Sodium aescinate microemulsion eye drops
A technique for sodium aescinate and eye drops, which is applied in the directions of emulsion delivery, cardiovascular system diseases, organic active ingredients, etc. Improved quality stability, uniform particle size distribution, and reduced eye irritation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
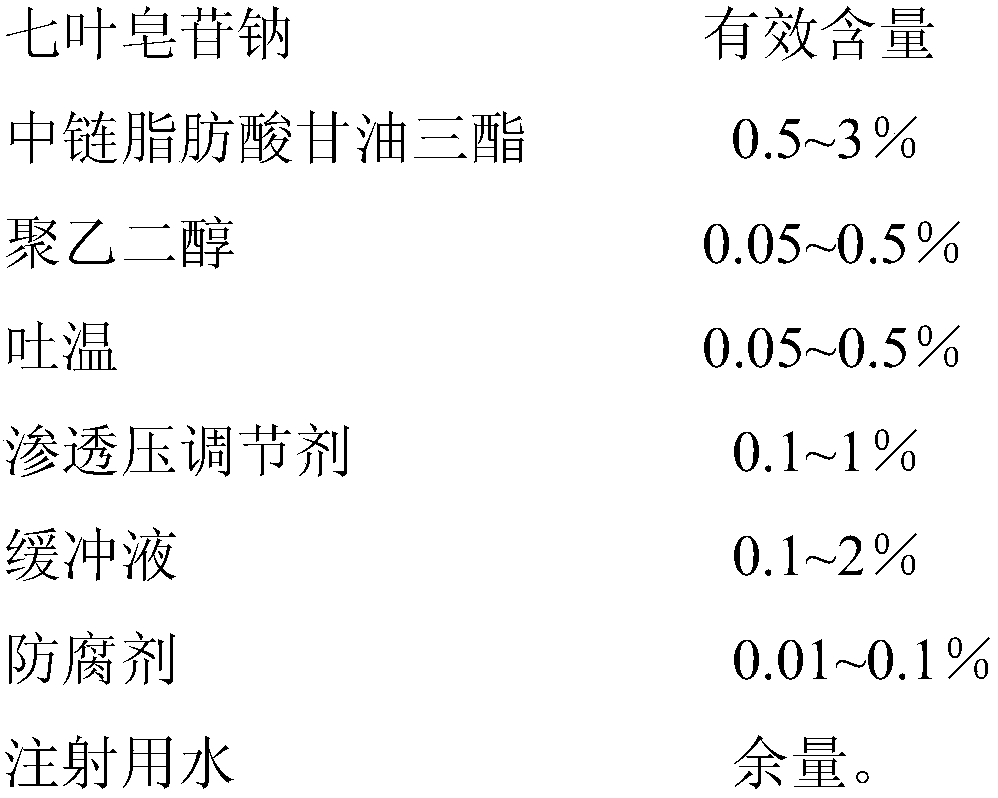
[0024]
[0025] Preparation method: 1) Take 8g of sodium aescinate, 3g of polyethylene glycol 400, 6g of sodium chloride, 9g of borate buffer (pH7.6), 0.5g of ethylparaben, and dissolve them in water for injection to prepare water box;
[0026] 2) Heat and stir 12 g of medium-chain fatty acid triglycerides and 2 g of Tween 80 to form an oil phase;
[0027] 3) Slowly add the oil phase to the water phase, and stir while adding, until a clear solution is formed, filter through a 0.22 μm microporous membrane, sterilize, and subpackage to obtain 1000 g of microemulsion eye drops.
Embodiment 2
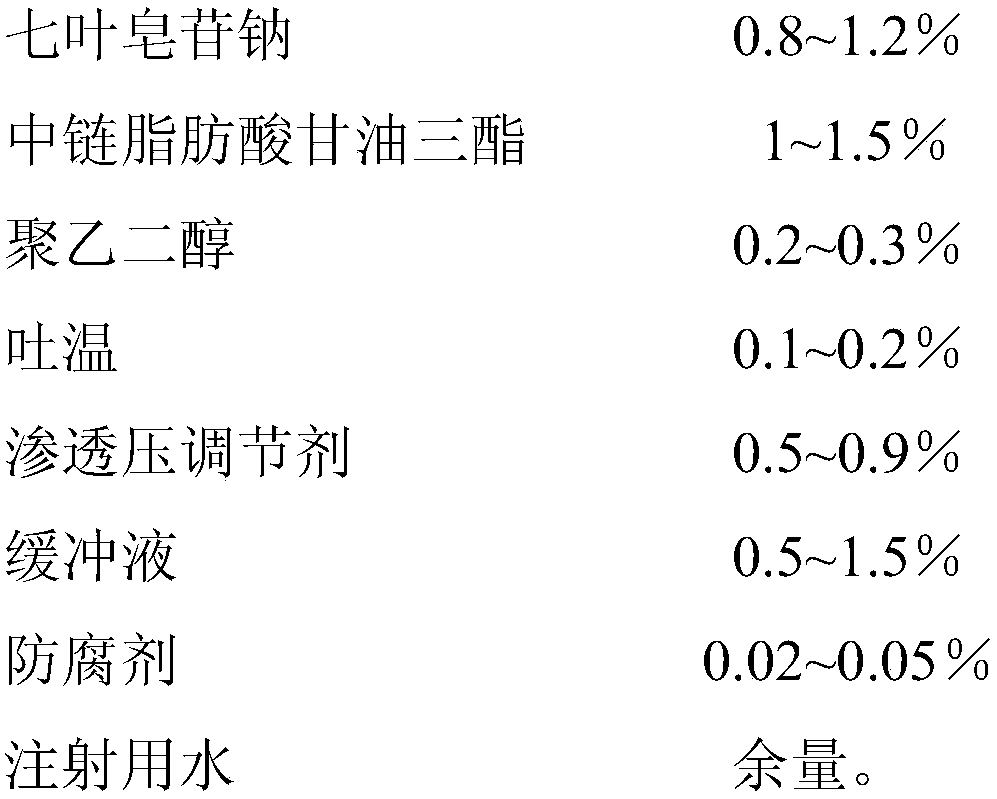
[0029]
[0030] Preparation method: 1) Take 12g of sodium aescinate, 2g of polyethylene glycol 600, 9g of potassium chloride, 6g of phosphate buffer (pH7.2), and 0.2g of benzalkonium bromide, dissolve them in water for injection, and make water Mutually;
[0031] 2) Heat and stir 15 g of medium-chain fatty acid triglycerides and 1 g of Tween 80 to form an oil phase;
[0032] 3) Slowly add the oil phase to the water phase, and stir while adding, until a clear solution is formed, filter through a 0.22 μm microporous membrane, sterilize, and subpackage to obtain 1000 g of microemulsion eye drops.
Embodiment 3
[0034]
[0035] Preparation method: 1) Take 6 g of sodium aescinate, 1 g of polyethylene glycol 200, 7 g of glucose, 5 g of borate buffer solution (pH8.0), and 0.8 g of benzyl alcohol, and dissolve them in water for injection to make an aqueous phase;
[0036] 2) Heat and stir 20 g of medium-chain fatty acid triglycerides and 0.8 g of Tween 80 to make an oil phase;
[0037] 3) Slowly add the oil phase to the water phase, and stir while adding, until a clear solution is formed, filter through a 0.22 μm microporous membrane, sterilize, and subpackage to obtain 1000 g of microemulsion eye drops.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com