Method for preparing sevoflurane
A technology of sevoflurane and fluoride, which is applied in the field of preparation of sevoflurane, can solve problems such as harsh requirements and difficult separation, and achieve the effects of increasing safety, reducing production costs and improving utilization rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
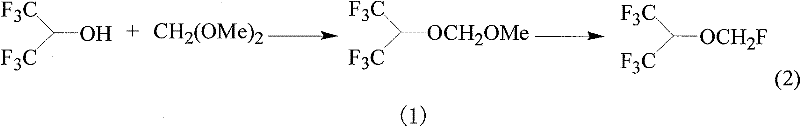
[0011] Example 1 (CF 3 ) 2 CHOCH 2 OCH 3 Synthesis
[0012] In a 500ml flask, add 168 grams of hexafluoroisopropanol, 100 grams of CH 2 (OCH 3 ) 2 and 2 grams of p-toluenesulfonic acid, stirred at room temperature for 24 hours, added 100ml of water, added 10% NaOH to adjust to PH=9, separated the organic layer, washed the organic layer with water, and distilled it at atmospheric pressure to recover the distillate at 76-78°C. The product was obtained to obtain 82 grams (CF 3 ) 2 CHOCH 2 OCH 3 (GC purity 98.3%).
Embodiment 2
[0013] Example 2 Synthesis of Sevoflurane
[0014] Add 21.2 grams (CF 3 ) 2 CHOCH 2 OCH 3 (GC purity 98.3%), 100 gram KF and 100 gram fuming sulfuric acid, gradually warming up to 50 ℃ in 8 hours, collect the steam that reaction produces with water trap, after the gained organic layer is washed with water, get 18 gram organic matter . GC analysis showed that the obtained organic matter contained 90.0% sevoflurane (yield 80%). The resulting organic matter was fractionally distilled, and the 50-54°C fraction was collected to obtain 14.5 g of sevoflurane product (99.6% purity by GC).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com