Natamycin-loaded alginic acid gel medicine membrane and a preparation method thereof
A technology of natamycin and alginic acid, applied in the field of medical materials, can solve the problems of poor solubility, large particle size of natamycin and many kinds of raw materials, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] This embodiment relates to the alginic acid gel film loaded with natamycin, and its composition and content are: every 1mL volume of the film contains 0.01-0.03g of sodium alginate, 0.0025-0.00755g of polyethylene oxide and 0.005 The natamycin of -0.015g; Concrete preparation process is as follows:
[0030] (1) Dissolve 0.256-0.768g of sodium alginate powder and 0.065-0.195g of polyethylene oxide powder into 20mL and 5mL of deionized water respectively to ensure that sodium alginate and polyethylene oxide are not attached to the bottom and side walls of the beaker, and then The two solutions are mixed and stirred gently;
[0031] (2) Place the above solution on a magnetic stirrer, stir at room temperature for 3 to 5 hours to obtain a uniform gel, add natamycin to the uniform gel, and the mass volume ratio of natamycin to gel is 0.005 ~0.015g: 1mL, then stir for 30 minutes at 50°C in a dark environment until uniform without precipitation;
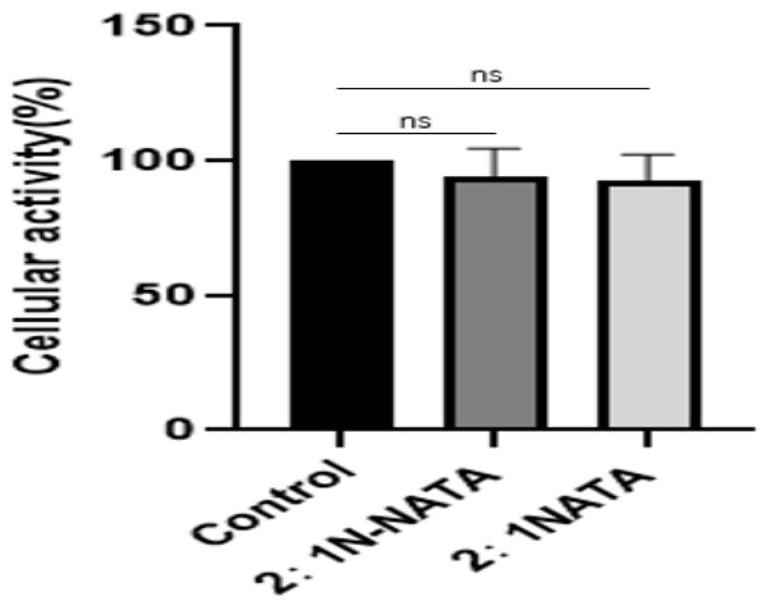
[0032] (3) Take 3-10 g of th...
Embodiment 2
[0037] This embodiment relates to the preparation process of the alginic acid gel film loaded with natamycin, and the specific steps are as follows:
[0038] (1) Dissolve 0.512g of sodium alginate powder and 0.127g of polyethylene oxide powder into 20mL and 5mL of deionized water respectively to ensure that sodium alginate and polyethylene oxide are not attached to the bottom and side walls of the beaker, and then mix the two solutions then stir gently;
[0039] (2) Place the above solution on a magnetic stirrer at room temperature and stir for 3 hours to obtain a uniform gel, add natamycin to the uniform gel, the mass volume ratio of natamycin to gel is 0.01g: 1mL , Stir for 30 minutes at 50°C in a dark environment until uniform and without precipitation;
[0040] (3) Spread 5 g of hydrogel containing natamycin on a petri dish with a diameter of 10 cm, and place it in the dark for defoaming;
[0041] (4) Mix 6 mL of absolute ethanol with 3 mL of deionized water to make an e...
Embodiment 3
[0045] This embodiment relates to the alginic acid gel film loaded with natamycin, and its specific preparation process is as follows:
[0046] (1) Dissolve 0.256g of sodium alginate powder and 0.065g of polyethylene oxide powder into 20mL and 5mL of deionized water respectively to ensure that sodium alginate and polyethylene oxide are not attached to the bottom and side walls of the beaker, and then mix the two solutions then stir gently;
[0047] (2) Place the above solution on a magnetic stirrer, stir at room temperature for 5 hours to obtain a uniform gel, add natamycin to the uniform gel, the mass volume ratio of natamycin to gel is 0.005g: 1mL, then stirred at 50°C for 30 minutes in a dark environment until uniform and without precipitation;
[0048] (3) Take 3 g of the hydrogel containing natamycin in step (2) and spread it on a petri dish with a diameter of 10 cm, and place it in the dark for defoaming;
[0049] (4) Get deionized water 9mL, then add calcium chloride ...
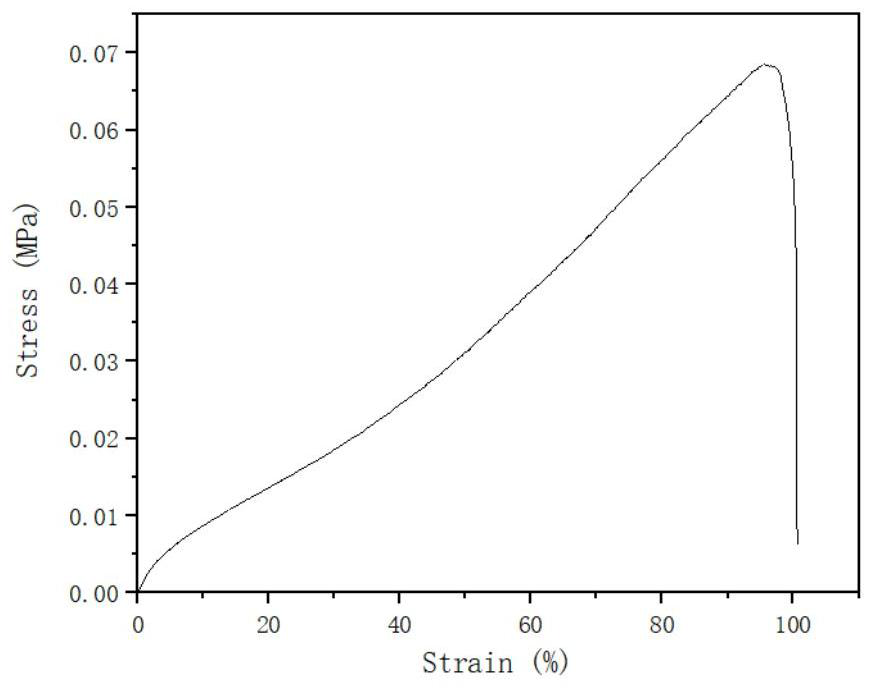
PUM
| Property | Measurement | Unit |
|---|---|---|
| Pull | aaaaa | aaaaa |
| Strength | aaaaa | aaaaa |
| Elastic modulus | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com