Pharmaceutical application of amniotic epithelial cell conditioned medium
A technology of amniotic membrane epithelial cells and conditioned medium, applied in the field of stem cells, can solve the problems of immune rejection, storage and transportation inconvenience, and achieve the effect of high product stability and low immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1: Preparation of amniotic membrane epithelial cell conditioned medium eye drops
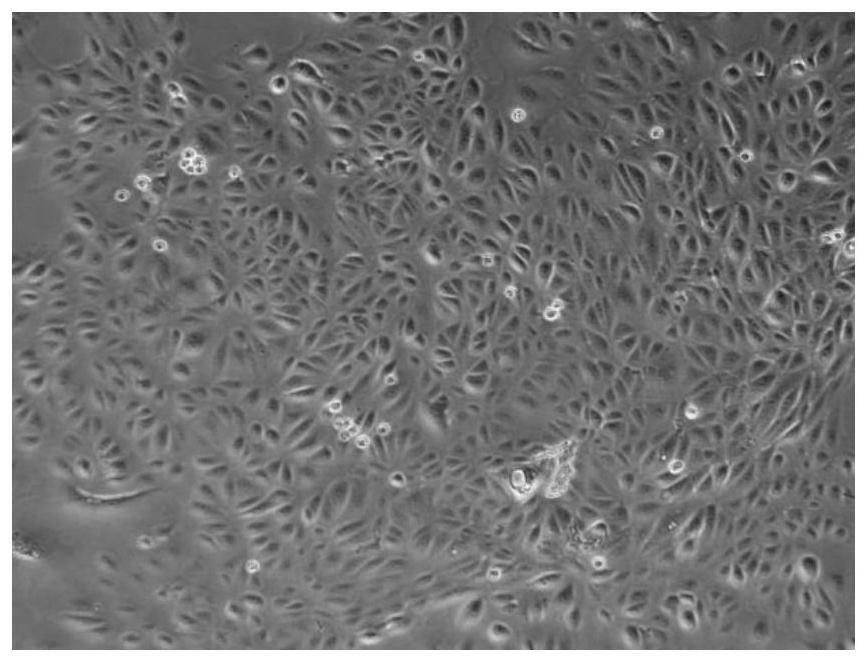
[0034] 1) Isolation and culture of amniotic membrane epithelial cells: Isolate the amniotic membrane from human placenta tissue, wash it with sterile saline, and obtain primary amniotic membrane epithelial cells by shear separation and trypsin digestion. Culture amnion epithelial cells in medium, when the growth of cell colonies is found, the adherent cells are fused to 70-80%, digested with 0.25% trypsin, and subcultured; the morphological characteristics of amnion epithelial cells are as follows figure 1 shown.
[0035] 2) Collection: continue to culture amnion epithelial cells to the third generation in a medium suitable for culturing amnion epithelial cells, discard the medium when the cell confluence reaches about 90%, wash the cells 3 times with sterile PBS, and replace with The serum-free medium was further cultured for 24 hours, the cells and cell debris were removed b...
Embodiment 2
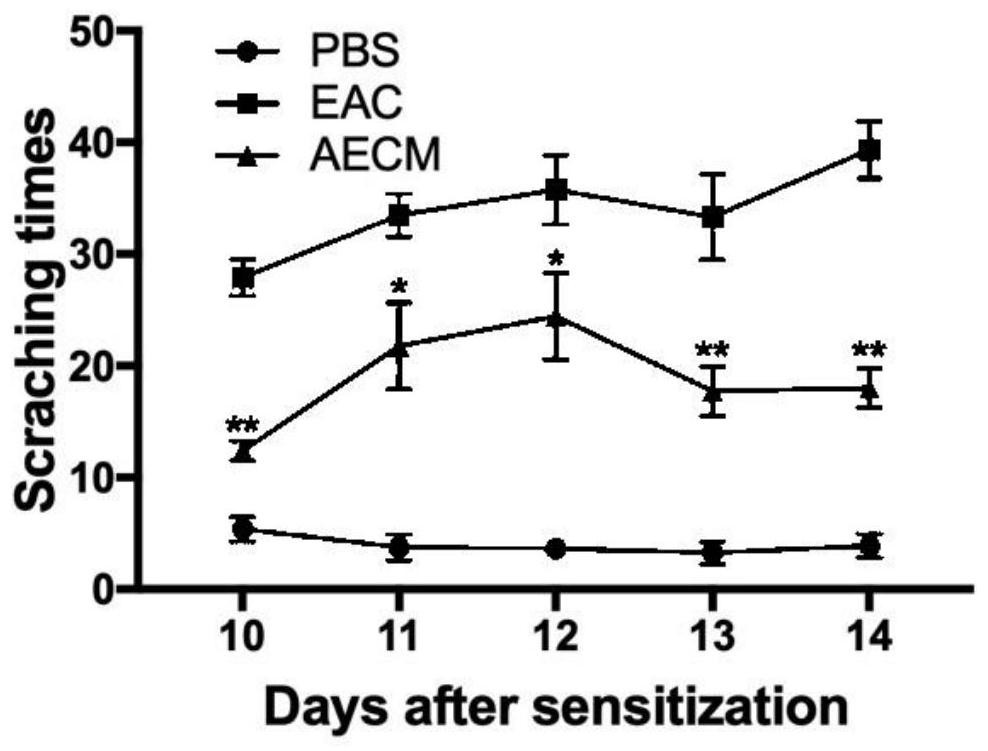
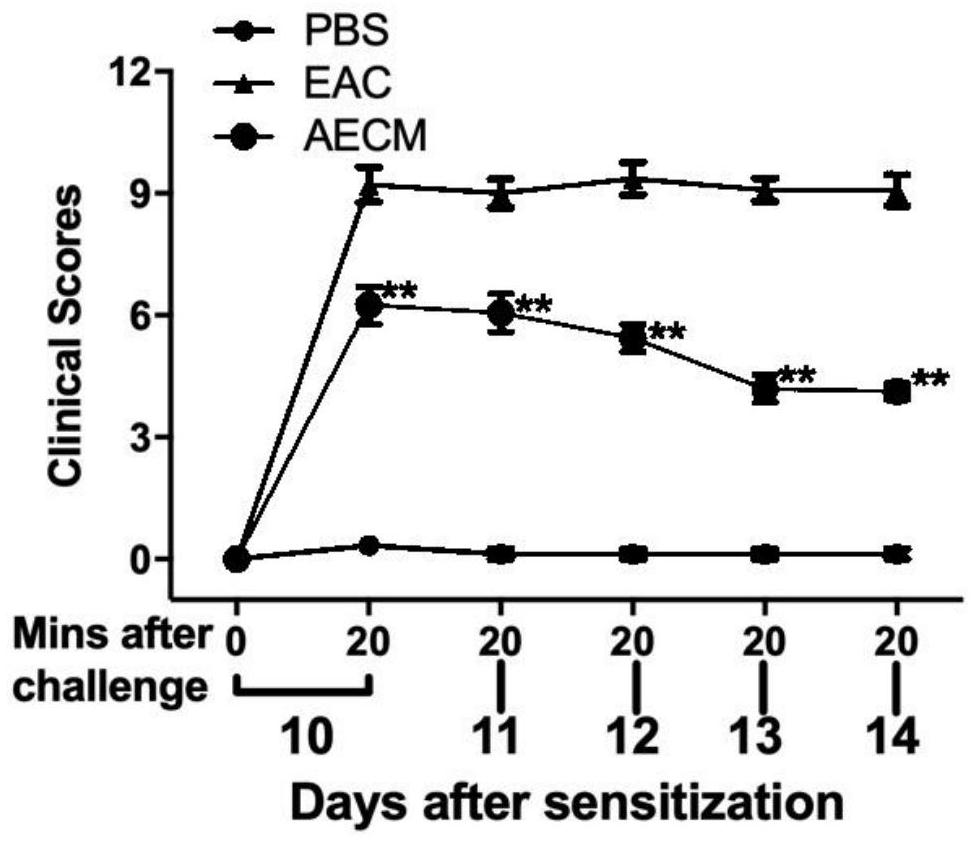
[0036] Example 2: Therapeutic Effect of Amniotic Epithelial Cell Conditioned Medium Eye Drops on Allergic Conjunctivitis
[0037] Experimental allergic conjunctivitis mouse model is a mouse model of experimental allergic conjunctivitis induced by ragweed pollen. The establishment method is as follows: 6-week-old Balb / c mice, male or female, were inoculated with 50 μg of SRW pollen (Greer Lab) into 5 ml of ImjectAlum (Thermo Scientific) by footpad injection on day 0, and the normal control group was injected with phosphoric acid Saline buffer solution (PBS). The sensitization procedure was repeated on day 5 to enhance the allergic response. From day 10 to day 14, 1.5 mg of SRW pollen (pH 7.2) suspended in 10 μl of phosphate buffered saline (PBS) was topically applied to both eyes of the mice, and the normal control group was administered phosphate buffered saline (PBS) . The method of eye drops is amniotic epithelial cell conditioned medium eye drops (10 μL) topically applie...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com