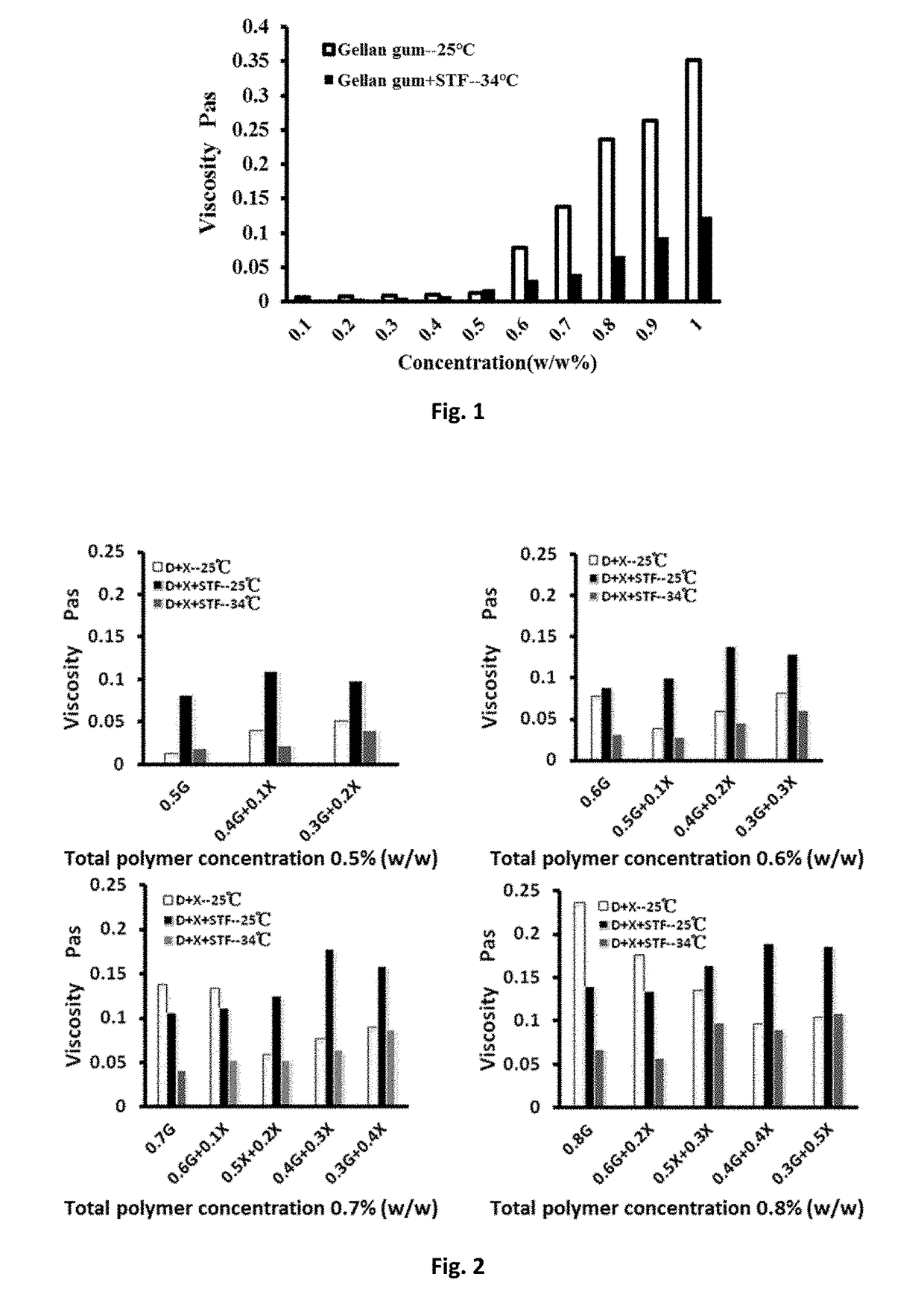
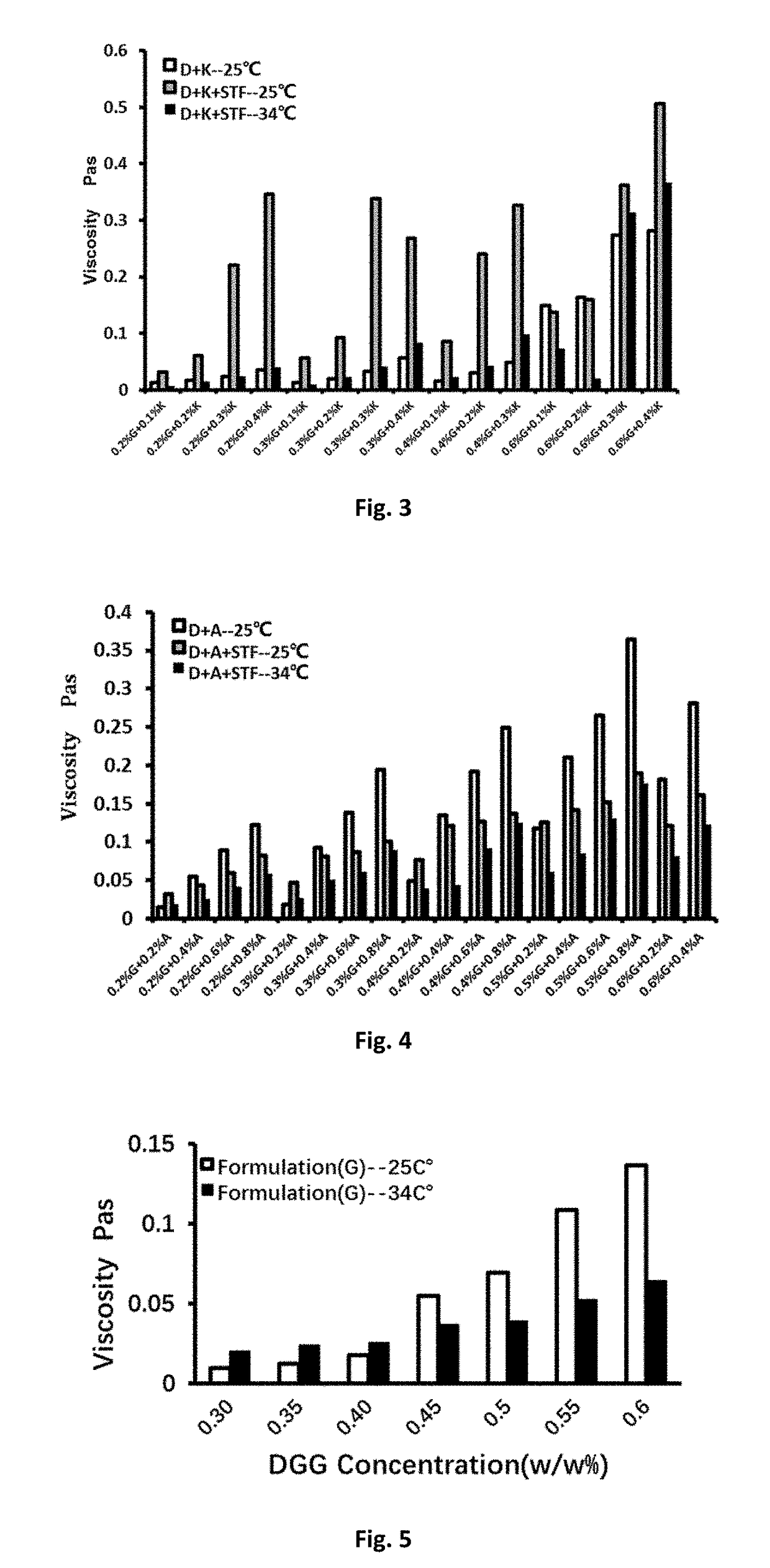
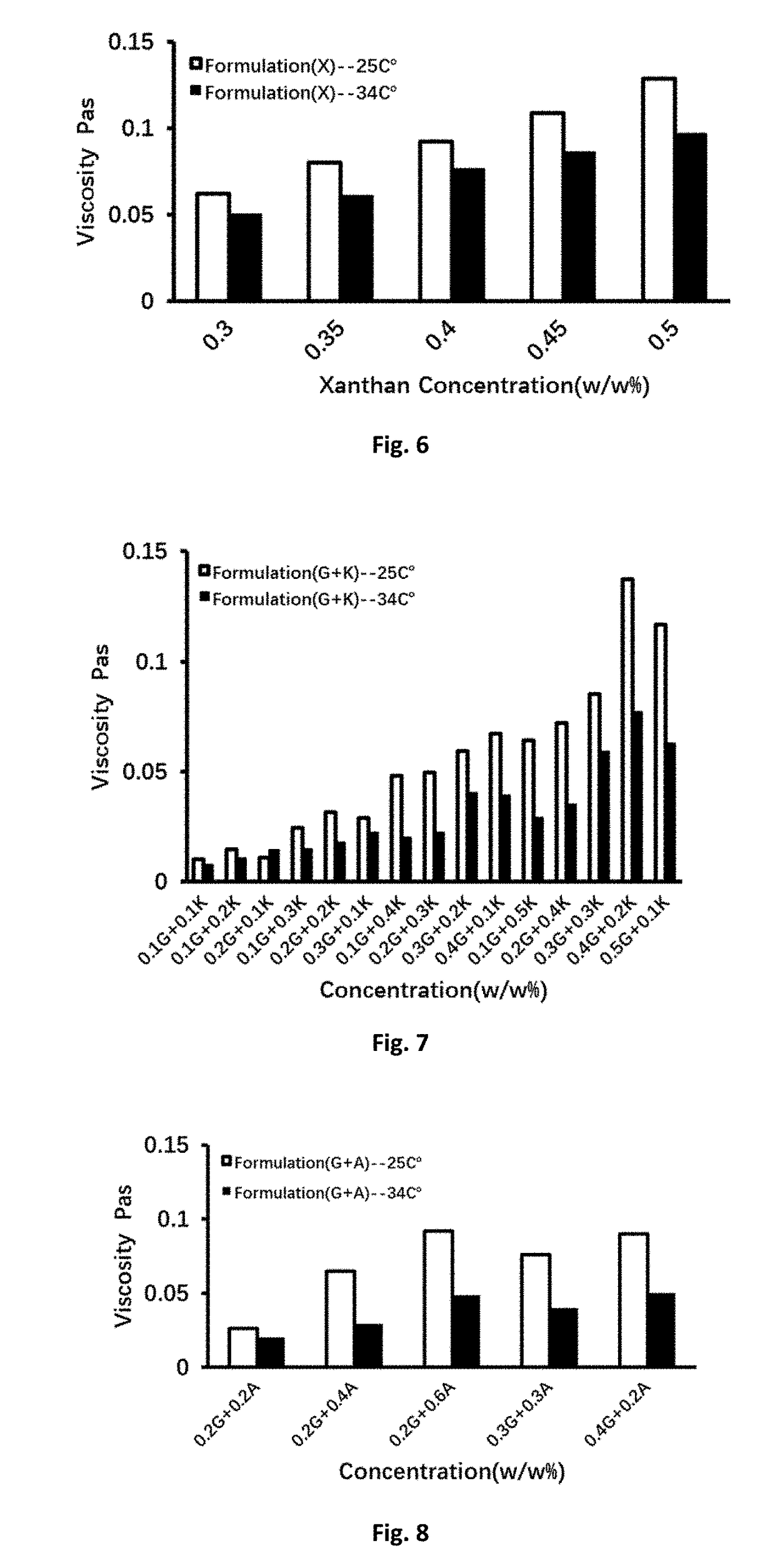
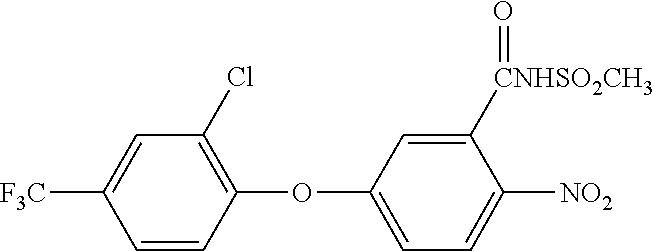
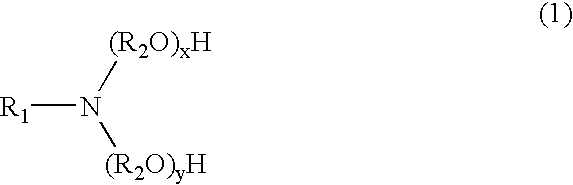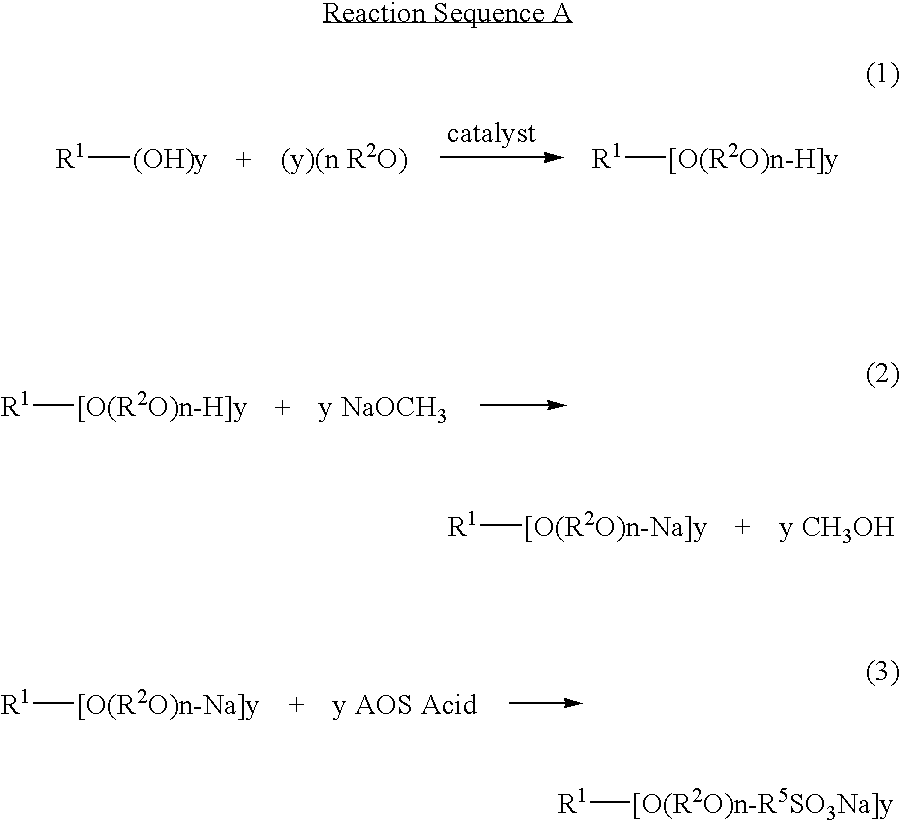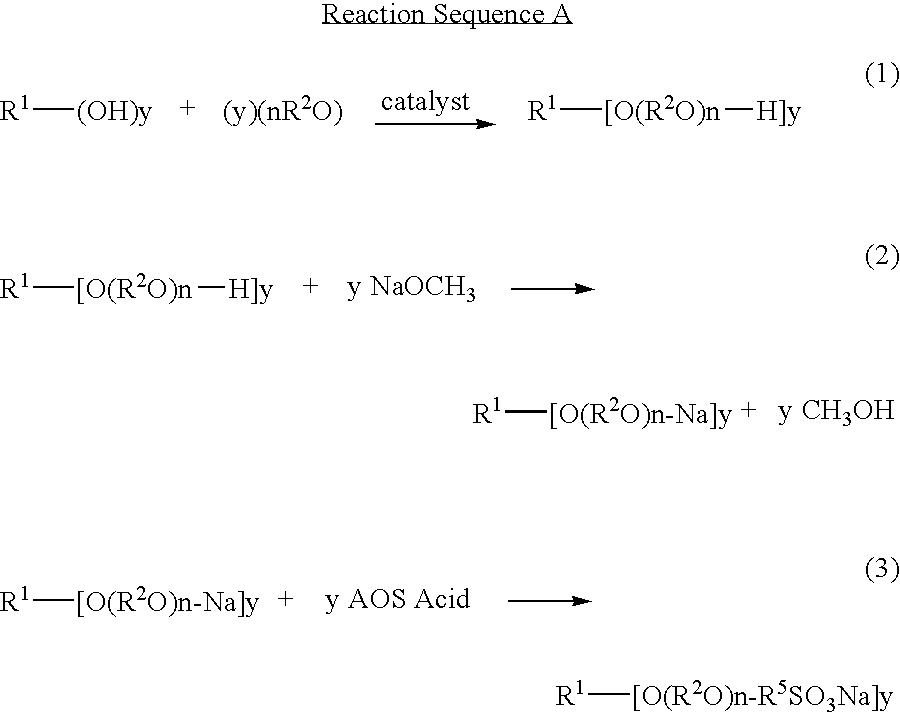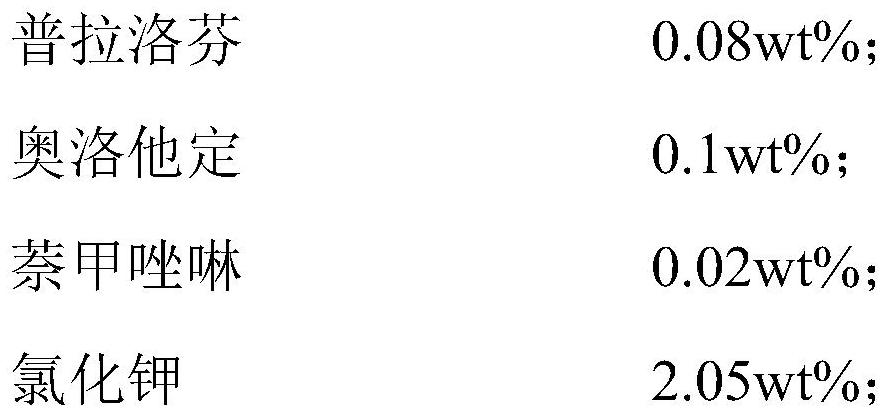Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
41results about How to "Reduce eye irritation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
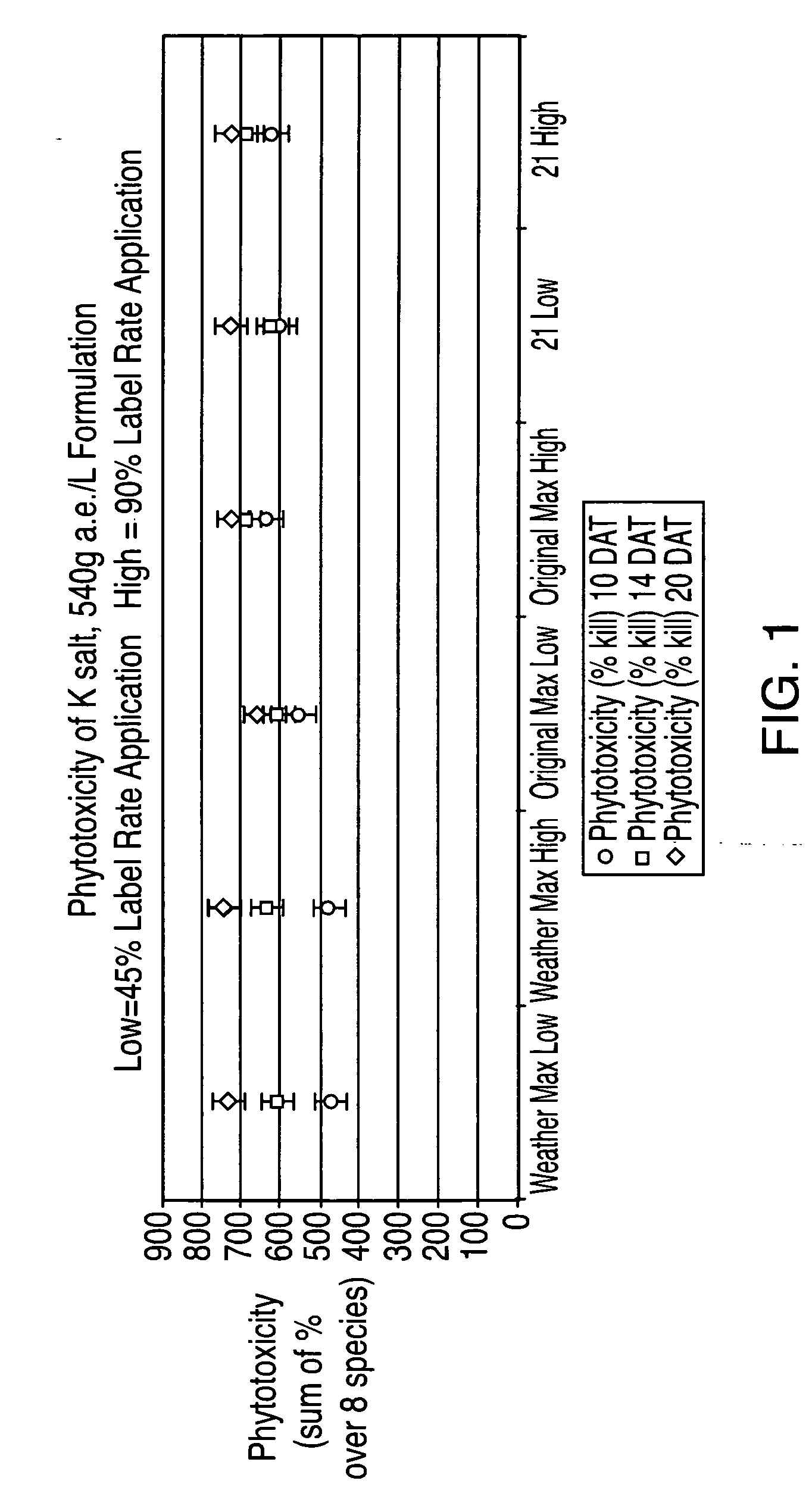
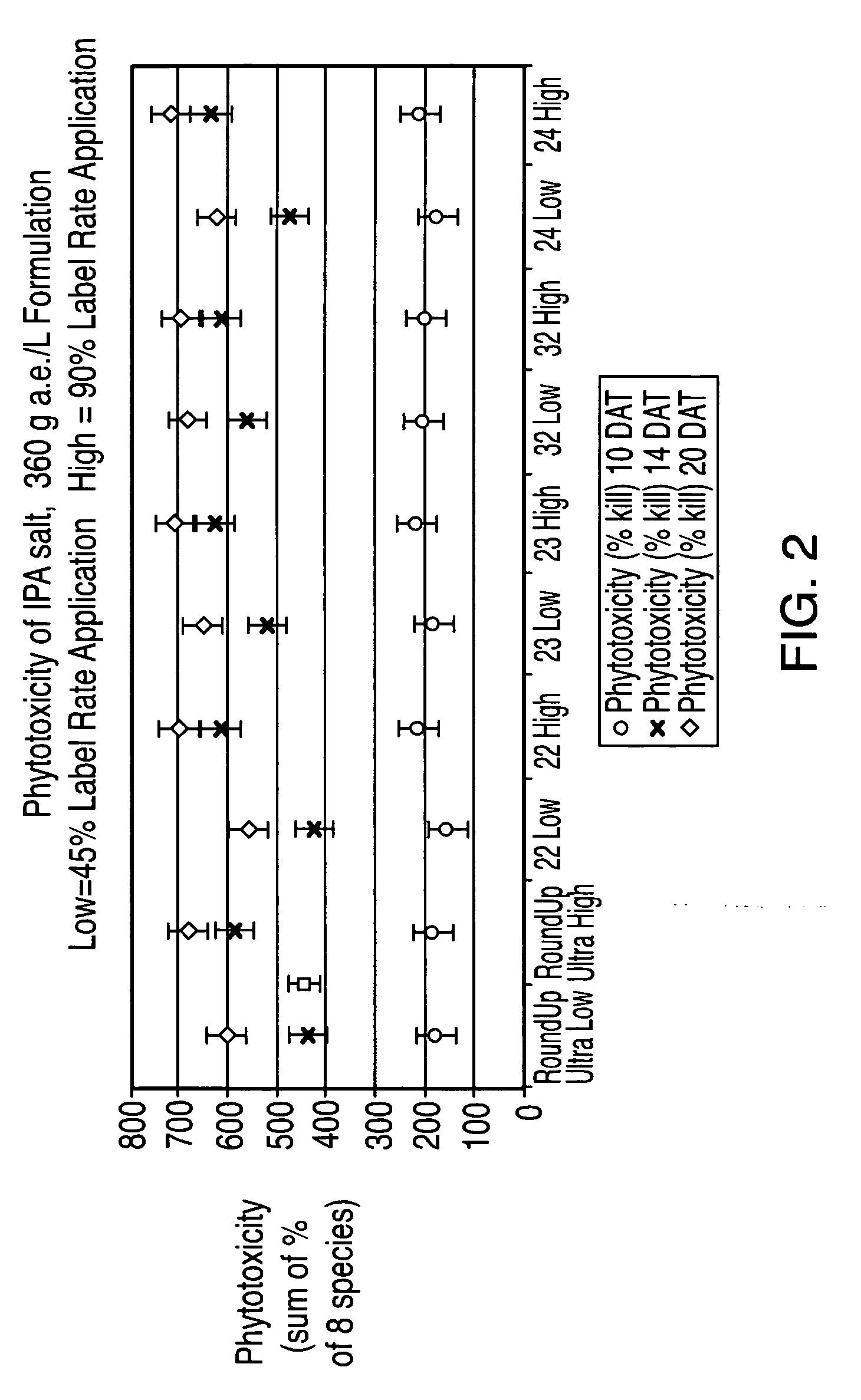
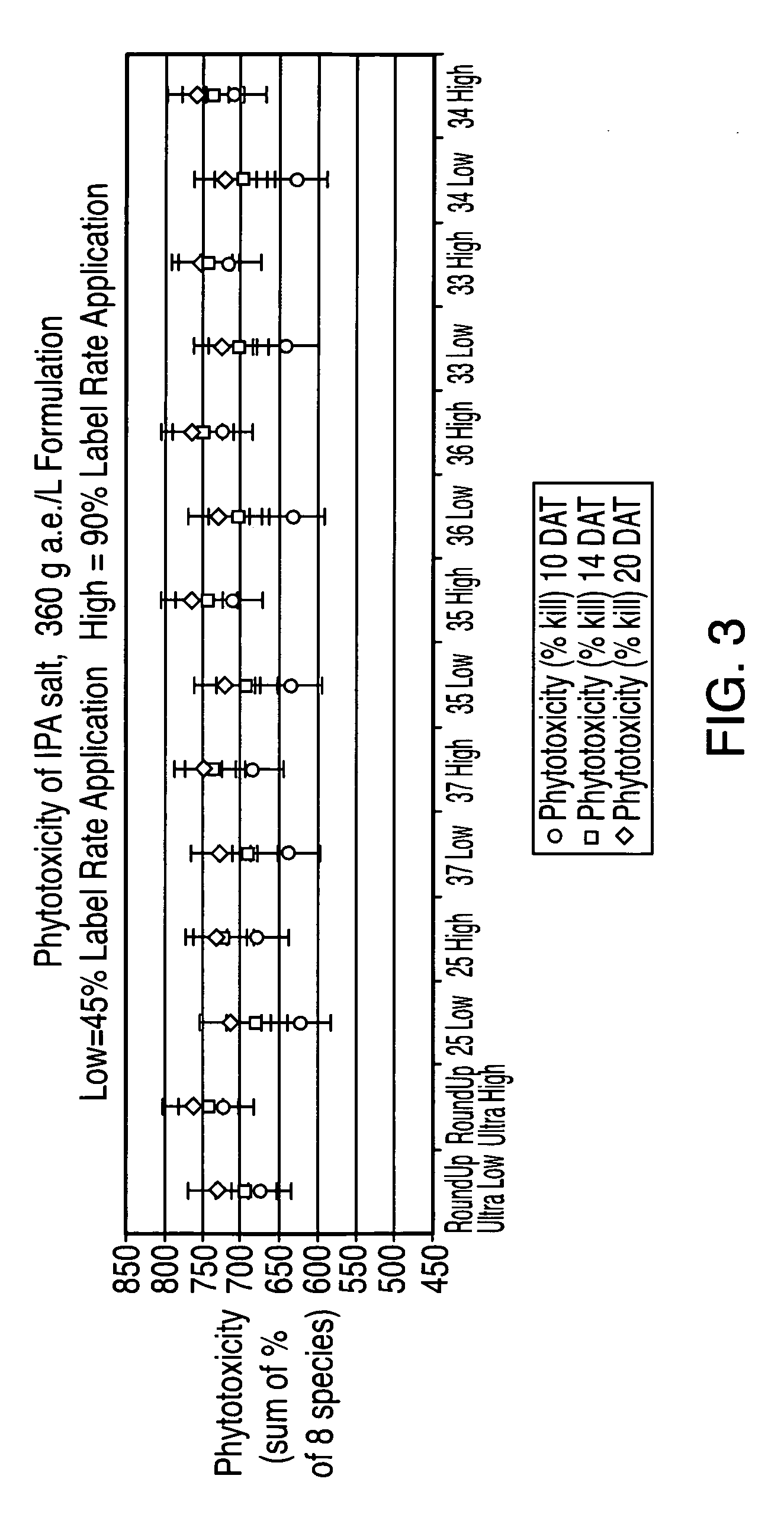
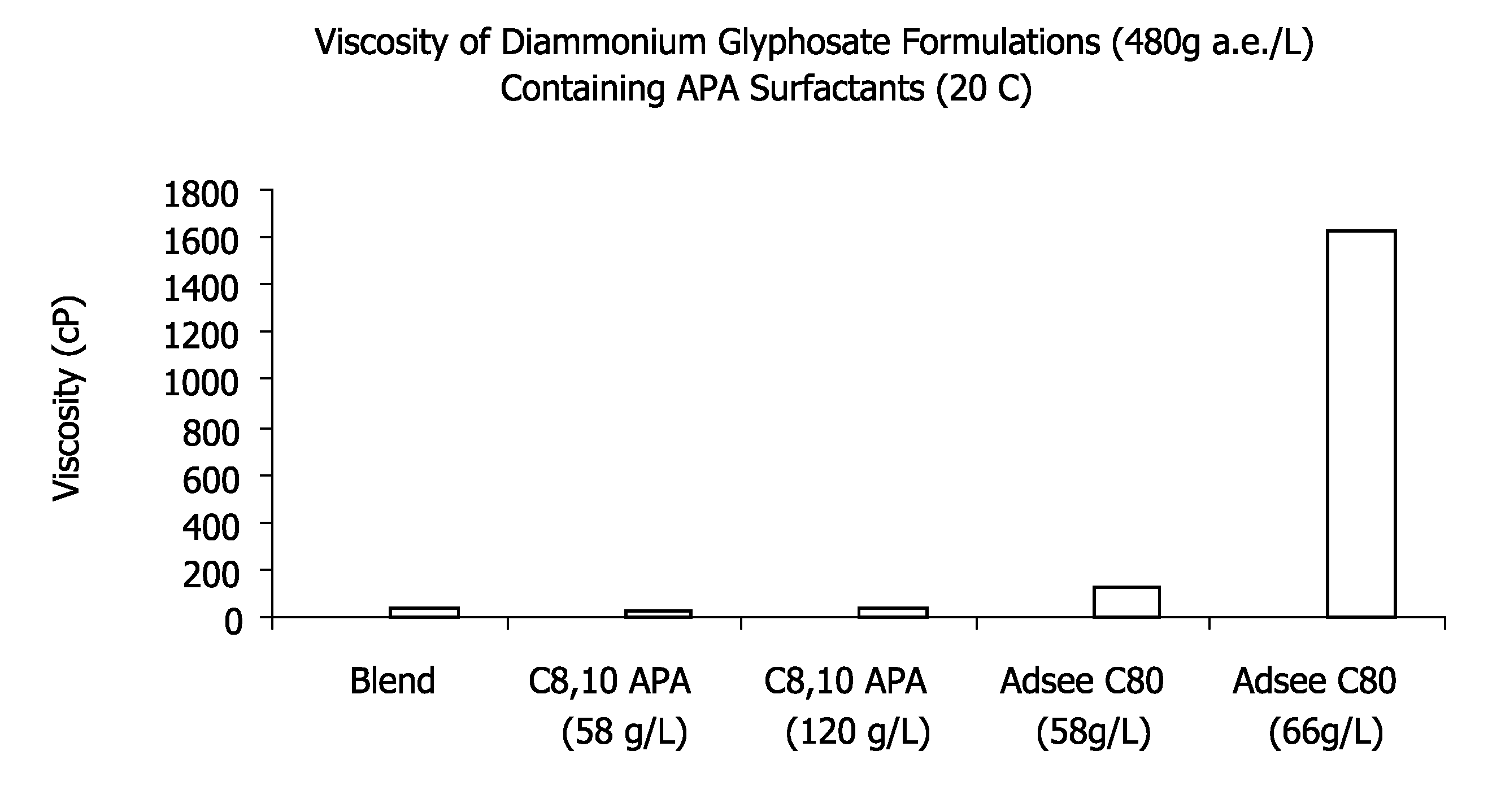
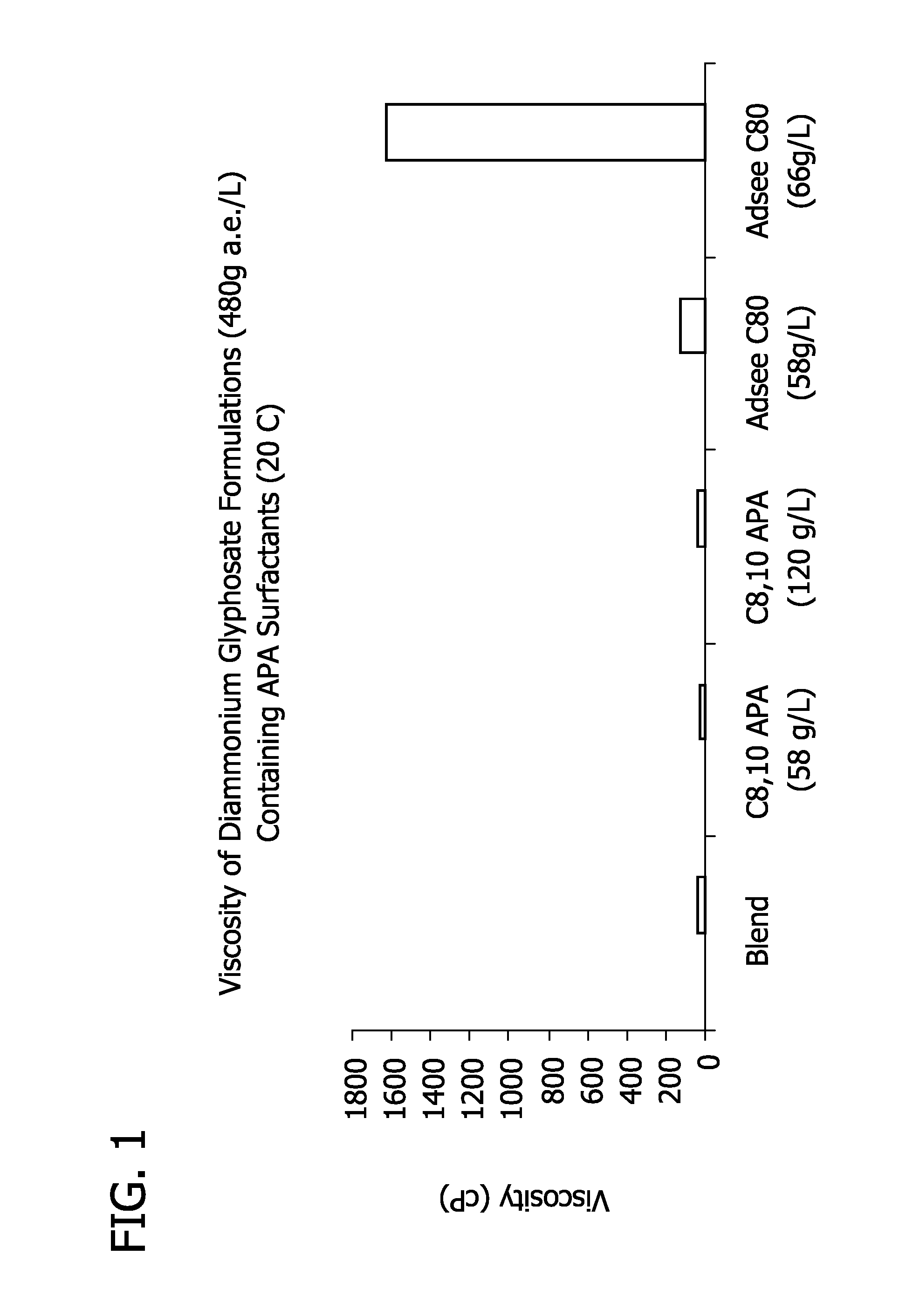
Ultra-high loading glyphosate concentrate
ActiveUS20090318294A1Much cycle timeMuch efficient productionBiocideDead animal preservationHigh concentrationActive agent
This invention relates to a storage stable, aqueous, herbicidal formulation containing an ultra-high concentration of glyphosate in the isopropylamine, potassium or mixed salt form in combination with a surfactant system, to a method of making the formulation, and to a method of treating unwanted vegetation employing the formulation. The surfactant system employed in the concentrate comprises dialkoxylated alkylamine, water miscible solubilizer and amine oxide. The surfactant system unexpectedly permits the formulation of storage stable, ultra-high loaded aqueous glyphosate salt concentrates possessing high or no cloud points.
Owner:STEPAN COMPANY
Glyphosate formulations containing amidoalkylamine surfactants
InactiveUS20100113274A1Low toxicityComposition is stableBiocideDead animal preservationSURFACTANT BLENDGlyphosate toxicity
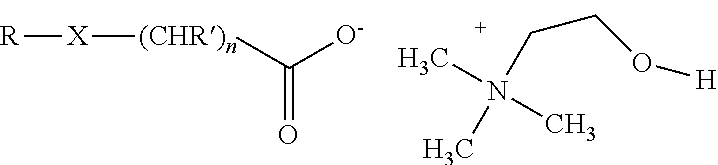
An herbicidal composition comprising (a) glyphosate or a derivative thereof, (b) an amidoalkylamine surfactants having the general structure:wherein R1 is a hydrocarbyl having from about 1 carbon atoms to about 22 carbon atoms, and R2, R3, and R4 are each independently hydrocarbyl having from about 1 carbon atom to about 6 carbon atoms; and (c) at least one co-surfactant.
Owner:MONSANTO TECH LLC
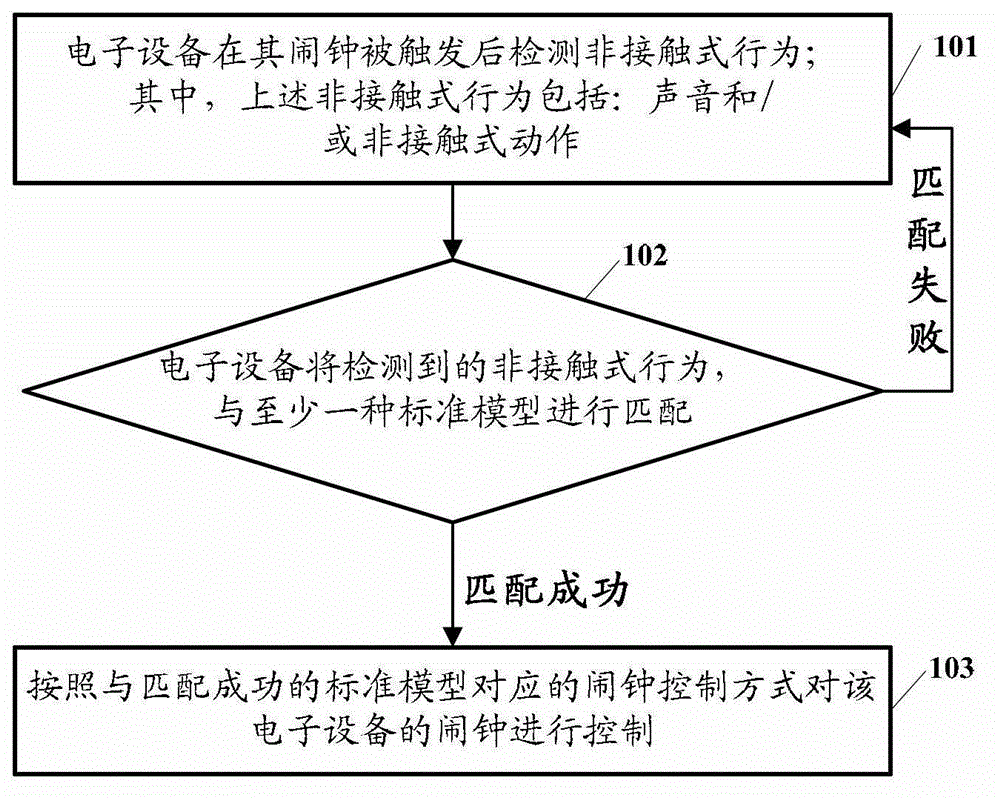
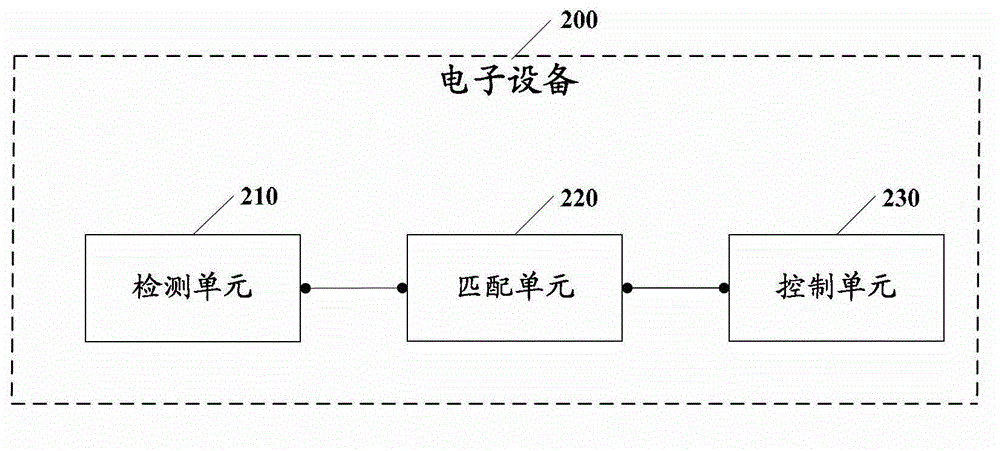
Electronic equipment alarm clock control method and electronic equipment
ActiveCN102866774AEasy to operateImprove experienceInput/output for user-computer interactionAcoustic time signalsEmbedded systemControl mode
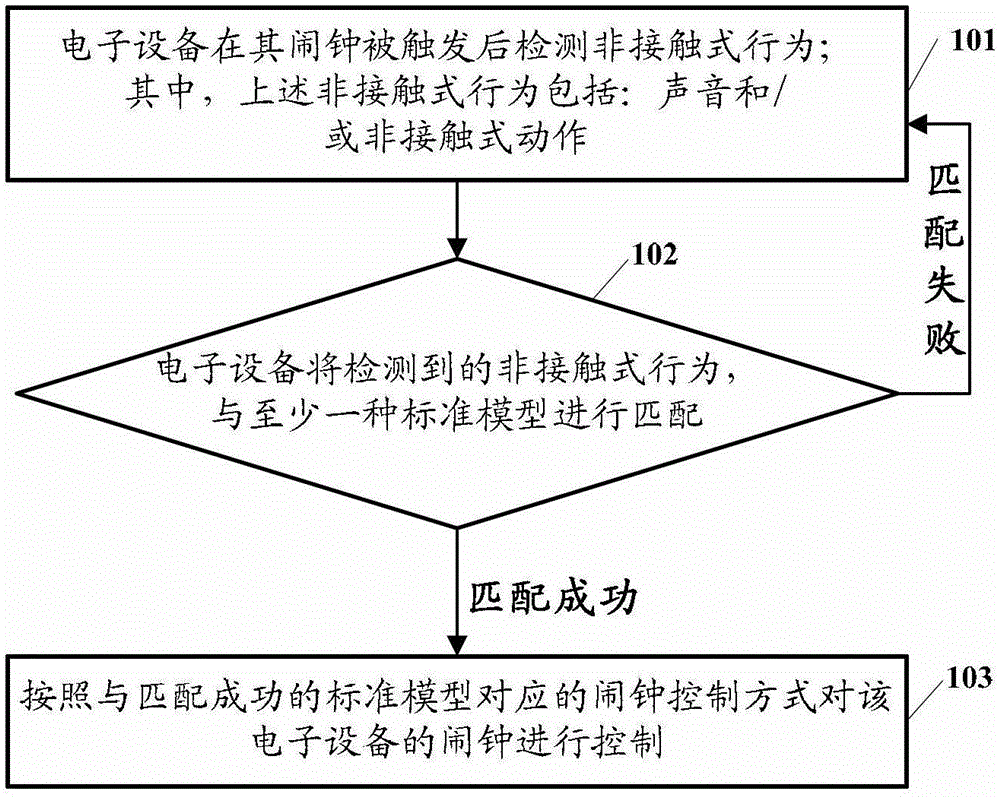
The embodiment of the invention discloses an electronic equipment alarm clock control method and electronic equipment. The method comprises the following steps of: after an alarm clock of the electronic equipment is triggered, detecting a non-contact behavior, wherein the non-contact behavior comprises voice and / or a non-contact action; matching the detected non-contact behavior with at least one standard model; and if the detected non-contact behavior is matched with at least one standard model, controlling the alarm clock of the electronic equipment according to an alarm clock control mode corresponding to the standard model which is matched with the non-contact behavior. By the technical scheme provided by the embodiment of the invention, the simplification of user operation for controlling the alarm clock of the electronic equipment can be facilitated, and user experiences can be improved.
Owner:GLOBAL INNOVATION AGGREGATORS LLC
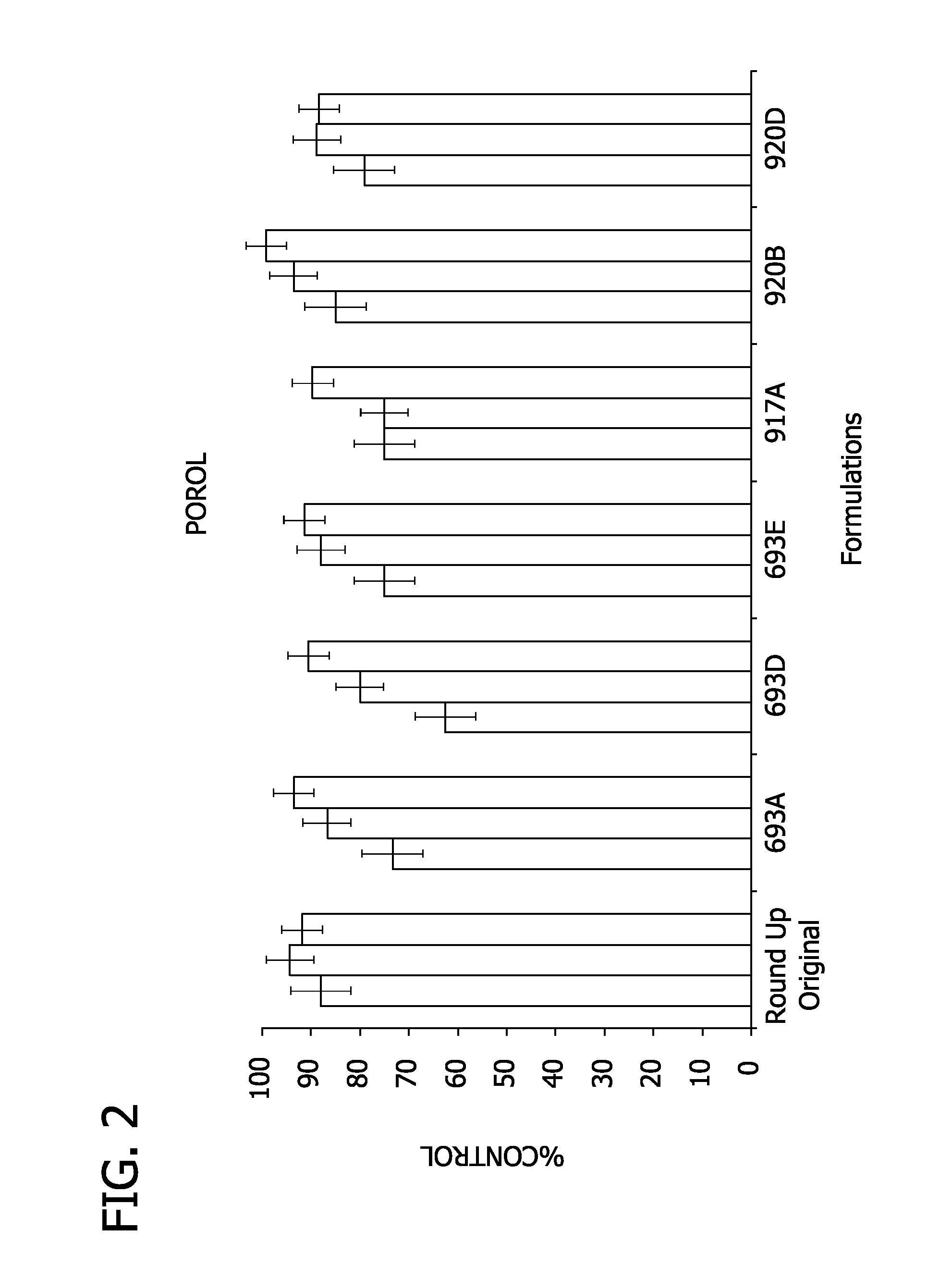
Antibacterial compositions comprising quaternary ammonium germicides and alkamine oxides having reduced irritation potential
Antibacterial compositions having antibacterial effectiveness and reduced eye irritation potential are disclosed. The antibacterial compositions contain a quaternary ammonium compound, an alkamine oxide, a nonionic compound, optional adjuvant materials known in the art, and water. The eye irritation is decreased by decreasing the amount, by weight, of alkamine oxide present in the composition and alternatively, or in combination therewith, increasing the ratio of nonionic material to alkamine oxide present in the composition.
Owner:DIAL CORPORATION
Aqueous herbicidal concentrates of auxinic carboxylic acids with reduced eye irritancy
ActiveUS20110257012A1Less irritatingReduce eye irritationBiocideDead animal preservationCarboxylic acidAmmonium
Owner:CORTEVA AGRISCIENCE LLC
Agricultural compositions which enhance performance of herbicides
InactiveUS20070049498A1Improved performance characteristicsReduced aquatic toxicityBiocideDead animal preservationAdjuvantIrritation
Agricultural compositions and / or tank mix adjuvants, which exhibit enhanced performance properties such as reduced eye irritation, reduced fish toxicity, and improved compatibility with other tank mixed pesticides and / or adjuvants while maintaining typical pesticidal performance characteristics containing at least one amine ethoxylate and glycerol.
Owner:ADJUVANTS UNLTD
Tacrolimus ophthalmic preparation and preparation method thereof
ActiveCN107929235AMinimal eye irritationImprove complianceOrganic active ingredientsSenses disorderConjunctival xerosisPatient compliance
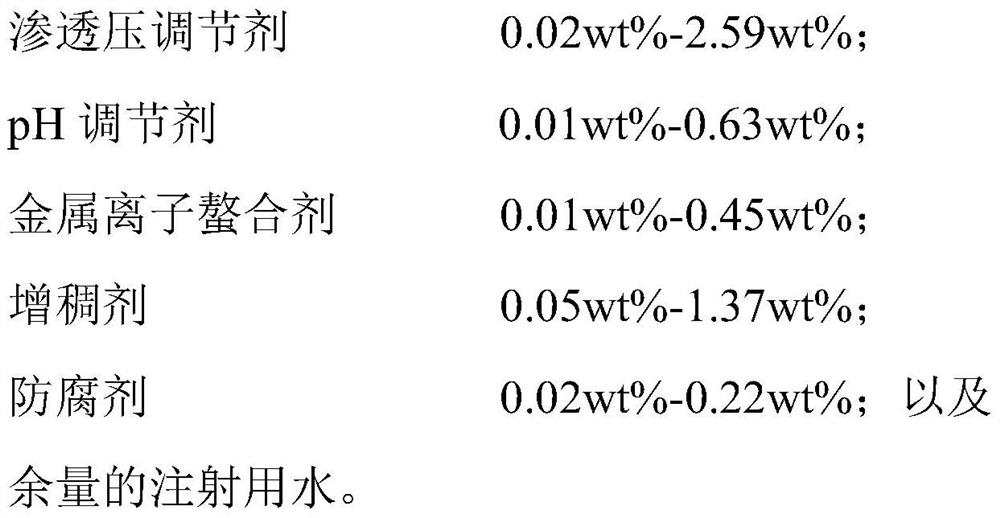
The invention provides a tacrolimus ophthalmic preparation and a preparation method thereof, and belongs to the technical field of pharmaceutical preparations. According to the present invention, thetacrolimus ophthalmic preparation has a micro-emulsion-like structure, is clarified and transparent, has good patient compliance, is prepared from tacrolimus, oil for injection, a surfactant, a stabilizer, an osmotic pressure regulator, a preservative, a pH value regulator, a tackifier and water for injection, and is used for preventing and treating immunological rejection after corneal transplantation or immunological corneal xerosis and conjunctival xerosis. According to the present invention, with the tacrolimus ophthalmic preparation, the problem of the water insolubilization of tacrolimusis solved, the tacrolimus is firstly prepared into the clarified and transparent eye drops with the micro-emulsion-like structure, the irritation on the eyes by the existing tacrolimus-suspended ophthalmic eye drops can be substantially reduced, and the patient compliance can be improved, such that the tacrolimus ophthalmic preparation of the present invention is suitable for the long-term use byimmunological rejection after corneal transplantation, corneal xerosis and conjunctival xerosis, and other patients with indications.
Owner:SHENYANG XINGQI PHARM CO LTD
Ether sulfonate surfactants and process for making same
InactiveUS20080171895A1Improve suppression propertiesGood wetting/foaming propertyOrganic chemistrySurface-active detergent compositionsPersonal careAlkaline earth metal
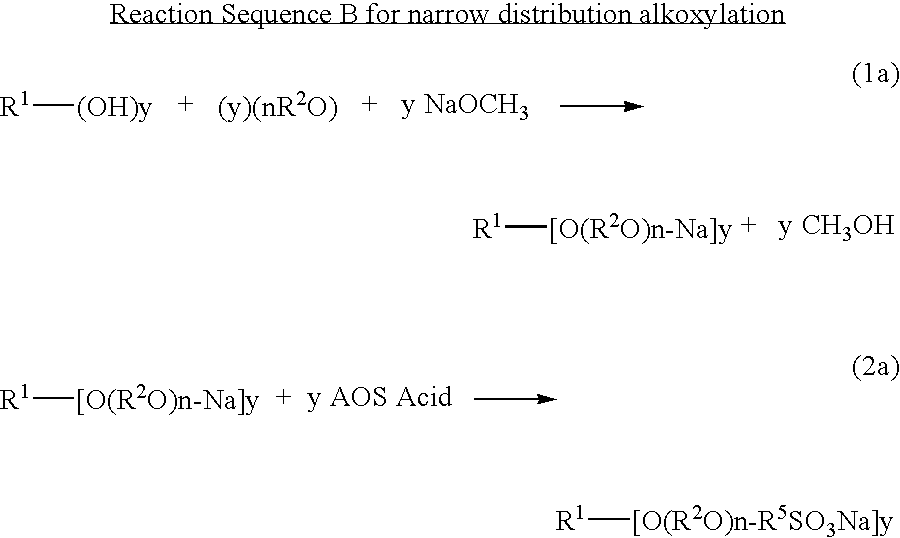
Novel compositions of matter and the process of preparing these surfactants having the structure:R1[—(O—(R2O)m—(R3O)n—(R4)]y where:R1=alkyl, alkenyl, amine, alkylamine, dialkylamine, trialkylamine, aromatic, polyaromatic, cycloalkane, cycloalkene,R2=C2H4 or C3H6 or C4H8,R3=C2H4 or C3H6 or C4H8,R4=linear or branched C7H14SO3X to C30H60 SO3X when y=1,R4=linear or branched C7H14SO3X to C30H60 SO3X or H when y>1 but at least one R4 must be linear or branched C7H14SO3X to C30H60 SO3X,m≧1,n≧0,n+m=1 to 30+,y≧1,X=alkali metal or alkaline earth metal or ammonium or amine.These novel ether sulfonate surfactants have excellent surfactant properties making them suitable for a variety of applications as surfactants including agriculture, adhesives, coatings, deinking, detergents, emulsion polymerization, laundry, lubricants, metal working, mining, oilfield, personal care, pharmaceuticals, and soil remediation.
Owner:BERGER PAUL DANIEL +1
Personal cleansing systems exhibiting low eye irritation potential
ActiveUS8470753B2Reduce eye irritationClear, viscous, effectiveCosmetic preparationsHair cosmeticsPersonal careCocamidopropyl hydroxysultaine
An ethylene oxide-free, dioxane-free, and formaldehyde-free personal care concentrate composition free of ethoxylated components which is non-irritating to eyes comprising water, sodium alkyl sulfate, propanediol, and a synthetic amphoteric surfactant selected from the group consisting of cocamidopropyl hydroxysultaine and cocamidopropyl betaine is disclosed. This composition is especially suitable for baby shampoos which are not irritating. In some embodiments the composition is free of formaldehyde.
Owner:SPECIALTY OPERATIONS FRANCE
Pharmaceutical formulations that form gel in situ
ActiveUS20170266294A1Slow down drug eliminationExtended staySenses disorderAerosol deliveryMedicineNose
The present invention provides aqueous formulations containing an anti-infection agent, a biocompatible polysaccharide, an osmotic pressure regulator, a pH regulator, and water, wherein a gel containing the therapeutic agent is formed in situ upon instillation of the formulations onto the skin and a body cavity of a subject. The formulations of this invention are useful for treating infectious diseases of skin or a body cavity (e.g., eye, nose, or vagina) of a subject.
Owner:IVIEW THERAPEUTICS
Ophthalmic composition and preparation method thereof
ActiveCN111407774AReduce evaporationStabilize tear filmOrganic active ingredientsSenses disorderOPHTHALMOLOGICALSOcular surface
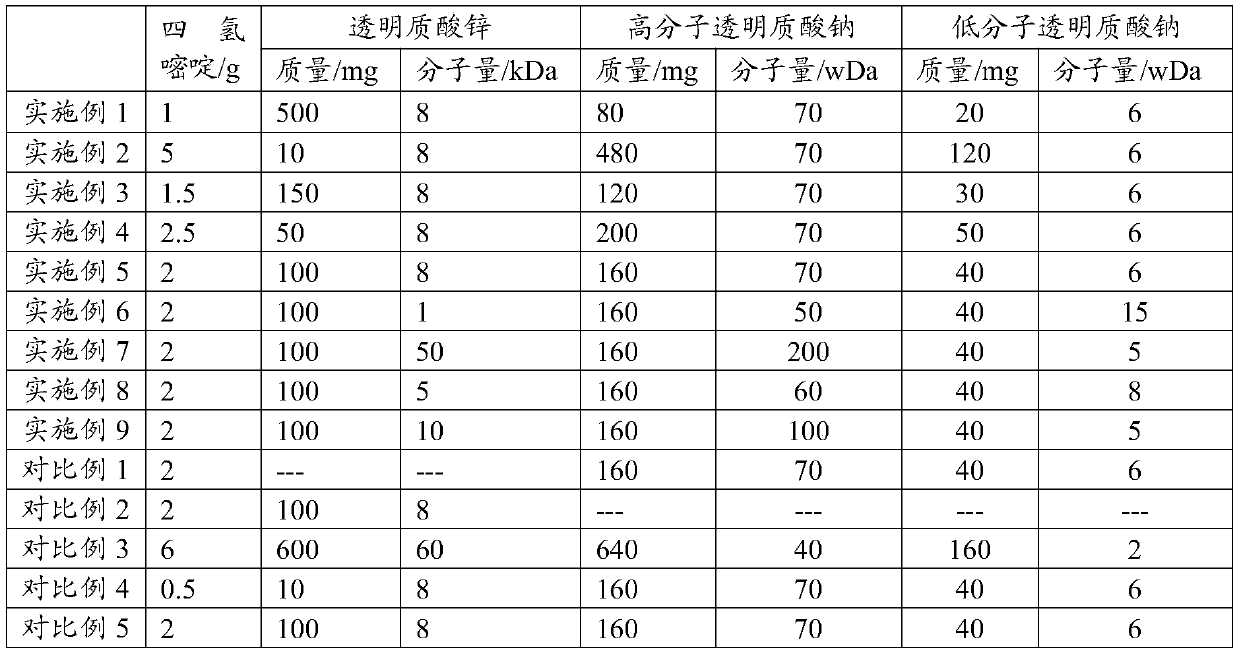
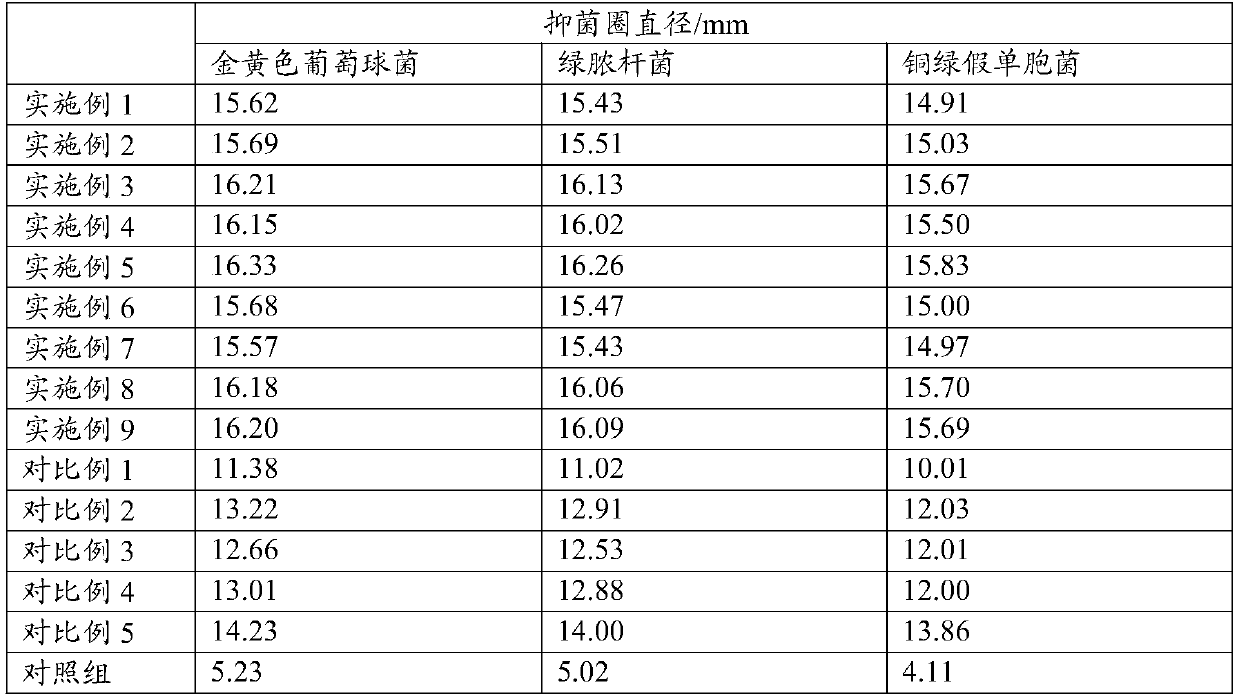
The present application discloses a preparation method for an ophthalmic composition. The method includes the following steps: dissolving a high-molecular-weight hyaluronate and a low-molecular-weighthyaluronate in water under heating conditions, adding sodium chloride, performing uniform stirring, adjusting the pH to be alkaline by using an inorganic alkali or salt, performing alcohol precipitation, performing washing, and performing drying to obtain compound hyaluronate powder; dissolving the compound hyaluronate powder in water, and adding zinc hyaluronate, tetrahydropyrimidine or a tetrahydropyrimidine derivative to obtain a mixed solution; and adjusting the pH of the mixed solution, and performing filter sterilization or heat press sterilization to obtain the ophthalmic composition.The present application also provides the ophthalmic composition. The ophthalmic composition provided in the present application contains the compounded sodium hyaluronate, the zinc hyaluronate and the tetrahydropyrimidine, does not contain preservatives, the sodium hyaluronate, the zinc hyaluronate and the tetrahydropyrimidine have synergistic effects, and the composition has the effects of reducing tear evaporation, protecting ocular surface cells, stabilizing tear films, and relieving visual fatigue.
Owner:BLOOMAGE BIOTECHNOLOGY CORP LTD
Personal cleansing systems exhibiting low eye irritation potential
ActiveUS20120196783A1Easy to transportReduce eye irritationCosmetic preparationsHair cosmeticsPersonal careHydroxysultaine
An ethylene oxide-free, dioxane-free, and formaldehyde-free personal care concentrate composition free of ethoxylated components which is non-irritating to eyes comprising water, sodium alkyl sulfate, propanediol, and a synthetic amphoteric surfactant selected from the group consisting of cocamidopropyl hydroxysultaine and cocamidopropyl betaine is disclosed. This composition is especially suitable for baby shampoos which are not irritating. In some embodiments the composition is free of formaldehyde.
Owner:RHODIA OPERATIONS SAS
Ether sulfonate surfactants and process for making same
InactiveUS7427588B2Improve suppression propertiesGood wetting/foaming propertyOrganic chemistrySurface-active detergent compositionsPersonal careAlkaline earth metal
Owner:BERGER PAUL DANIEL +1
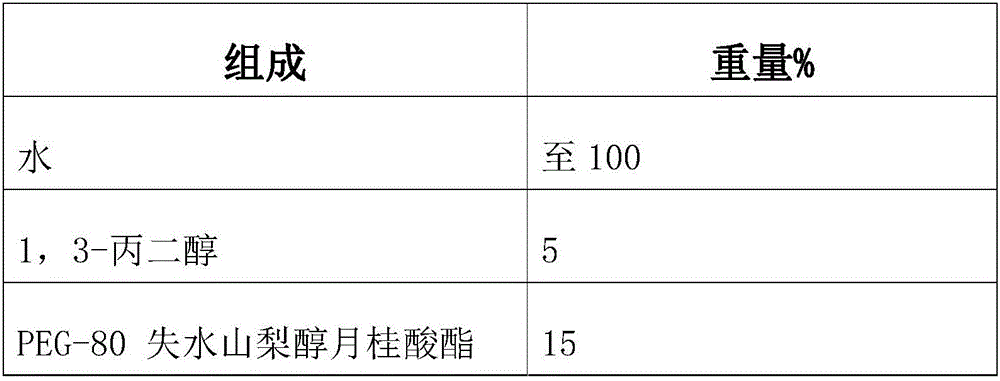
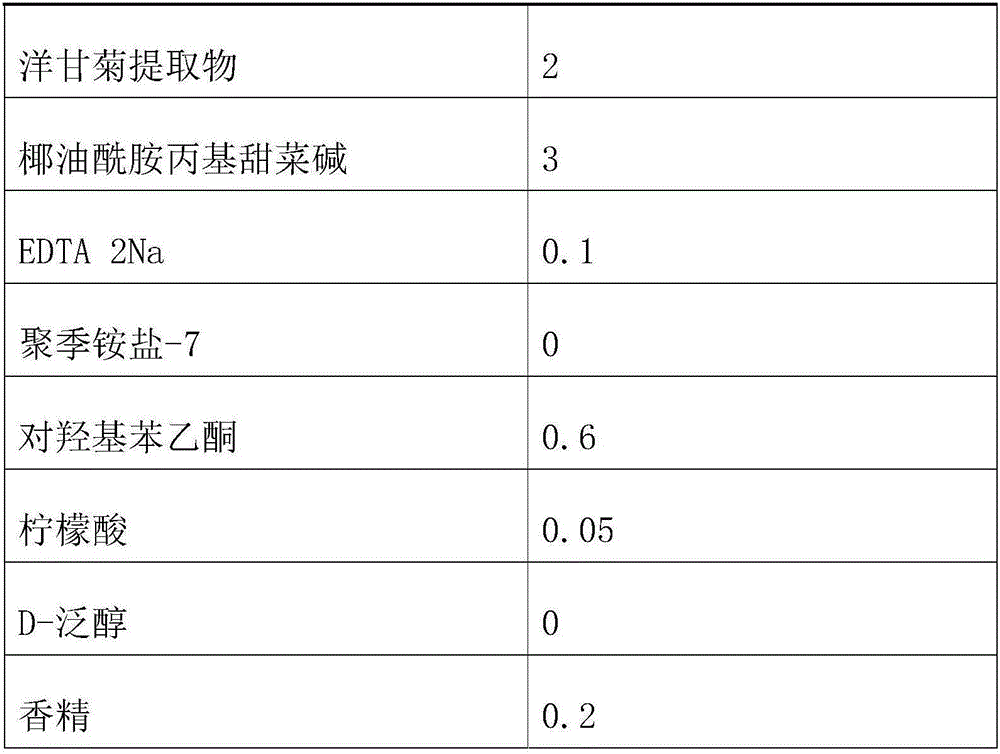
Tear-free formula shampoo composition
ActiveCN106309241AReduce eye irritationReduce manufacturing costCosmetic preparationsHair cosmeticsPolyethylene glycolChamomile extract
The invention discloses a tear-free formula shampoo composition. The composition is prepared from the following components: water, polyethylene glycol (PEG)-80 sorbitan laurate, 1,3-propylene glycol, a chamomile extract, cocamidopropyl betaine, and the like. The composition does not contain an anionic surfactant or only contains cationic surface active matters, and does not contain formaldehyde, thus being especially suitable for tear-free baby shampoo.
Owner:珠海姗拉娜化妆品有限公司
Oxidative hair dye composition
InactiveCN105392467AReduce nasal irritationReduce eye irritationCosmetic preparationsHair cosmeticsHair dyesNOSE IRRITATION
The present invention relates to an oxidative hair dye composition and, more specifically, to an oxidative hair dye composition which contains at least one kind of ester having 30 or more carbon atoms, thereby suppressing the volatilization of ammonia and thus reducing eye and nose irritation.
Owner:AMOREPACIFIC CORP
Ophthalmic solution comprising diquafosol
ActiveUS9486529B2Improve anti-corrosion performancePerformance deteriorationOrganic active ingredientsSenses disorderFiltrationEye irritation
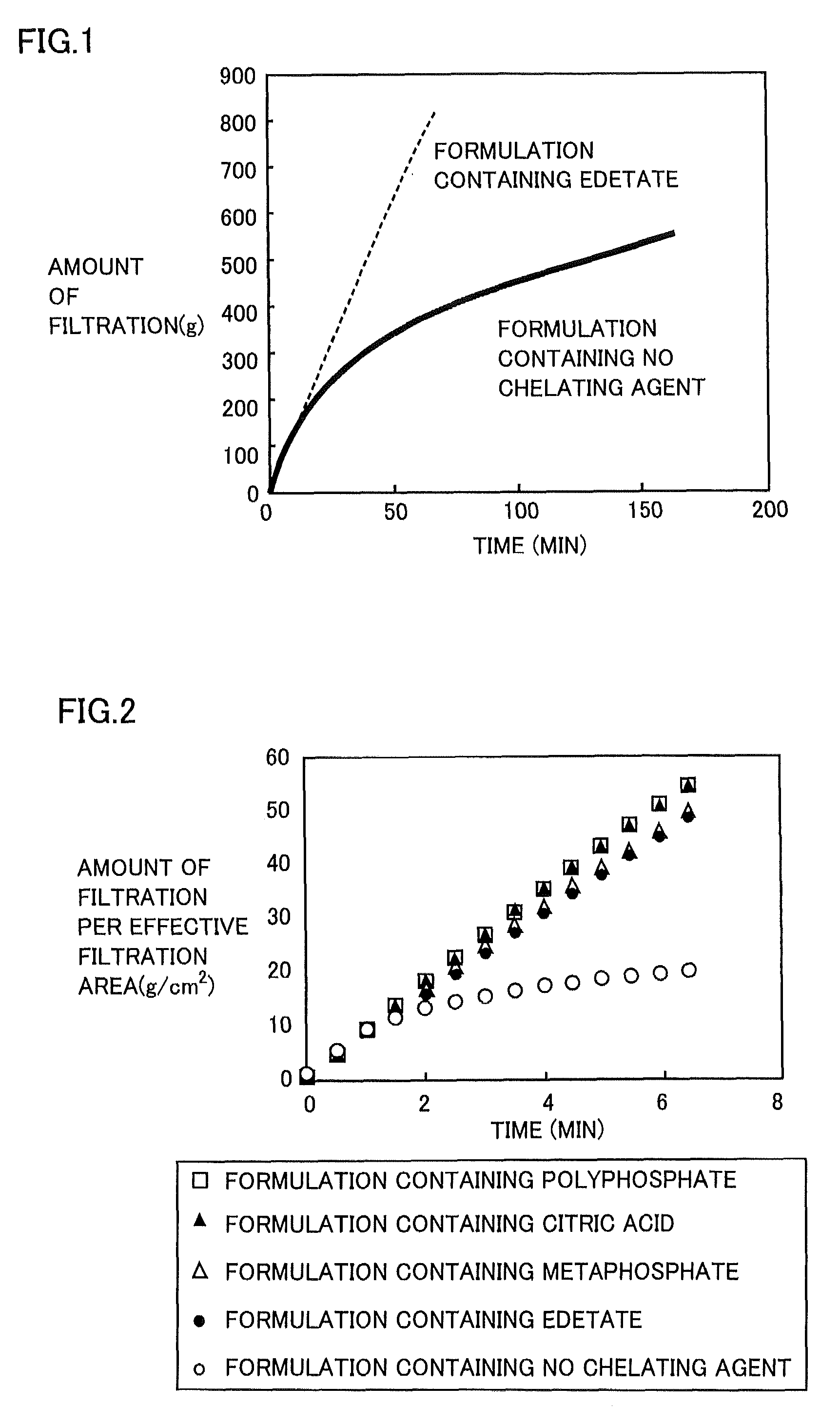
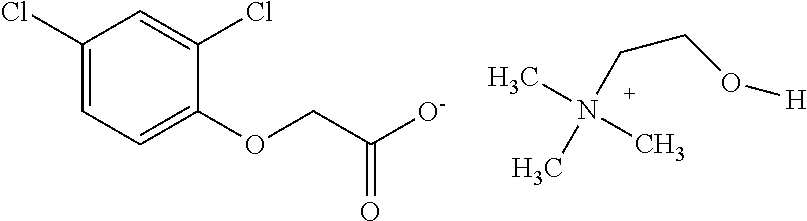
Regarding Diquafosol ophthalmic solution comprising a chelating agent at a concentration of 0.0001 to 1% (w / v), formation of insoluble precipitates found in Diquafosol ophthalmic solution during storage of the solution, as well as deterioration of the filtration performance in the course of production (course of filtration sterilization), have been inhibited. Further, in Diquafosol ophthalmic solution comprising a chelating agent, reduction of eye irritation and enhancement of the preservative effectiveness have been confirmed, in comparison to Diquafosol ophthalmic solution comprising no chelating agent. Accordingly, the present invention has been confirmed to provide physicochemical properties that are stable during the courses of production and distribution as well as the course of storage by a patient, and also reduce eye irritation and enhance preservative effectiveness.
Owner:SANTEN PHARMA CO LTD
Aqueous herbicidal concentrates of auxinic carboxylic acids with reduced eye irritancy
ActiveUS8563473B2Less irritatingReduce eye irritationBiocideDead animal preservationCarboxylic acidChemistry
Owner:CORTEVA AGRISCIENCE LLC
BDZL (bendazac lysine) eye drops
InactiveCN105687129AImprove bioavailabilityPromote absorptionOrganic active ingredientsSenses disorderTreatment effectIrritation
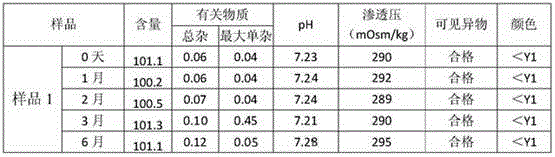
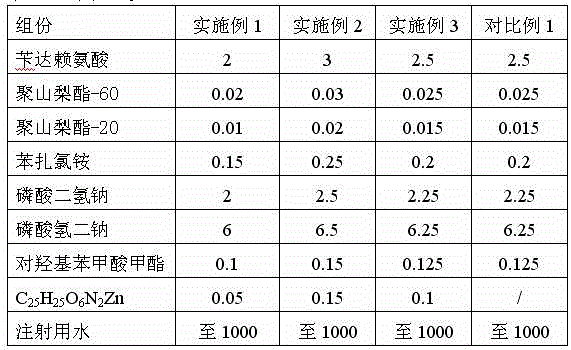
The invention relates to the technical field of pharmaceutical preparations, in particular to BDZL (bendazac lysine) eye drops. The BDZL eye drops are used for preventing and treating senile cataract and are prepared from components in parts by mass as follows: 2-3 parts of BDZL, 0.02-0.03 parts of polysorbate-60, 0.01-0.02 parts of polysorbate-20, 0.15-0.25 parts of benzalkonium chloride, 2-2.5 parts of monosodium phosphate, 6-6.5 parts of sodium hydrogen phosphate, 0.1-0.15 parts of methyl 4-hydroxybenzoate, 0.05-0.15 parts of C25H25O6N2Zn and up to 1,000 parts of water for injection. The BDZL eye drops have the advantages that the quality is stable, irritation for eyes is remarkably lower than that of like products sold in the market, the bioavailability is improved remarkably by means of the effective component BDZL under matching of C25H25O6N2Zn, the absorption is good, the effect is taken rapidly, and the treatment effect is stable.
Owner:GUANGDONG YIMING PHARMA
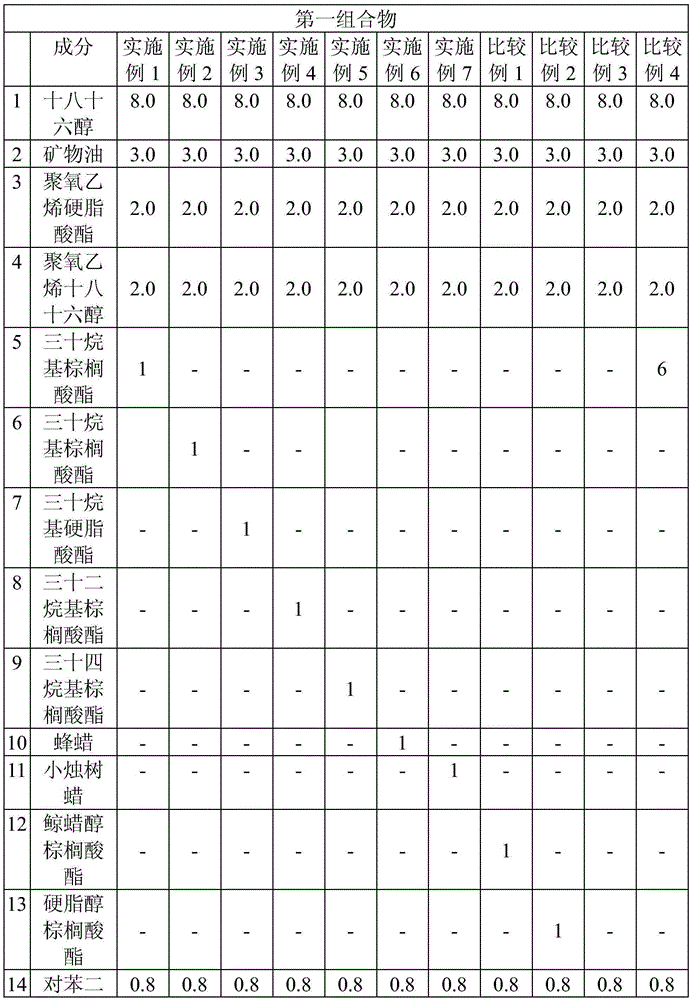
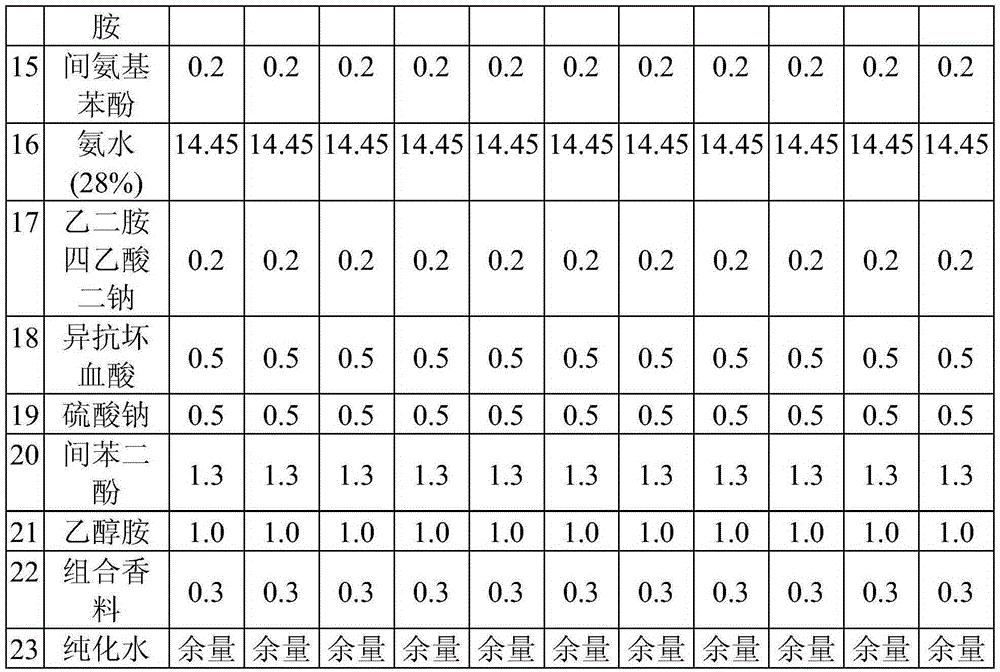
High concentrate fomesafen herbicide formulations
PendingUS20200296961A1Reduce application rateReduce storageBiocideAnimal repellantsActive agentFatty amine
The invention relates to compositions containing the non-salt form of the herbicide fomesafen solubilized with at least one surfactant selected from the group consisting of fatty amine, alkanolamine, fatty amine alkoxylate and alkoxylated quaternary ammonium compounds. Advantages provided by these compositions include higher active ingredient levels, improved cold temperature stability, and enhanced weed control performance.
Owner:HELENA AGRI ENTERPRISES LLC
Natamycin-loaded alginic acid gel medicine membrane and a preparation method thereof
ActiveCN112891326AReduce eye irritationLess discomfortOrganic active ingredientsSenses disorderEye irritationDrug release
The invention relates to the technical field of medical materials, in particular to a natamycin-loaded alginic acid gel medicine membrane and a preparation method thereof. The natamycin-loaded alginic acid gel medicine membrane comprises the following components and contents: every 1mL of the medicine membrane contains 0.01-0.03 g of sodium alginate, 0.0025-0.0075 g of polyoxyethylene and 0.005-0.015 g of natamycin. Alginic acid gel is used as a carrier, natamycin is loaded, and an ethanol solution containing calcium ions is used for crosslinking, so that the spatial structure of the material is more cohesive, the medicine-loaded gel is more stable after membrane formation, the size of pores in a medicine membrane is reduced, membrane release is delayed, and the action time of natamycin is prolonged; alginic acid is good in histocompatibility, and eye irritation and discomfort of natamycin are reduced; the medicine membrane can be better attached to the cornea ulcer surface, and has long medicine action time, good effects, no irritation and discomfort and high acceptability of patients; and the medicine membrane is a novel ophthalmic medicine membrane which is simple in preparation method, low in cost and wide in market prospects.
Owner:THE AFFILIATED HOSPITAL OF QINGDAO UNIV
Natamycin-grafted oxidized alginic acid fiber membrane and preparation method thereof
ActiveCN113069556AReduce eye irritationLess discomfortOrganic active ingredientsSenses disorderFiberEye irritation
The invention relates to the technical field of medical materials, and relates to a natamycin-grafted oxidized alginic acid fiber membrane and a preparation method thereof. The preparation method comprises specific preparation process as follows: preparing oxidized alginic acid fiber: reacting sodium periodate with alginic acid fiber to obtain the oxidized alginic acid fiber; putting the oxidized alginic acid fiber into a glycerol aqueous solution to be soaked, conducting rinsing and drying, and putting the oxidized alginic acid fiber into a dry and sterile centrifugal tube to be stored; and soaking the oxidized alginic acid fiber with a natamycin aqueous solution, oscillating and incubating the oxidized alginic acid fiber at 50 DEG C in a dark place for 36 hours, then washing the oxidized alginic acid fiber twice with an ethanol aqueous solution and deionized water, and drying to obtain the oxidized alginic acid fiber membrane. The surface of the oxidized alginic acid fiber membrane is grafted with natamycin, so that the eye irritation and discomfort caused by the natamycin can be reduced, the half-life period of the medicine is prolonged, the utilization rate of the medicine is increased, and the treatment effect of the fungal keratitis is improved; and the preparation process is simple, the cost is low, and the product is easy to preserve and has wide market prospects.
Owner:THE AFFILIATED HOSPITAL OF QINGDAO UNIV
A tacrolimus ophthalmic preparation and preparation method thereof
ActiveCN107929235BReduce eye irritationComfortable to useOrganic active ingredientsSenses disorderConjunctival xerosisConjunctiva
The invention provides a tacrolimus ophthalmic preparation and a preparation method thereof, belonging to the technical field of pharmaceutical preparations. The tacrolimus ophthalmic preparation provided by the present invention has a microemulsion-like structure, and the prepared tacrolimus ophthalmic preparation is clear and transparent, and has good patient compliance. The preparation is prepared from tacrolimus, oil for injection, surfactant, stabilizer, osmotic pressure regulator, preservative, pH regulator, thickener and water for injection, and is used for preventing and treating immune rejection after keratoplasty or Immune keratoconjunctivitis sicca. The tacrolimus ophthalmic preparation provided by the present invention solves the water-insoluble problem of tacrolimus. For the first time, tacrolimus is made into a clear and transparent eye drop with a microemulsion-like structure. Tacrolimus suspension-type eye drops are irritating to the eyes, which improves the compliance of patients, and thus is more suitable for long-term use of patients with indications such as immune rejection after keratoplasty and keratoconjunctival sicca.
Owner:SHENYANG XINGQI PHARM CO LTD
Illumination system for ophthalmic examination
InactiveCN111938564ANarrow down the range of lightReduce eye irritationEye diagnosticsElectric machineryLighting system
The invention discloses a illumination system for ophthalmic examination. The illumination system comprises a base and an inspection mirror, wherein a first gear ring is arranged outside the inspection mirror in a sleeving manner, the first gear ring is rotatably connected with the outer side wall of the inspection mirror, a first motor is arranged at the end, close to the inspection mirror, of asupporting rod, an output shaft of the first motor is in driving connection with a first gear, the first gear is in engaged connection with the first gear ring, the first gear drives the first gear ring to rotate, a plurality of fixing rods are connected to the outer side wall of the first gear ring, a plurality of first illuminating lamps are arranged on the fixing rods, the end, away from the first motor, of the inspection mirror is sleeved with a lantern ring, the lantern ring is rotationally connected with the outer wall of the inspection mirror, and the outer side wall of the lantern ringis connected with a shading plate. According to the illuminating system, the illumination adjusting angle is large, different illumination angles can be adjusted according to the requirements of doctors, the brightness of local diffused light is increased, a uniform light source is generated in a small brightness area, and the inspection accuracy and the inspection efficiency can be improved.
Owner:ZHENGZHOU RAILWAY VOCATIONAL & TECH COLLEGE
Bionic sebum film composition as well as preparation method and application thereof
ActiveCN113081905AImprove permeabilityGood moisturizing effectCosmetic preparationsHair cosmeticsIrritationSqualane
The invention provides a bionic sebum film composition as well as a preparation method and application thereof. The bionic sebum film composition is prepared from the following components in parts by weight: 5 to 50 parts of simmondsia chinensis seed oil, 5 to 50 parts of plant squalane, 2 to 30 parts of olive fruit oil, 2 to 30 parts of prunus amygdalus dulcis oil, 1 to 20 parts of Marula oil and 1 to 10 parts of meadowfoam seed oil. The bionic sebum film composition provided by the invention has a strong moisturizing effect and has the effects of relieving irritation, calming and diminishing inflammation.
Owner:媞颂日化用品(广州)有限公司
Preparation method of eye drops
PendingCN113509436ASolve the technical problems of poor water solubilityRich varietyOrganic active ingredientsSenses disorderCyclodextrinAqueous solubility
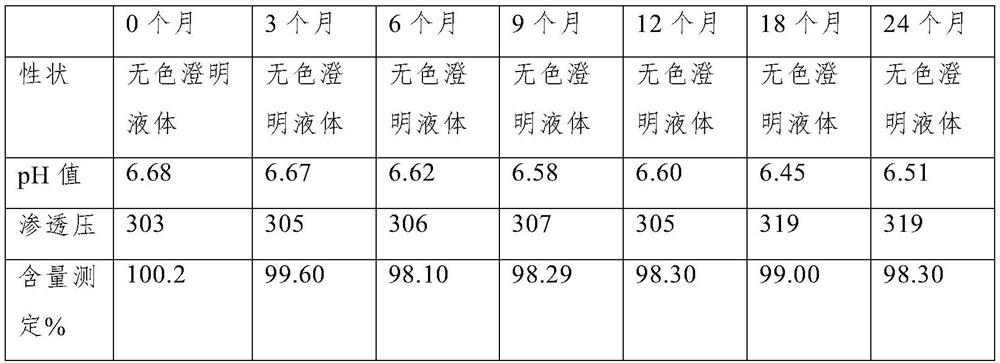
The invention discloses a preparation method of eye drops, and the eye drops comprise a voriconazole solution clathrated by sulfobutyl-beta-cyclodextrin. The preparation method of the voriconazole solution clathrated by sulfobutyl-beta-cyclodextrin comprises the following steps: 1, dissolving voriconazole with an acid solution to obtain a solution A; 2, dissolving sulfobutyl-beta-cyclodextrin by using water for injection to obtain a solution B; 3, adding the solution A into the solution B under room-temperature stirring or ultrasonic conditions to obtain a mixed system; 4, stirring the mixed system in the step 3 at room temperature to obtain the voriconazole solution included by the sulfobutyl-beta-cyclodextrin. According to the voriconazole oral liquid, the voriconazole solution included by the sulfobutyl-beta-cyclodextrin serves as a main component, the defect that voriconazole is poor in water solubility can be effectively overcome, the voriconazole oral liquid is non-toxic and non-irritant to ocular surfaces and has an obvious sterilization and bacteriostasis effect on various common clinical fungi, the content of the voriconazole oral liquid can still be kept at about 98.3% after the voriconazole oral liquid is placed for two years, and the voriconazole oral liquid has high stability and clarity.
Owner:陕西省眼科研究所
Alarm clock control method of electronic device and electronic device
InactiveCN102866774BEasy to operateImprove experienceInput/output for user-computer interactionAcoustic time signalsControl mannerContact behavior
Owner:GLOBAL INNOVATION AGGREGATORS LLC
Ophthalmic composition as well as preparation method and application thereof
PendingCN114099505ASuppress generationGood treatment effectAntibacterial agentsOrganic active ingredientsConjunctivaOcular inflammation
The present invention relates to an ophthalmic composition comprising pranoprofen and olopatadine and / or naphazoline. The invention also relates to a preparation method of the ophthalmic composition, which comprises the following steps: mixing the pranoprofen, the olopatadine and / or the naphazoline, the osmotic pressure regulator, the pH regulator, the metal ion chelating agent, the thickening agent, the preservative and the water for injection to obtain the ophthalmic composition. The invention also relates to an application of the ophthalmic composition in preparation of a medicine for treating ocular inflammation, and the ocular inflammation comprises external eye and / or anterior segment inflammation caused by gram-positive bacterium and / or gram-negative bacterium infection. When the ophthalmic composition provided by the invention is used as eye drops, the treatment effect on eye inflammation of a patient can be improved, and adverse effects of eye stimulation, conjunctival congestion, edema and the like after medication can be avoided, so that the medication compliance of the patient is improved, and the recovery time of the patient is shortened.
Owner:湖北远大天天明制药有限公司
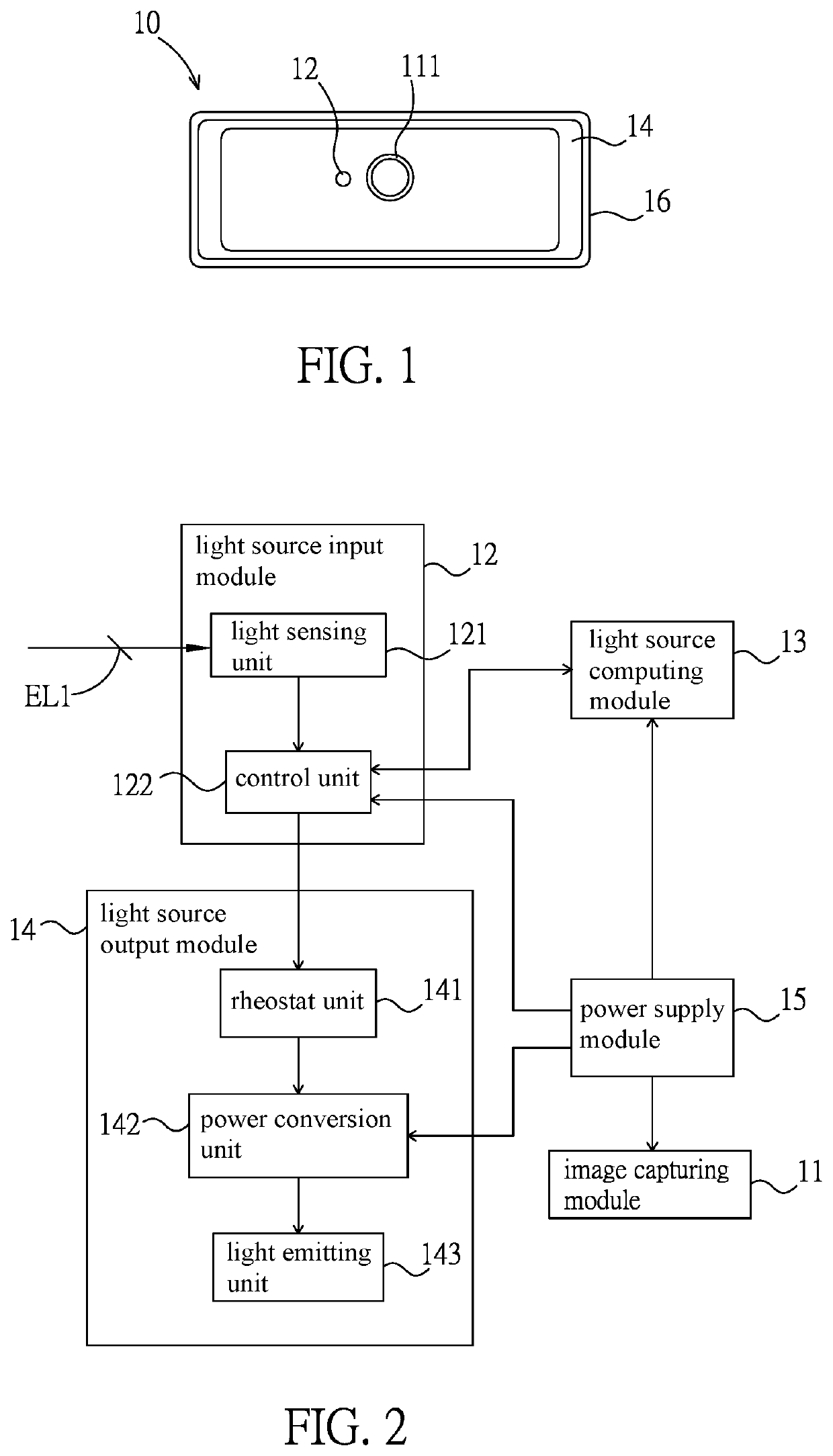
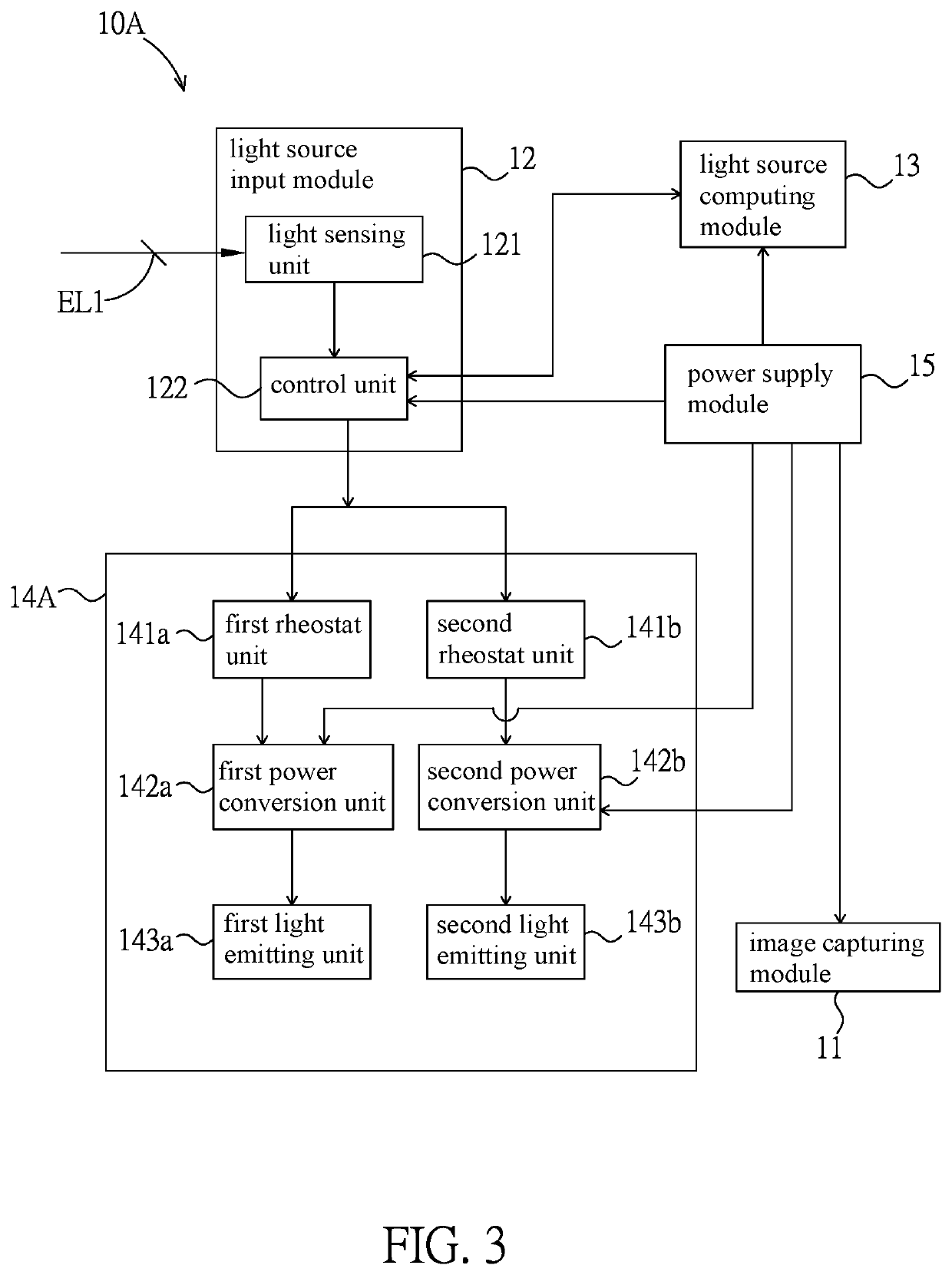
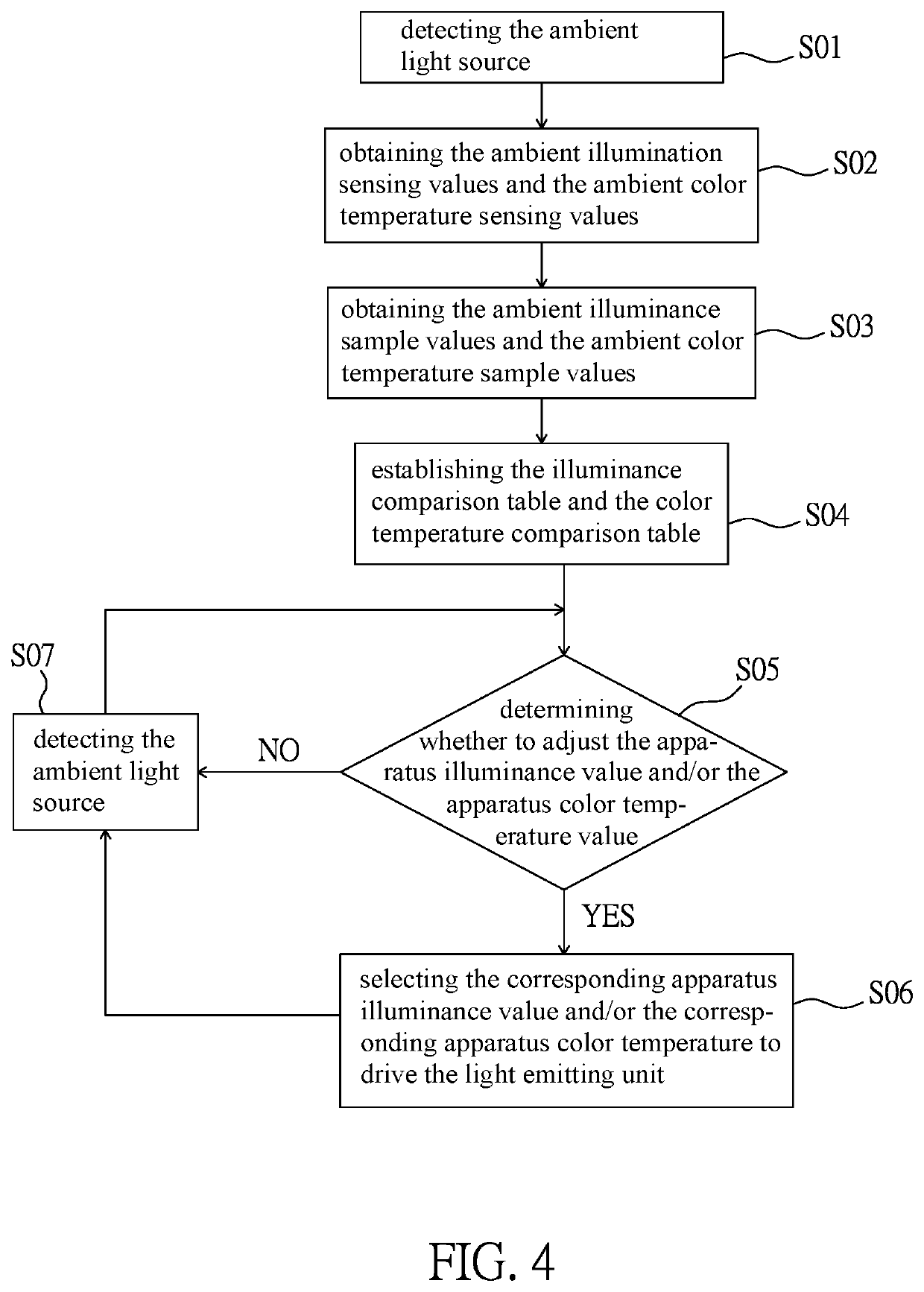
Intelligent light supplement device, video apparatus and intelligent light supplement method thereof
PendingUS20220283479A1Reduce eye irritationTelevision systemsExposure controlLight sensingIlluminance
An intelligent light supplement device, video apparatus, and an intelligent light supplement method are disclosed. The intelligent light supplement device includes a light source input module, a light source computing module, and a light source output module. The light source input module has a light sensing unit, which receives an ambient light source. The light source computing module is electrically connected to the light source input module, compares the ambient light source with a content of an illuminance comparison table to generate an illuminance control signal corresponding to an apparatus illuminance value, and / or compares the ambient light source with a content of a color temperature comparison table to generate a color temperature control signal corresponding to an apparatus color temperature value. The light source output module has a light emitting unit and drives the light emitting unit according to the illuminance control signal and / or the color temperature control signal.
Owner:AVER INFORMATION INC
Antibacterial compositions comprising quaternary ammonium germicides and alkamine oxides having reduced irritation potential
ActiveUS20110313049A1Reduce eye irritationAntibacterial agentsCosmetic preparationsAdjuvantAmmonium compounds
Owner:HENKEL KGAA
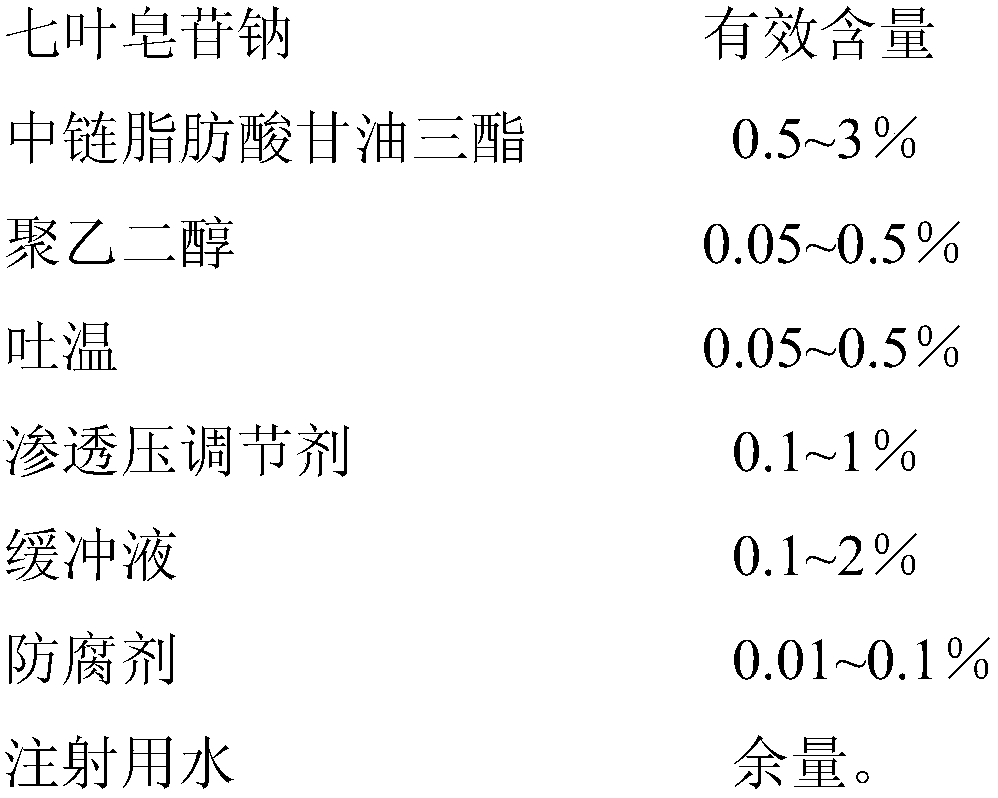
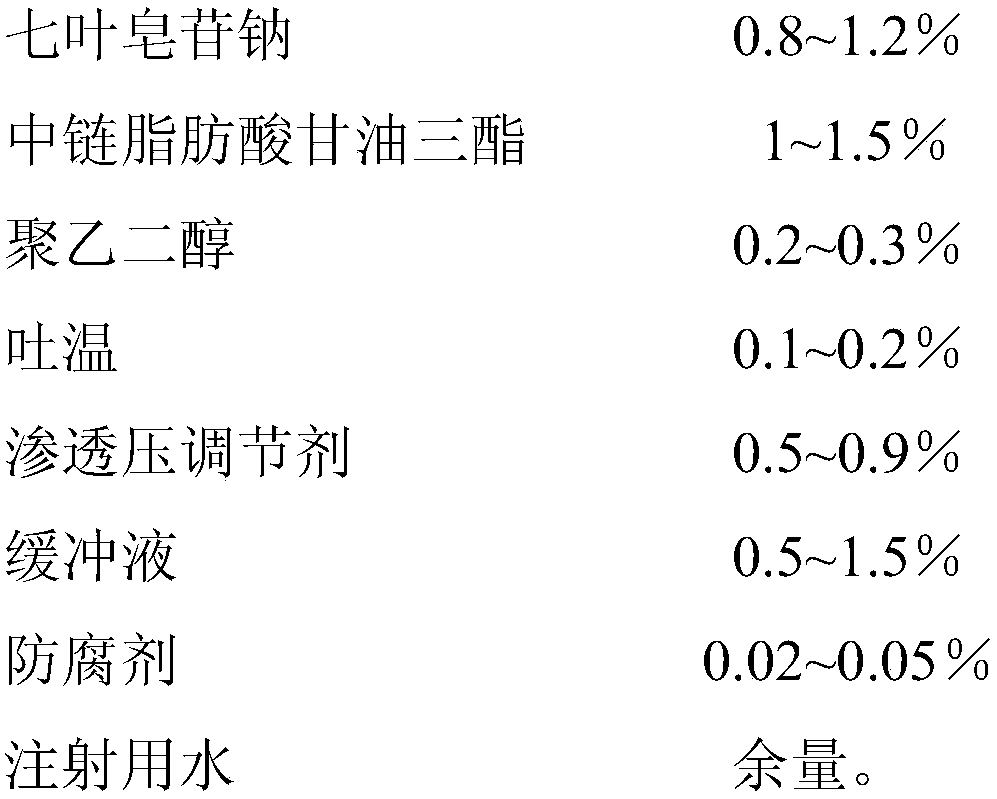
Sodium aescinate microemulsion eye drops
InactiveCN107837232AImprove quality stabilityReduce eye irritationOrganic active ingredientsSenses disorderChemistryEye drops solution
The invention discloses sodium aescinate microemulsion eye drops, which are prepared from effective content of sodium aescinate, 0.5-3% of medium-chain triglycerides, 0.05-0.5% of polyethylene glycol,0.05-0.5% of Tween, 0.1-1% of an osmotic pressure regulator, 0.1-2% of a buffer solution, 0.01-0.1% of a preservative and the balance of water for injection. The sodium aescinate is micro-emulsifiedso as to be prepared into the eye drops according to the special properties, so that the mass stability of the sodium aescinate is effectively improved, and the eye irritation is reduced.
Owner:WUHAN AIMIN PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com