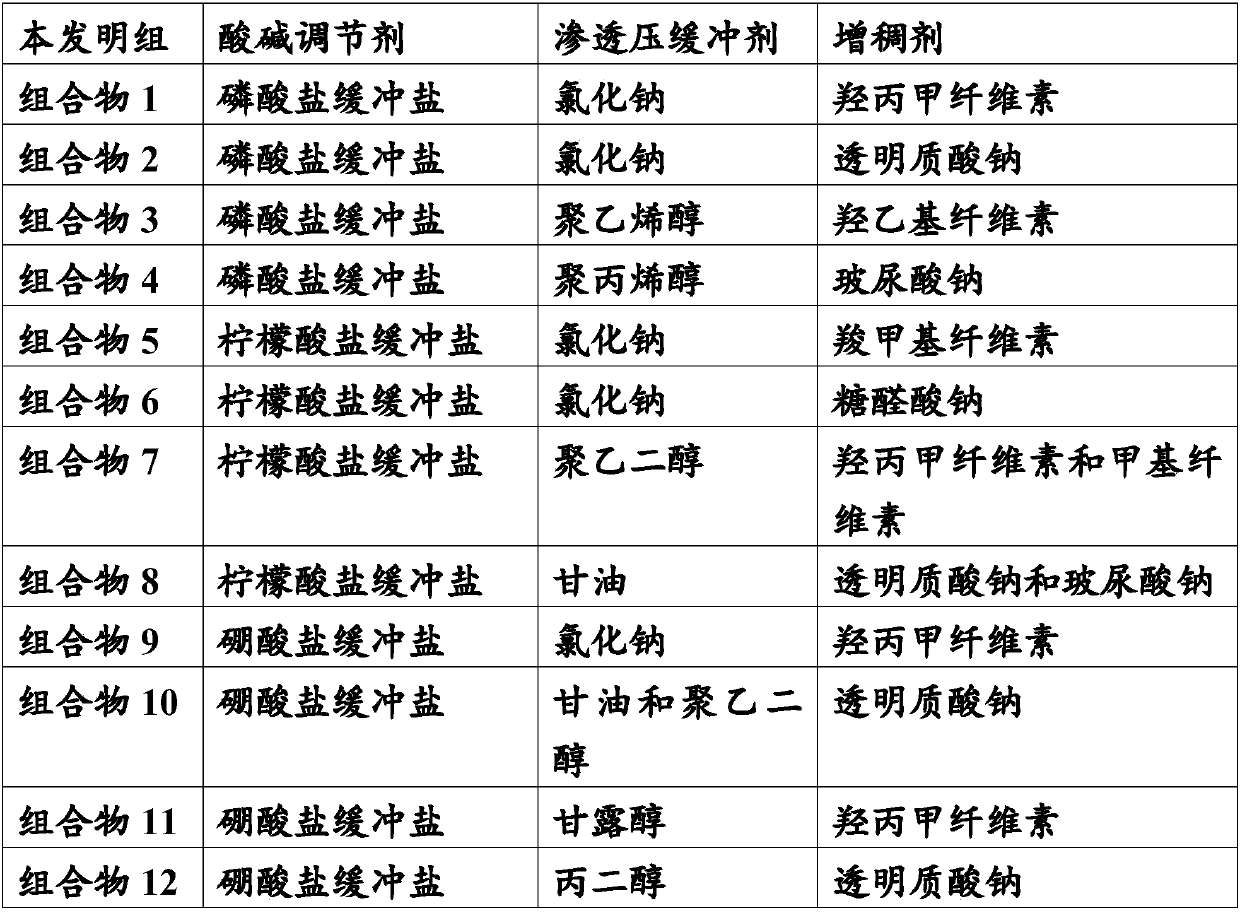
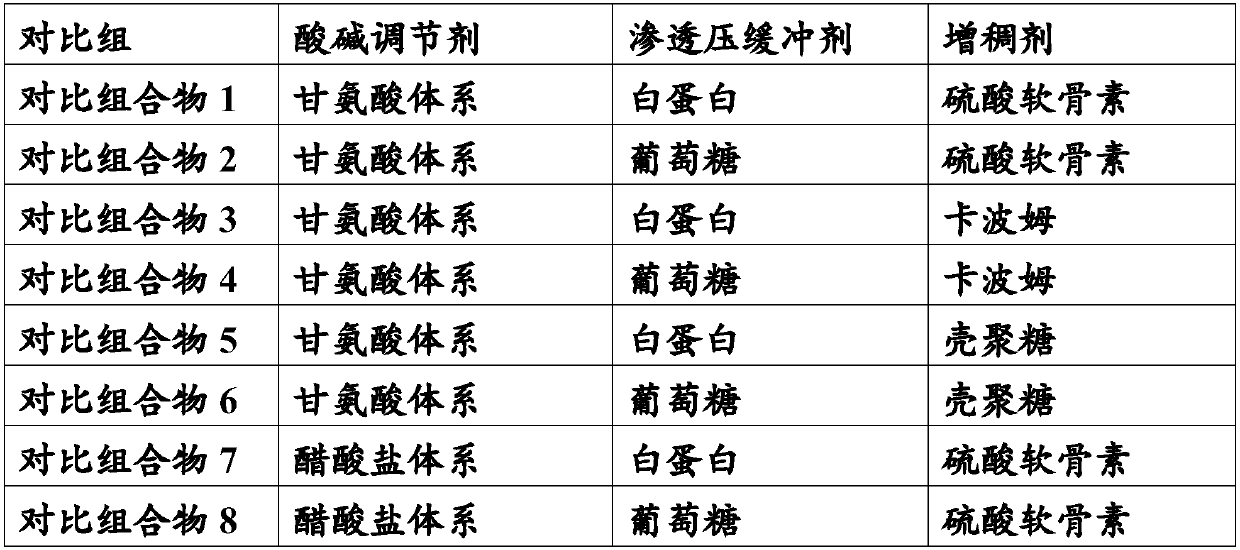
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
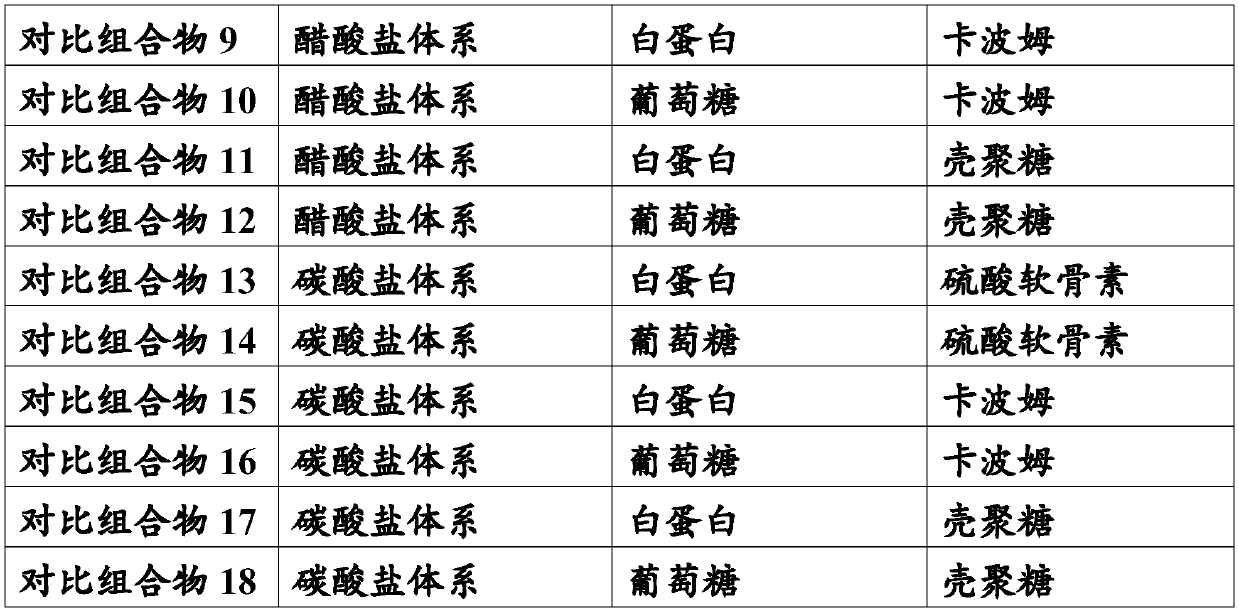
65 results about "Instilling eye drops" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Vitamin A liposome artificial lacrimal eye drops
ActiveCN1850054ASenses disorderHydroxy compound active ingredientsSodium bicarbonateAdditive ingredient
The present invention relates to a vitamin A liposome artificial tears eye drops for preventing and curing xerophthalmia and ophthalmokopia. Its composition includes fat-soluble vitamin A, liposome encapsulating material, vitamin E and artificial tears hydrated liquor, in which the fat-soluble vitamin A is vitamin A palmitate or vitamin A acetate, the liposome encapsulating material is soya bean lecithin and cholesterol, and the composition of artificial tears hydrated liquor includes sodium hydrogen carbonate, sodium chloride, glucose, potassium chloride, calcium chloride and water. Besides, according to requirements one or several kinds of auxiliary components of menthanol, PVP and HPMC, etc. also can be added.
Owner:CHINA RESOURCES SAIKE PHARMA
Derivatives of new chitosan, preparation method, and application in use for making ophthalmic preparation
ActiveCN1760212AOrganic active ingredientsSenses disorderPerylene derivativesPharmaceutical Substances
Owner:HAICHANG CONTACT LENSES
Ophthalmic solution comprising diquafosol
ActiveUS20150072951A1Reduce eye irritationImprove anti-corrosion performanceBiocideSenses disorderFiltrationEyes irritation
Regarding Diquafosol ophthalmic solution comprising a chelating agent at a concentration of 0.0001 to 1% (w / v), formation of insoluble precipitates found in Diquafosol ophthalmic solution during storage of the solution, as well as deterioration of the filtration performance in the course of production (course of filtration sterilization), have been inhibited. Further, in Diquafosol ophthalmic solution comprising a chelating agent, reduction of eye irritation and enhancement of the preservative effectiveness have been confirmed, in comparison to Diquafosol ophthalmic solution comprising no chelating agent. Accordingly, the present invention has been confirmed to provide physicochemical properties that are stable during the courses of production and distribution as well as the course of storage by a patient, and also reduce eye irritation and enhance preservative effectiveness.
Owner:SANTEN PHARMA CO LTD
Liposome artificial tear eye drops
InactiveCN101095695AAvoid damageExtended staySenses disorderHydroxy compound active ingredientsEye FatigueLiposome
The invention relates to an artificial eye tear drop of liposome for preventing and treating dry eye disease and eye fatigue, which comprises soybean lecithin, cholesterin, anti-oxidizing agent and artificial tear hydrated liquid, whose constituents include sodium hydrogen carbonate, sodium chloride, glucose, potassium chloride, water, or one or more auxiliary compositions of mint, PVP and HPMC.
Owner:孙勐
Composition for enhancing drug safety and clinical efficacy of low concentration atropines
InactiveCN109675038AImprove securityImprove clinical efficacySenses disorderInorganic non-active ingredientsSide effectPediatrics
The present invention discloses a composition capable of enhancing the drug safety and clinical efficacy of low concentration atropines, and especially when the composition is used in low concentration atropines eye drops, the fact that the composition used in the low concentration atropines eye drops provided by the invention can effectively improve the drug safety of low concentration atropinesby adjusting key quality parameters such as pH, osmotic pressure and viscosity of the eye drops is creatively discovered, especially for improving clinical efficacy, so as to effectively solve the problems in the use of current low-concentration atropines eye drops, such as poor safety, large efficacy differences, unstable main drugs, safety of impurities degradation, concomitant adverse reactionsand other side effects. The composition enhances the clinical efficacy of low concentration atropines eye drops in the prevention and treatment of myopia of adolescents and children and provides a safer and healthier guarantee for the eyesight of adolescents and children, and has a very broad application prospect.
Owner:杭州赫尔斯科技有限公司
Eye drops for treatment of conjunctivochalasis
An ophthalmic preparation and method, usable to treat conjunctivochalasis. The ophthalmic preparation comprises an aqueous solution of glycerol. The preparation may also include additional components including high molecular weight polymers for viscosity control and pharmacologically active substances. The method includes administering an ophthalmic preparation including an aqueous solution of glycerol to a patient.
Owner:RESDEVCO RES & DEV
Compound allantoin vitamin B6-E and ammoniation ethyl sulfate eye drops and preparation method thereof
ActiveCN102441001ASolve turbiditySolve easy discolorationSenses disorderPharmaceutical delivery mechanismVitamin b6Disodium Edetate
The invention discloses compound allantoin vitamin B6-E and ammoniation ethyl sulfate eye drops and a preparation method thereof. The compound allantoin vitamin B6-E and ammoniation ethyl sulfate eye drops are proportionally prepared from the main components of chondroitin sulfate, allantoin, taurine, vitamin B6 and vitamin E and the auxiliary materials of edetate disodium, boric acid, borax, polysorbate-80, benzalkonium chloride, natural borneol, ethanol and water for injection, and a preparation method is also provided. After the components of the compound allantoin vitamin B6-E and ammoniation ethyl sulfate eye drops and the eye drops which are prepared from the preparation method are applied, the medicine compatibility problem of five main components is solved, so the liquid medicine is clear, the contents of all the main medicines are stable, the aseptic and visible foreign matter inspection qualification rate of a product is ensured to be high, the cost is low, and the compound allantoin vitamin B6-E and ammoniation ethyl sulfate eye drops are worth being popularized in the industrial production field.
Owner:江西珍视明药业有限公司
Ophthalmic solution
InactiveCN1438886AEliminate or relieve congestionSenses disorderOrganic chemistryConjunctivaBlood vessel
The present invention provides an ophthalmic solution containing pranoprofen or a pharmacologically acceptable salt thereof, and a vasoconstrictor, which is effective for ameliorating congestion in outer ocular areas, particularly conjunctiva and pericorneal area.
Owner:SENJU PHARMA CO LTD
Steroid compound as well as preparation method and application thereof
PendingCN114591389AReduce turbidityOrganic active ingredientsSenses disorderBULK ACTIVE INGREDIENTActive ingredient
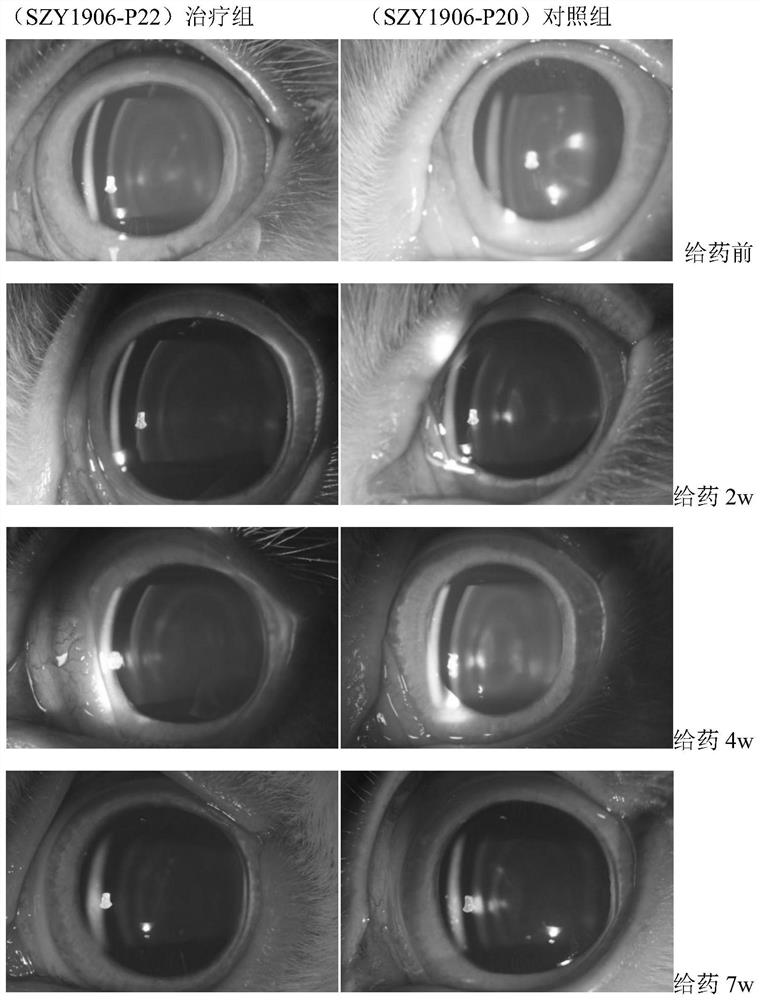
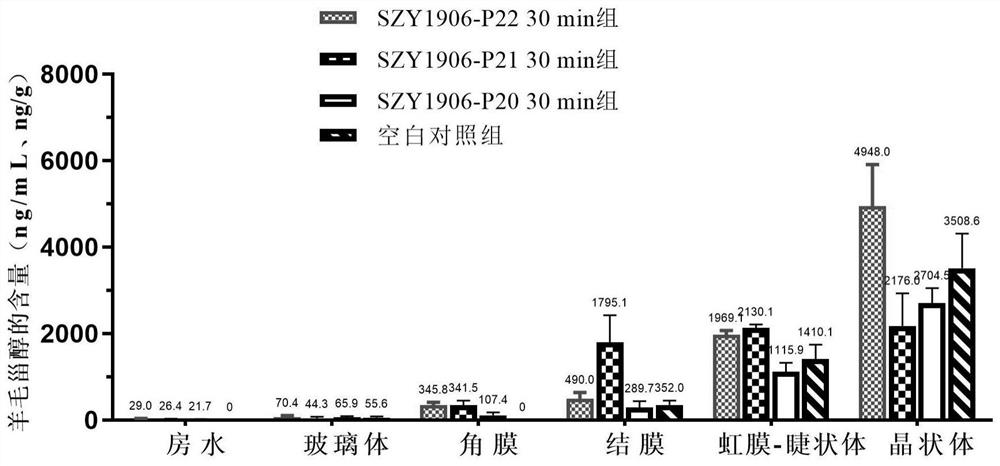
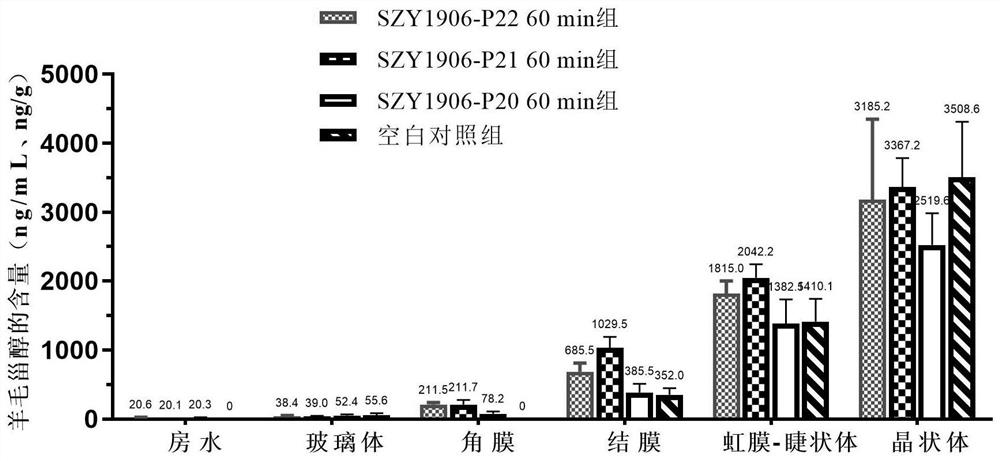
The invention discloses a steroid compound and pharmaceutically acceptable salt thereof, and a preparation method and application thereof. The structural formula of the steroid compound is shown as a formula I ', in the formula I', R is dimethylamino or methylamino, and the eye drops taking the compound (SZY1906-P22) of the formula I with R being dimethylamino as an active ingredient can reduce the lens opacity of New Zealand rabbits with spontaneous senile cataract.
Owner:GUANGZHOU OCUSUN OPHTHALMIC BIOTECHNOLOGY CO LTD
Nutritious supplement for improving eyesight and preparation method and application thereof
InactiveCN110151866AImprove eyesightReasonable compositionSenses disorderMetabolism disorderSide effectMedicine
The invention discloses a nutritious supplement for improving eyesight and a preparation method and application thereof. Raw materials of the nutritious supplement comprise mulberries, radix rehmanniae preparata, semen astragali complanati, fructus lycii, semen euryales, semen cassiae, fructus schisandrae chinensis, semen plantaginis, flos buddlejae, flos tagetis erectae, ginseng and fructus ligustri lucidi. The invention also provides the preparation method and application of the nutritious supplement. The nutritious supplement can be used for preparing an oral or external preparation for improving the eyesight. Preferably, the oral preparation is added into a dairy beverage and mixed uniformly for drinking. The external preparation is used as an eye drop. The nutritious supplement for improving the eyesight and the preparation method and application thereof have the advantages of reasonable formula, small dosage, obvious effect and the like. The Chinese medicinal essence is extracted, preparation is convenient and easy to achieve, the cost is low, the healing rate is high, and the preparation is safe and free of toxic and side effects and can avoid generation of drug resistance and achieve the effect of fundamentally improving the eyesight.
Owner:李美霖
Eyelid opening device for ophthalmic nursing
InactiveCN111214263AEffective and stableLarge operating spaceSurgeryEye treatmentOphthalmic nursingEyelid
The invention discloses an eyelid opening device for ophthalmologic nursing, and relates to the technical field of ophthalmologic nursing. The an eyelid opening device comprises a first opening mechanism and a second opening mechanism, wherein the first opening mechanism is fixedly connected to the second opening mechanism through an adjusting mechanism, a rotating disc is separately arranged on the first opening mechanism and the second opening mechanism through rotating shafts, a connecting rod is fixedly arranged on one side of the top of each rotating disc, a dropping funnel is fixedly arranged on the top of each connecting rod, the first opening mechanism and the second opening mechanism are also connected through an elastic rope, and the adjusting mechanism comprises a left threadedrod, a right threaded rod and an adjusting pipe. The eyelid opening device is reasonable in structure, convenient and fast to operate and capable of effectively and stably opening eyelids without an need of long-time hand-held operations by a doctor, so that a large operating space is saved for the doctor, meanwhile, the eyelid opening device has an auxiliary effect of dropping eye drops, each dropping funnel is of a rotating structure, the dropping funnels can assist dropping the eye drops and can be rotated to one side without affecting observation of the doctor, thus the eyelid opening device is high in practical value.
Owner:陈晓娟
Application of inhibiting myopia by adjusting eye sclera lipid metabolism
The invention relates to an application of inhibiting myopia by adjusting eye sclera lipid metabolism. The invention discovers a new mechanism causing myopia, namely close connection between sclera lipid metabolism abnormality and myopia, thereby revealing a brand new myopia prevention and control target; meanwhile, the invention provides eye drops which can effectively prevent and control shortsightedness and can also avoid the problem of eye allergy.
Owner:极目峰睿(上海)生物科技有限公司
Use of cerebrosid-kinin in preparation of medicine for preventing and treating peripheral nerve disease
InactiveCN1762486AReduce water contentAvoid Metabolic DisordersNervous disorderPeptide/protein ingredientsDiseaseKinin
Disclosed is the novel use of glycoside and ignotin in the preparation of medicaments for treating diseases of peripheral nerves, wherein the glycoside and ignotin comprises active compounds such as polypeptides and ganglioside. The cattle encephalon glycoside and ignotin can be mixed with medically acceptable auxiliary materials to obtain medicinal composition in the dosage forms of tablets, capsules, granules, drop pills, injections and eye drops.
Owner:诺氏制药(吉林)有限公司
Ophthalmic solution of difluprednate
The present invention provides an ophthalmic solution comprising a. therapeutically effective concentration of difluprednate, a crystal growth inhibitor and pharmaceutically acceptable amounts of a solubilizer comprising a mixture of i. quaternary ammonium compound and ii. polyethoxylated castor oil, b. in an aqueous vehicle. wherein the crystal growth inhibitor is polyvinyl alcohol or its derivatives. Also, the present invention provides a method of treatment of inflammatory disorder of the eye, said method comprising administering into the eye of a person in need thereof, an aqueous solution comprising difluprednate as the sole active ingredient at a concentration of 0.02% to 0.04% weight by volume in an aqueous vehicle, wherein the solution is free of oil and wherein the solution is administered twice-a-day.
Owner:SUN PHARMA INDS
Ophthalmic cetirizine hydrochloride liposome, in-situ gel and preparation method of ophthalmic cetirizine hydrochloride liposome
ActiveCN114392235AReduce releaseImprove permeabilityOrganic active ingredientsSenses disorderCholesterolPhospholipid
The invention discloses cetirizine hydrochloride ophthalmic liposome, in-situ gel and a preparation method of cetirizine hydrochloride ophthalmic liposome, and belongs to the technical field of pharmaceutical preparations. In order to overcome the defects that existing cetirizine hydrochloride eye drops are short in residence time, poor in compliance of semi-solid dosage forms, low in bioavailability, not easy to accept by patients and the like, an ethanol injection method is combined with an ammonium sulfate gradient method to obtain the liposome, and the liposome comprises 0.28-0.30% of cetirizine hydrochloride, 1.4-6.0% of phospholipid, 0.14-1.8% of cholesterol, 0.1-0.15% of an osmotic pressure regulator, 0.05-0.1% of a bacteriostatic agent and the balance of water. And a solvent. The invention further provides the cetirizine hydrochloride liposome-in-situ gel and a preparation method thereof. By optimizing the prescription and the process, the obtained liposome and in-situ gel can delay the drug release, increase the retention time of eyes, improve the cornea permeability and improve the bioavailability.
Owner:CHENGDU UNIV
Loteprednol etabonate suspension eye drops
InactiveCN103565740AResolve Drug ConcentrationSolve the problem that the nominal value does not matchOrganic active ingredientsSenses disorderActive componentParticle-size distribution
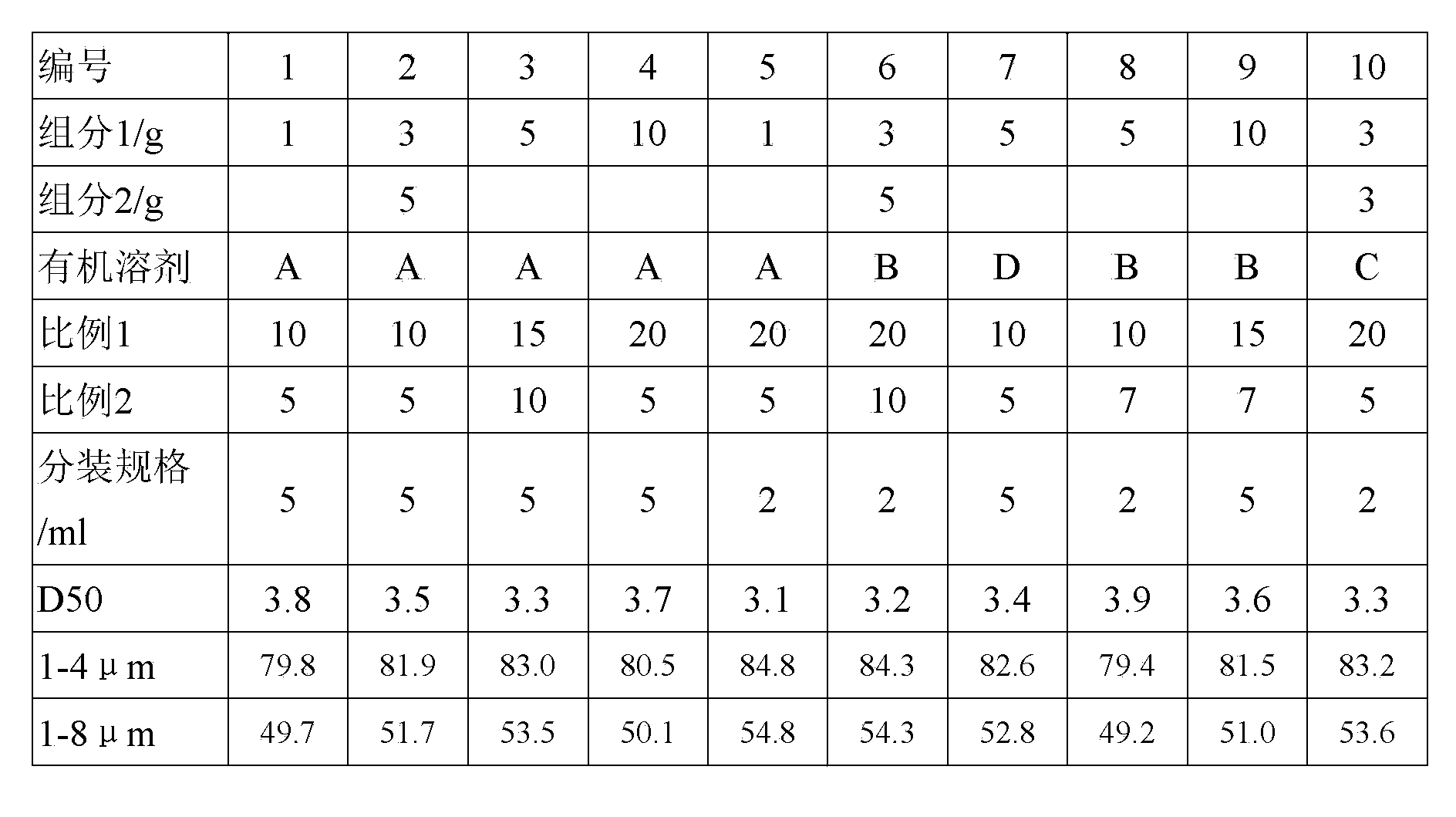
The invention discloses loteprednol etabonate suspension eye drops. The loteprednol etabonate suspension eye drops contain loteprednol etabonate as an active component, water and one or more accessory materials for suspension eye drops. The used loteprednol etabonate micro-powder meets the particle size distribution conditions comprising that D50 is in a range of 3.0-4.0 microns, the maximum particle size is less than 30 microns, a ratio of micro-powder particles having sizes of 1-8 microns is in a range of 79-85%, and a ratio of micro-powder particles having sizes of 1-4 microns is in a range of 49-56%.
Owner:TIANJIN JINYAO GRP
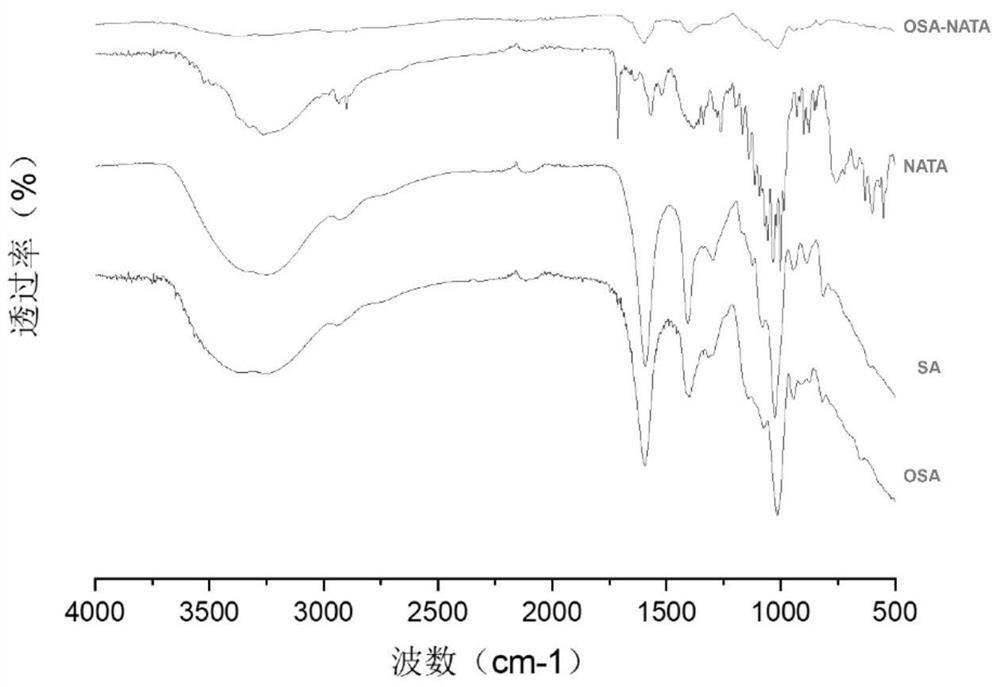
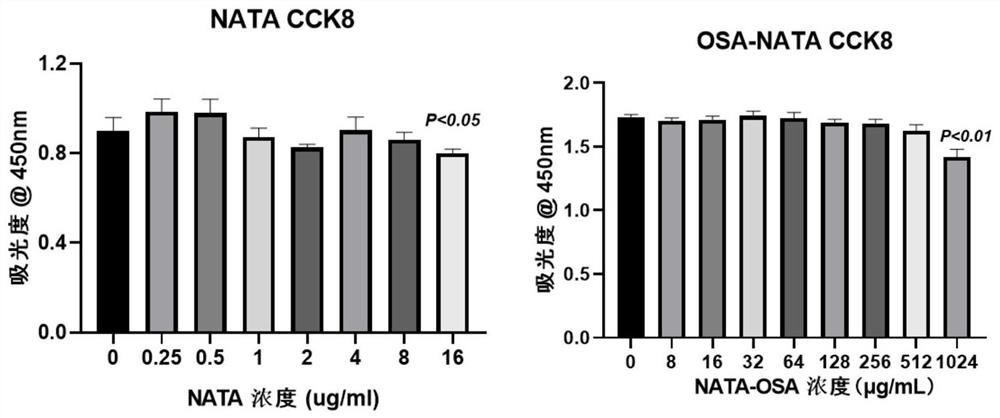
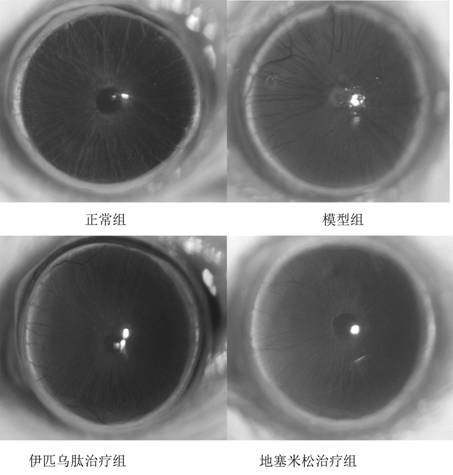
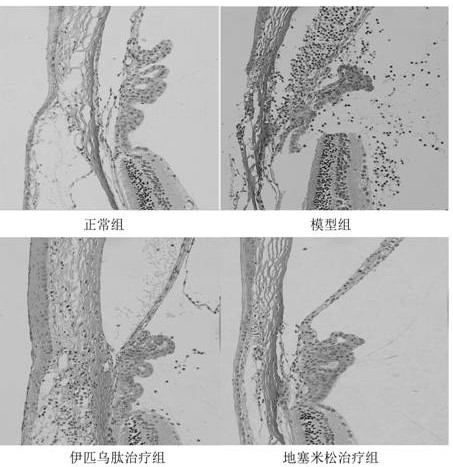
Oxidized sodium alginate modified natamycin eye drops and preparation method thereof
PendingCN113081956AGood tissue compatibilitySimple preparation processOrganic active ingredientsSenses disorderEyes irritationEye drop
The invention relates to the technical field of medical materials, in particular to oxidized sodium alginate modified natamycin eye drops and a preparation method thereof. The oxidized sodium alginate modified natamycin eye drops has the component and content that 1 mL of sterilized deionized water contains 0.5-1.5 mg of oxidized sodium alginate-natamycin freeze-dried powder; the oxidized sodium alginate-natamycin is a product obtained by carrying out a Schiff base reaction on oxidized sodium alginate and natamycin; the preparation method comprises the following specific steps: preparing oxidized sodium alginate solid, preparing oxidized sodium alginate-natamycin medicine powder, and preparing the eye drops. The property of the oxidized sodium alginate is similar to that of a human extracellular matrix, so that the oxidized sodium alginate is good in histocompatibility and can be degraded into non-toxic polysaccharide which does not participate in metabolism; the water solubility and permeability of the natamycin are improved, the eye irritation and discomfort of the natamycin are reduced, and the treatment effect of the fungal keratitis is improved; the preparation process is relatively simple, low in cost and easy to store.
Owner:THE AFFILIATED HOSPITAL OF QINGDAO UNIV
Application of polypeptide in medicine for treating eye inflammation
PendingCN111956781APharmacologically clearGood curative effectSenses disorderPeptide/protein ingredientsSide effectPharmacologic action
The invention discloses an application of a polypeptide in a medicine for treating eye inflammation. The amino acid sequence of the polypeptide is YGRKKRRQRRRMMPYSTELIFYIEMDP. Eye drops prepared fromthe polypeptide comprise the following components in percentage by weight: 0.05-1% of polypeptide, and the eye inflammation is conjunctivitis, keratitis and / or uveitis. In the polypeptide eye drops, the pharmacological action of the polypeptide in treatment of inflammation is to inhibit inflammation from participating in cell proliferation, inhibit NF-kB activation, inhibit the generation and secretion of various inflammatory mediators, inhibit the growth of new capillaries, inhibit the aggregation and infiltration of inflammatory cells and the like. The polypeptide eye drops are clear in pharmacology, good in curative effect, low in side effect, stable in biological activity of bulk drugs, simple in production process, low in cost and beneficial to large-scale clinical application and reduction of burden of patients.
Owner:WUHAN YICHENG BIOTECH CO LTD
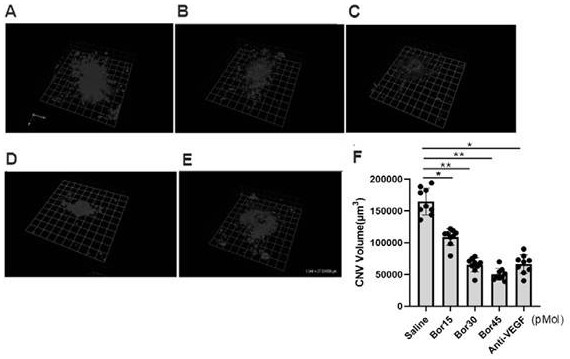
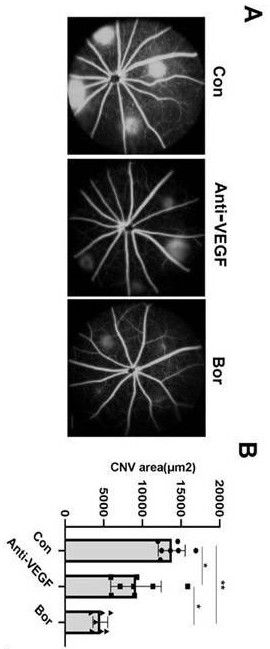
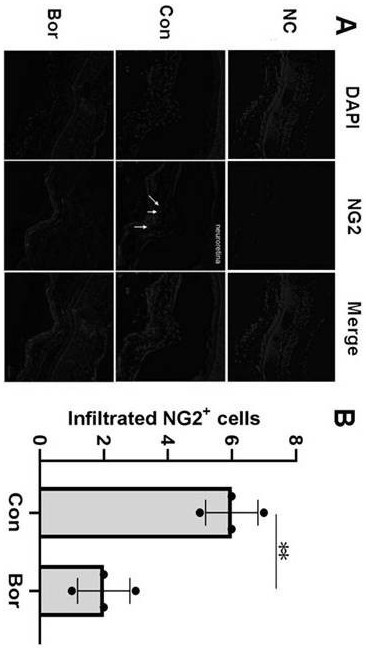
Application of bortezomib and preparation of bortezomib to preparation of medicine for treating choroidal neovascularization related diseases and preparation method of eye drops
PendingCN112263670ALittle side effectsGood curative effectSenses disorderDipeptide ingredientsChoroid membranePharmaceutical drug
The invention discloses application of bortezomib and preparation of bortezomib to preparation of a medicine for treating choroidal neovascularization related diseases and a preparation method of eyedrops. A protease complex inhibitor bortezomib is injected into a vitreous cavity or bortezomib eye drops are used on the ocular surface, the bortezomib and the preparation of bortezomib can be used for treating choroidal neovascularization related fundus diseases such as treatment of wet age-related macular degeneration, the side effect is small, and the curative effect is good.
Owner:WENZHOU MEDICAL UNIV
Traditional Chinese medicine effective components for treating eye diseases, and composition thereof
ActiveCN112656788AClarify effectivenessElucidate pharmacological effectsAntibacterial agentsSenses disorderDiseaseInflammatory factors
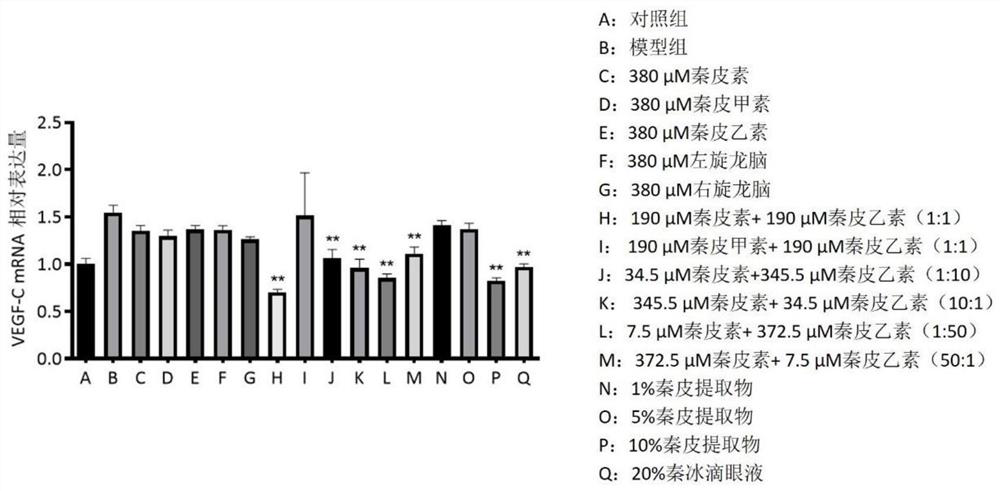
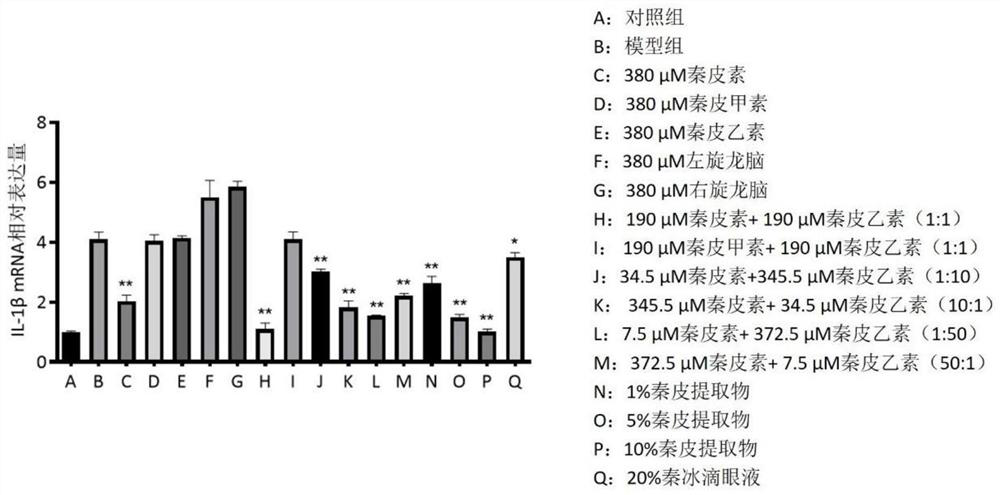
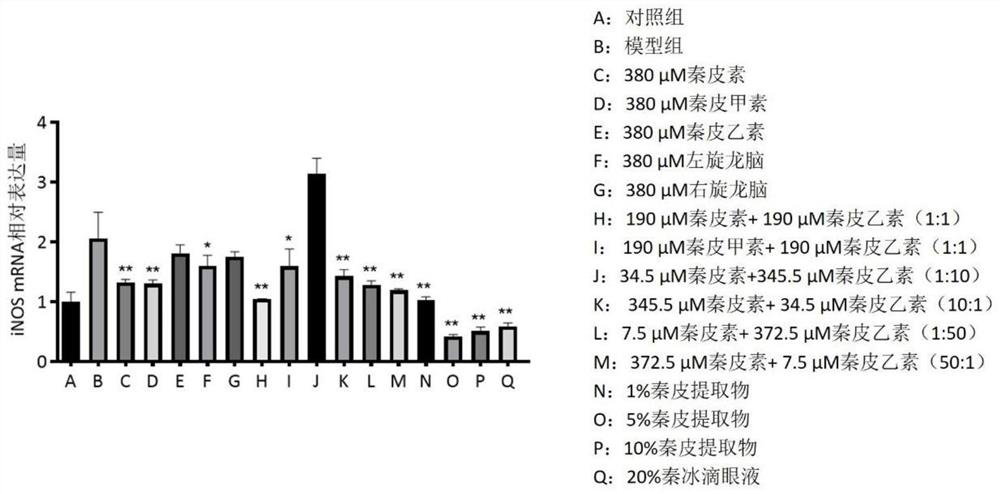
The invention relates to traditional Chinese medicine effective components for treating eye diseases, and a composition thereof. The composition contains the effective components including aesculin and aesculin. The invention breaks through the conventional cognition in the field, clarifies the actual effective components and pharmacological action of the Qin ice eye drops, verifies that the composition is stable in action effect and clear in action mechanism, can inhibit the expression of lymphatic neogenesis related factors and inflammatory factors in macrophages, the growth of methicillin-resistant staphylococcus aureus MRSA and pseudomonas aeruginosa PA14 is inhibited, and lymphatic vessel neogenesis caused by mouse corneal alkali burn is inhibited, so that the traditional Chinese medicine effective components and the composition can be used for treating lymphatic vessel neogenesis, inflammation and immune-related eye diseases.
Owner:SHANGHAI UNIV OF T C M +1
Chinese medicine for treating eye diseases and its prepn process
InactiveCN1436544AIn line with physiological characteristicsWide range of treatmentSenses disorderLeech/worm material medical ingredientsCentipedeCornea diseases
The present invention relates to Chinese medicine, and is especially one kind of eye drop for treating eye diseases and its preparation process. The Chinese medicine is prepared with sesame oil, scorpion, centipede and earth worm and through special technological process. It has wide treating range, and may be used in treating cornea disease, iritis and secondary glaucoma, and has high effective rate and no toxic side effect and drug fastness.
Owner:孟昭风
Nimodipine ophthalmic preparation and preparation method thereof
InactiveCN101961332ALittle side effectsMedication convenienceOrganic active ingredientsSenses disorderGlaucomaSide effect
The invention relates to a drug effective ingredient, in particular to a nimodipine (NMD) ophthalmic preparation and a preparation method thereof. The nimodipine ophthalmic preparation comprises a medicinal auxiliary and an effective quantity of nimodipine ingredient used for protecting optic nerves and dispersed to the auxiliary. In the embodiment of the invention, the NMD ophthalmic preparation comprises a 0.09% NMD eye drop and a 0.3% NMD eye ointment. The preparation method of the 0.09% NMD eye drop comprises the following steps of: adding 0.9 g of NMD and 0.3 g of ethylparaben into 50 ml of tween-80, and uniformly stirring, adding 600 ml of double buffer solution, heating to dissolve, adding the double buffer solution to 1000 ml, and adjusting the pH value to 6.7-7.2; filtering, sterilizing by flowing stream, and canning. The preparation method of the 0.3% NMD eye ointment comprises the following steps of: placing 3g of nimodipine in a sterilizing mortar in sterile operation, adding 5 ml of sterilized liquid paraffin, grinding into a fine paste, adding into 0.3g of ethylparaben and 50 ml of tween-80, uniformly stirring, adding an eye ointment matrix several times, uniformly grinding while adding till being 1000g, and subpackaging in a sterile way. The nimodipine ophthalmic preparation has the advantages of high efficiency, small side effect and convenient medication, and has good application prospect in the prevention and treatment of eye diseases, especially the protection of glaucoma optic nerves.
Owner:耿燕
Medicinal composition for treating chronic conjunctivitis
InactiveCN103169760ARelieves redness and relieves itchingSignificant effectSenses disorderHydroxy compound active ingredientsEye strainingTraditional medicine
The invention discloses a medicinal preparation for treating chronic conjunctivitis. The medicinal composition comprises pearl, astragalus and other traditional Chinese medicines, and eye drops can be prepared from the medicinal composition according to clinical requirements. The medicinal composition has an excellent effect for treating or relieving symptoms of chronic conjunctivitis, asthenopia and ocular dryness.
Owner:翁捷
Pharmaceutical application of amniotic epithelial cell conditioned medium
ActiveCN112716979AInhibit allergic conjunctivitisAvoid using directlySenses disorderAntipyreticPharmaceutical drugImmunogenicity
The invention provides a pharmaceutical application of amniotic epithelial cell conditioned medium eye drops, an application of the amniotic epithelial cell conditioned medium in preparation of the eye drops and an application of the amniotic epithelial cell conditioned medium in preparation of drugs for allergic conjunctivitis. The invention also discloses a preparation method of the amniotic epithelial cell conditioned medium eye drops. According to the pharmaceutical application of the amniotic epithelial cell conditioned medium eye drops, it is found and proved for the first time that the conditioned medium derived from human amniotic epithelial cells can inhibit allergic conjunctivitis. When the amniotic epithelial cell conditioned medium eye drops are used for treating conjunctivitis allergic conjunctivitis, the immunogenicity is low, direct use of cells is avoided, and the amniotic epithelial cell conditioned medium eye drops are safe, effective and high in product stability.
Owner:上海优祺生物医药科技有限公司
Application of exenatide to preparation of medicines for treating ocular ischemia diseases and improving ocular blood circulation in eye drip way
PendingCN111166870ABroaden the field of studyReduce fearSenses disorderPeptide/protein ingredientsRetinal capillaryBlood circulating
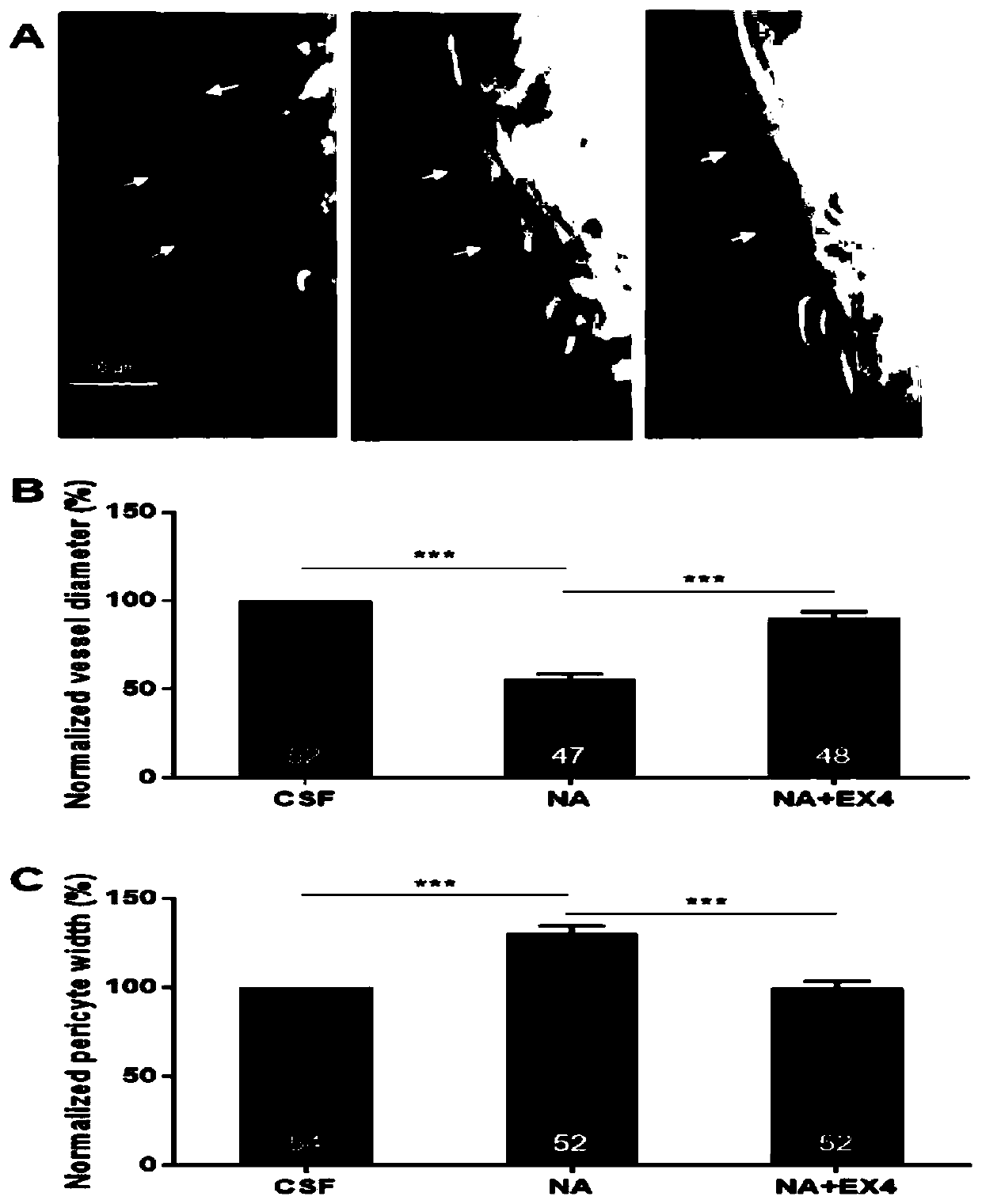
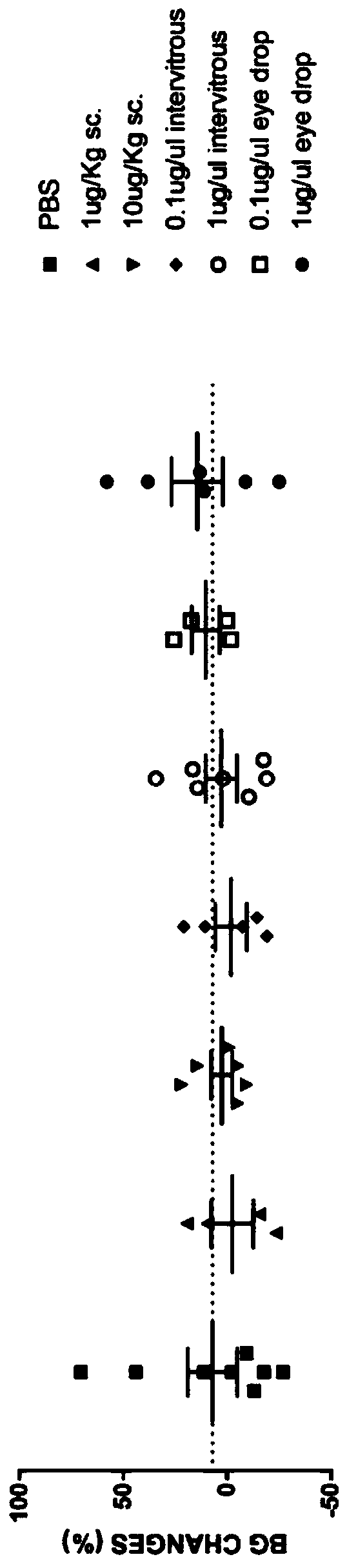
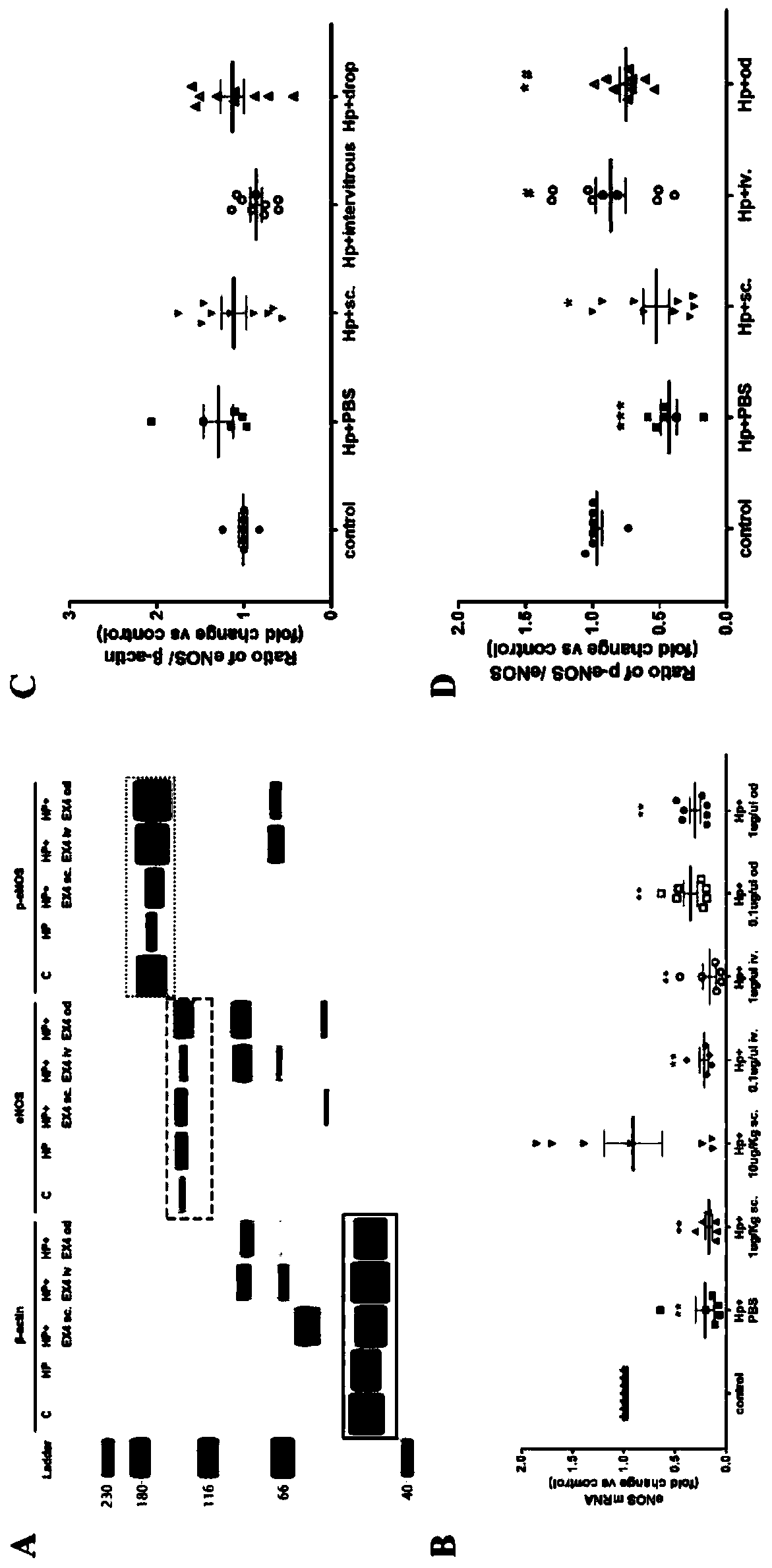
The invention relates to the technical field of medicines, in particular to application of exenatide to preparation of medicines for treating ocular ischemia diseases and improving ocular blood circulation in an eye drip way. The exenatide can be used for treating the ocular ischemia diseases such as onset of acute glaucoma and improving the ocular blood circulation . According to the applicationdisclosed by the invention, through building an acute ischemia reperfusion model and simulating damage of the onset of the acute glaucoma, findings prove that an eye drop preparation of Exendin-4 canimprove a retinal vascular endothelial function, dilating retinal capillary and recovering fundus blood circulation, so that the damage to the fundus blood supply caused by the ocular ischemia diseases such as acute glaucoma can be effectively alleviated, and simultaneously, the findings prove that a new medicine dosage form (eye drop preparation) can achieve an effective local action under the premise of avoiding systemic action. The exenatide disclosed by the invention can be used for treating deficiency of the fundus blood supply such as the acute glaucoma, retinopathy of prematurity and retinal vascular occlusion diseases, and thus, the visual function is saved.
Owner:EYE & ENT HOSPITAL SHANGHAI MEDICAL SCHOOL FUDAN UNIV
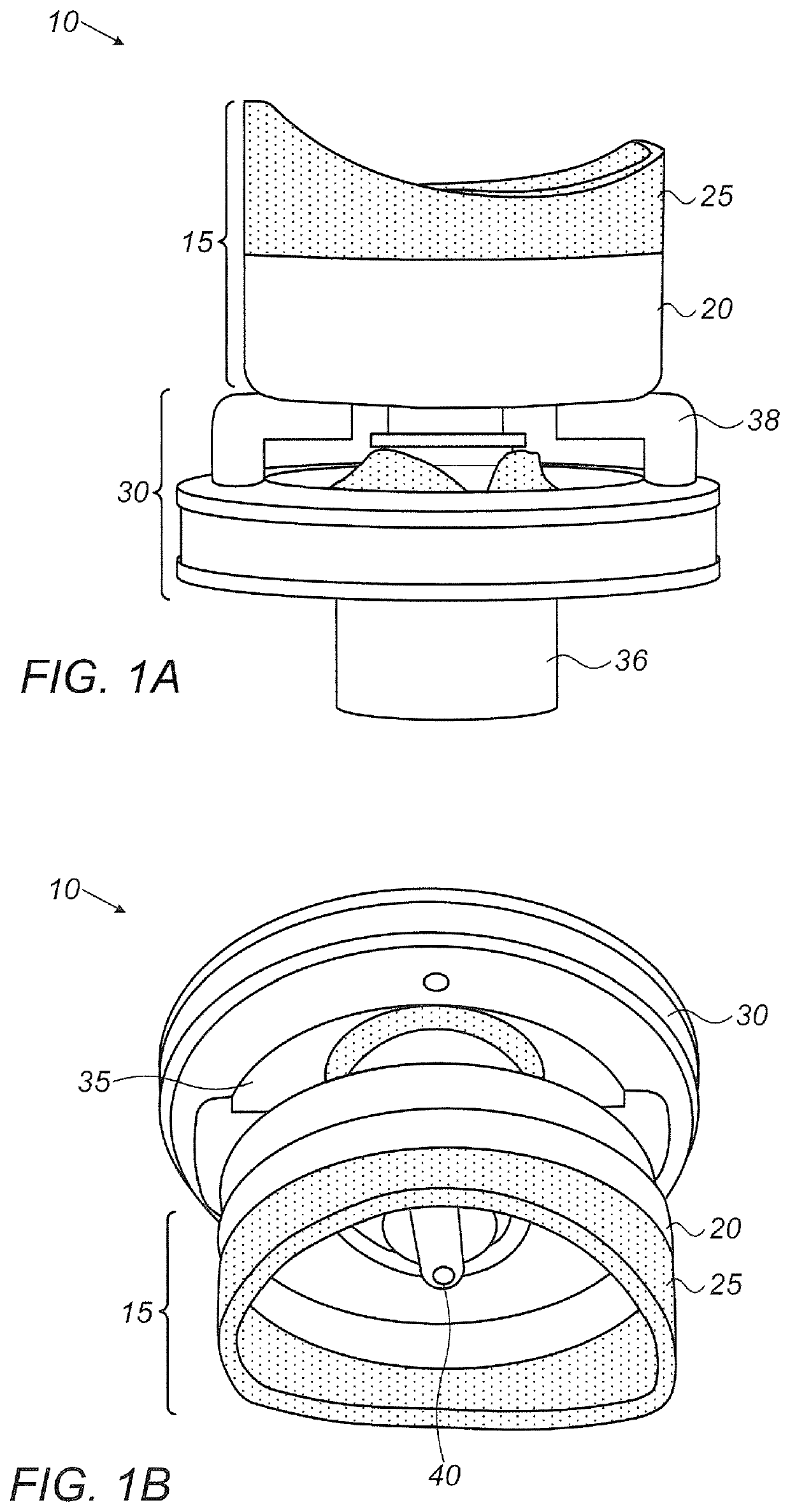
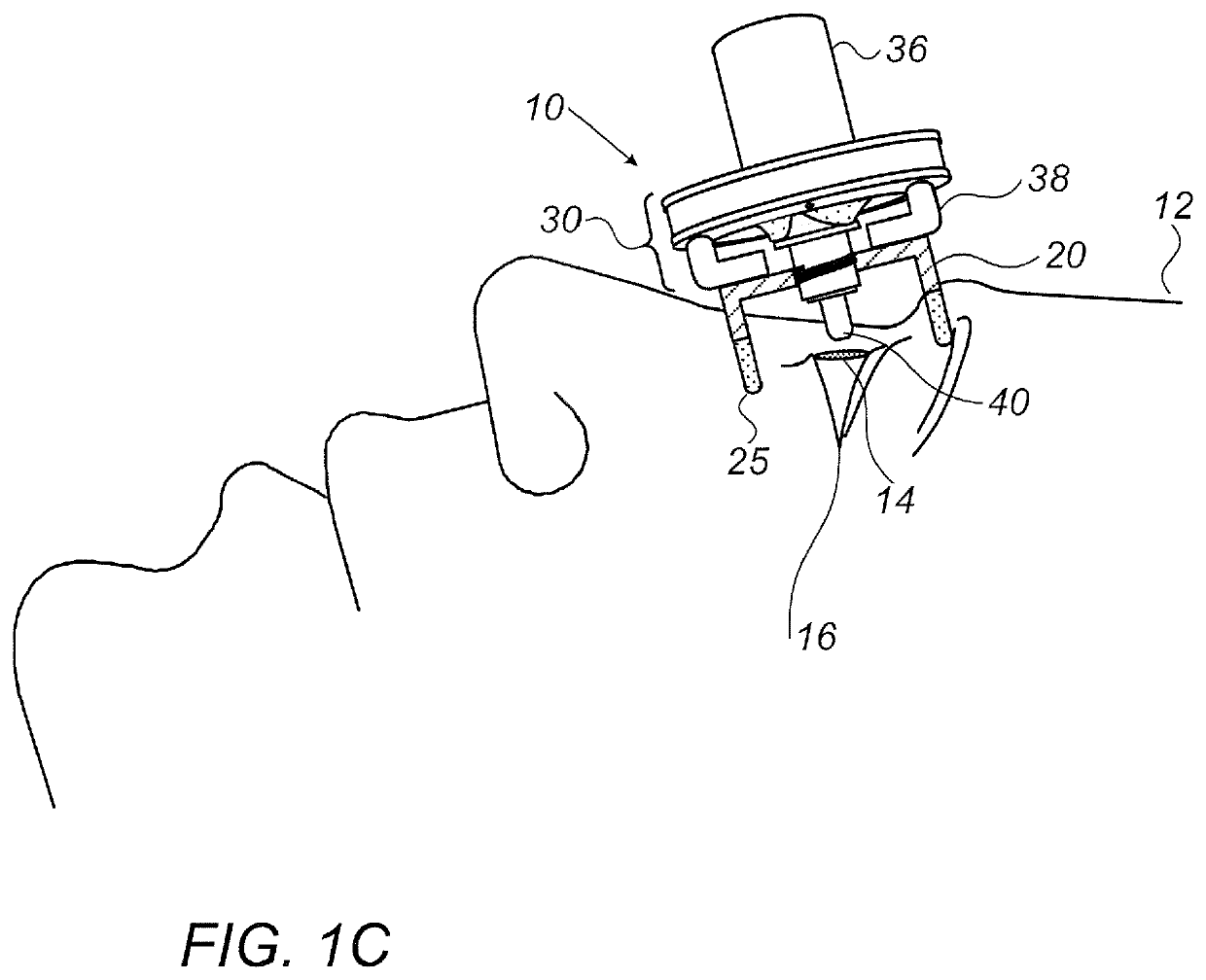
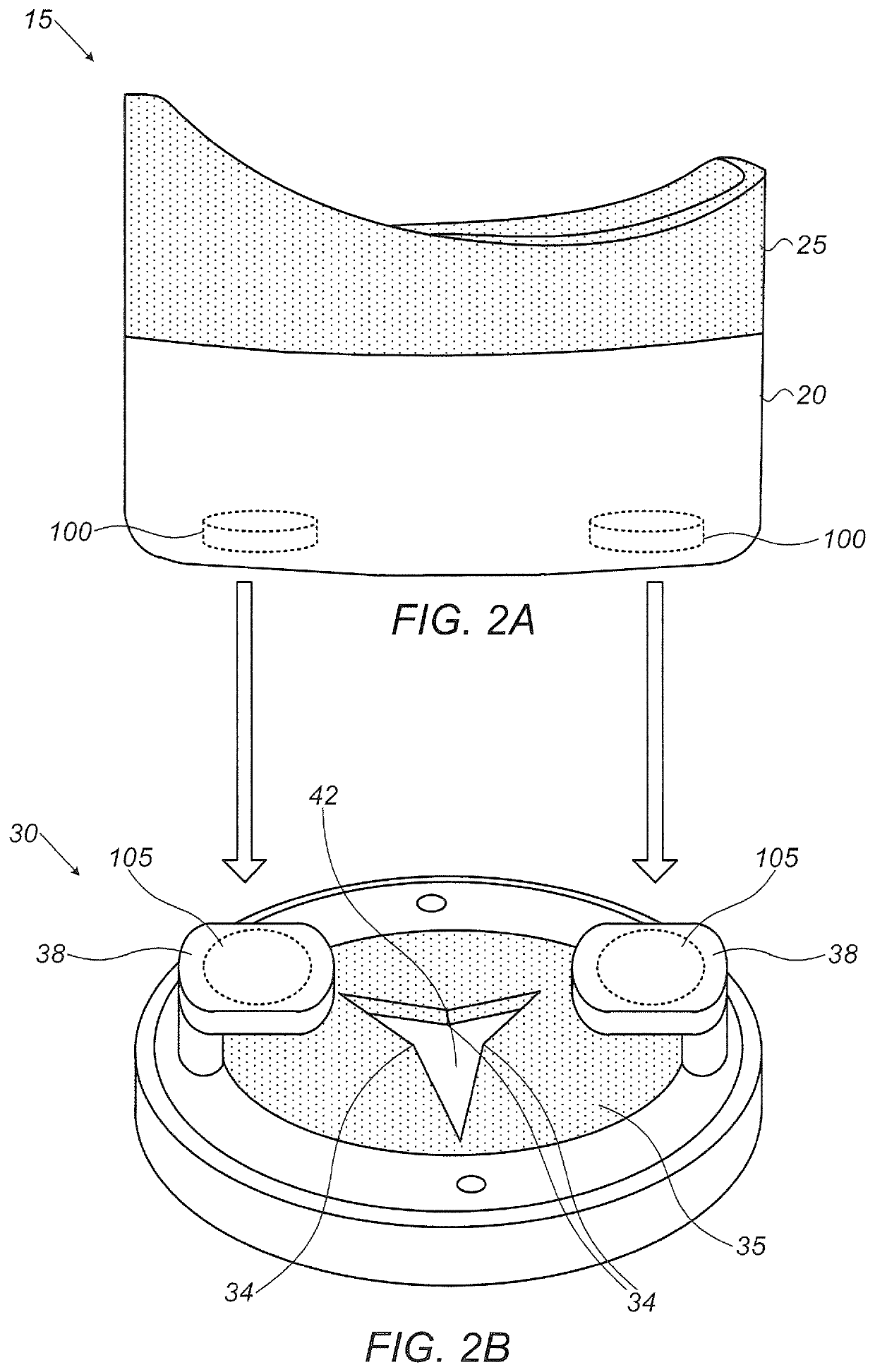
Eye drop guide device for instilling eye drops
An eye drop guide for instilling eye drops to an eye includes an eye cup housing and an eye drop dispenser holder. The eye cup housing includes an eye cup at a first end of the eye cup housing configured to be placed over an eye, a hole through the eye cup housing at a second end of the eye cup housing, and a magnet fixed to the eye cup housing at the second end. The eye drop dispenser holder may include a magnet fixed to the eye drop dispenser holder. The magnet in the eye cup housing and the magnet in the eye drop dispenser holder are configured to be attracted to one another so as to removably attach the eye cup housing to the eye drop dispenser holder.
Owner:SINGAPORE NAT EYE CENT PTE LTD +1
Ophthalmic solution of lissamine green and its use in ophthalmology
InactiveUS20200009270A1Improve stabilityNotable ease of useLuminescence/biological staining preparationInorganic non-active ingredientsConjunctivaLISSAMINE GREEN
Owner:OFTALAB SRL
Meloxicam eye drops and preparation method and use thereof
ActiveUS20160022822A1Improve solution stabilityImprove stabilitySenses disorderAntipyreticSolventSodium hyaluronate
Disclosed are meloxicam eye drops and the preparation method and use thereof. The eye drops contain active ingredient meloxicam or a pharmaceutically acceptable salt thereof, a solubilizer, a stabilizer, a pH adjusting agent, an antimicrobial agent and an osmotic pressure adjusting agent, wherein the solubilizer is one of hydroxypropyl-β-cyclodextrin, sulfobutylether-β-cyclodextrin, and β-cyclodextrin or a mixture thereof, and the stabilizer is one of polymer povidone, sodium hyaluronate, and hypromellose or a mixture thereof.
Owner:SEEFUNGE PHARM TECH CO LTD
Method for preparing eyesight-improving wet tissue
InactiveCN106038803AEasy to carryTo promote metabolismSenses disorderUnknown materialsPeppermintsContact eye
The invention relates to a method for preparing eyesight-improving wet tissue, and belongs to the field of traditional Chinese medicine health protection. Regarding the problem that eye drops directly act on eye balls, mucous membranes of eyes are irritated and feel paining when the liquid and the eye balls are directly contacted, the skin of eyelid is flushing, and the tunica conjunctiva is swelling, the method for preparing the eyesight-improving wet tissue is provided. According to the wet tissue, chamomile, frosted mulberry leaf, peppermint, antelope horn, unprocessed rehmannia root, prunella vulgaris and the like with effects of relieving tension, alleviating eye fatigue, clearing liver, improving eyesight and lowering liver-fire are adopted to prepare an extracting medicinal liquid, and the extracting medicinal liquid is loaded on a non-woven fabric to prepare the wet tissue. The wet tissue is used for wiping eyes or directly applied to eyelids, and does not directly contact eye balls, so that eye balls are prevented from being irritated. The wet tissue can be used for improving metabolism of eyes and promoting blood circulation and is beneficial to eye health protection.
Owner:梅庆波
Eye drops for alleviating ocular inflammation and allergic symptoms and preparation method thereof
ActiveCN110179748BRelieve inflammationReduce congestionSenses disorderHydroxy compound active ingredientsOcular inflammationAllergic symptoms
The invention discloses an eye drop for relieving ocular inflammation and allergic symptoms and a preparation method thereof, and mainly relates to the field of external ophthalmic medicaments. Including the following components by weight percentage: 69% to 99% of purified water, 0.01% to 10% of sodium hyaluronate, 0.01% to 10% of glycyrrhizinate, 0.1% to 5% of sodium chloride, and 0.1% of Beta dextran %~5%, taurine 0.01%~1%, 1,3-butanediol 0.005%~0.2%. The invention does not contain any prohibited ingredients, has no toxic and side effects, is safe and reliable.
Owner:吉林省华恩生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com