A tacrolimus ophthalmic preparation and preparation method thereof
A technology for crolimus eye and tacrolimus, which is applied in the field of tacrolimus ophthalmic preparations and its preparation, and can solve the problems that the clinical needs of ophthalmic preparations cannot be met, the degradation products of tacrolimus are increased, and the like.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
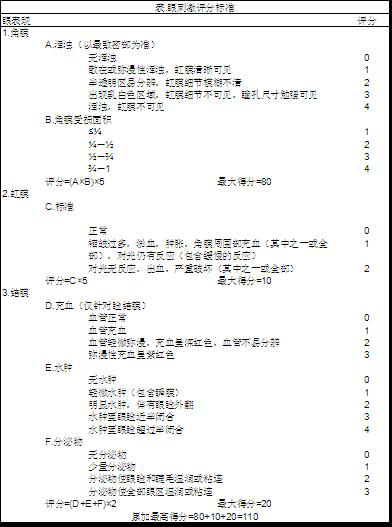
[0050] Prepare the ophthalmic preparation of the present invention according to the corresponding prescription proportioning in Table 1.
[0051] Preparation method: (1) Take an appropriate amount of water for injection and add the prescribed amount of sodium hyaluronate, fully swell for later use; (2) Mix the prescribed amount of MCT, Tween80 and polyethylene glycol evenly, add the prescribed amount of tacrolimus, fully dissolve, and set aside; (3) Take an appropriate amount of water for injection, and dissolve the prescribed amount of glycerin and benzalkonium chloride sequentially; (4) mix the solutions obtained from (2) and (3) and stir thoroughly to obtain a clear solution; (5) mix the sodium hyaluronate solution obtained from (1) with the solution obtained from (4) evenly; (6) Add water for injection to the solution obtained in (5) to 100ml, stir evenly, and adjust the pH value to 5.0 if necessary. (7) Filter and sterilize the obtained medicinal solution through a 0.22 μ...
Embodiment 2~ Embodiment 4
[0053] Prepare the ophthalmic preparation of the present invention according to the corresponding prescription proportioning in Table 1.
[0054] The preparation method is the same as in Example 1. Wherein, without changing the dissolving sequence of surfactant osmotic pressure regulator and osmotic pressure regulator, the type of osmotic pressure regulator and surfactant is replaced according to the preparation method of Example 1; for the prescription without adding sodium hyaluronate, omit the example 1 Step (1) in the preparation method.
[0055] Table 1 Prescription ratio
[0056]
Embodiment 5~ Embodiment 8
[0058] Prepare the ophthalmic preparation of the present invention according to the corresponding prescription proportioning in Table 2.
[0059] Preparation method: (1) Take an appropriate amount of water for injection and add the prescribed amount of sodium hyaluronate to fully swell for later use; (2) Add the prescribed amount of MCT, Solutol ® Mix HS15 and polyethylene glycol evenly, add the prescribed amount of tacrolimus, fully dissolve, and set aside; (3) take an appropriate amount of water for injection, and dissolve the prescribed amount of glycerin and benzalkonium chloride in sequence; (4) mix the solutions obtained from (2) and (3) , fully stirred to obtain a clear solution; (5) mix the sodium hyaluronate solution obtained from (1) with the solution obtained from (4) evenly; (6) add water for injection to the solution obtained from (5) to 100ml, stir evenly, and adjust the pH value to 4.8 if necessary . (7) Filter and sterilize the obtained medicinal solution thr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com