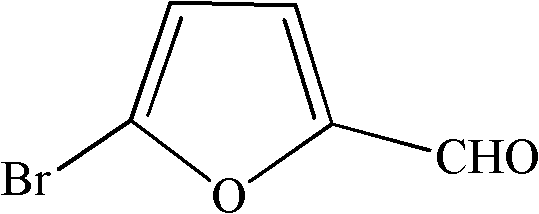
Synthesis method of 5-bromo-2-furaldehyde
A technology of furan formaldehyde and synthesis method, applied in the field of preparation of known compounds, can solve problems such as unfavorable sustainable development and economic development requirements, poor bromination reaction selectivity, serious environmental pollution, etc. Product yield and purity, the effect of reducing environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
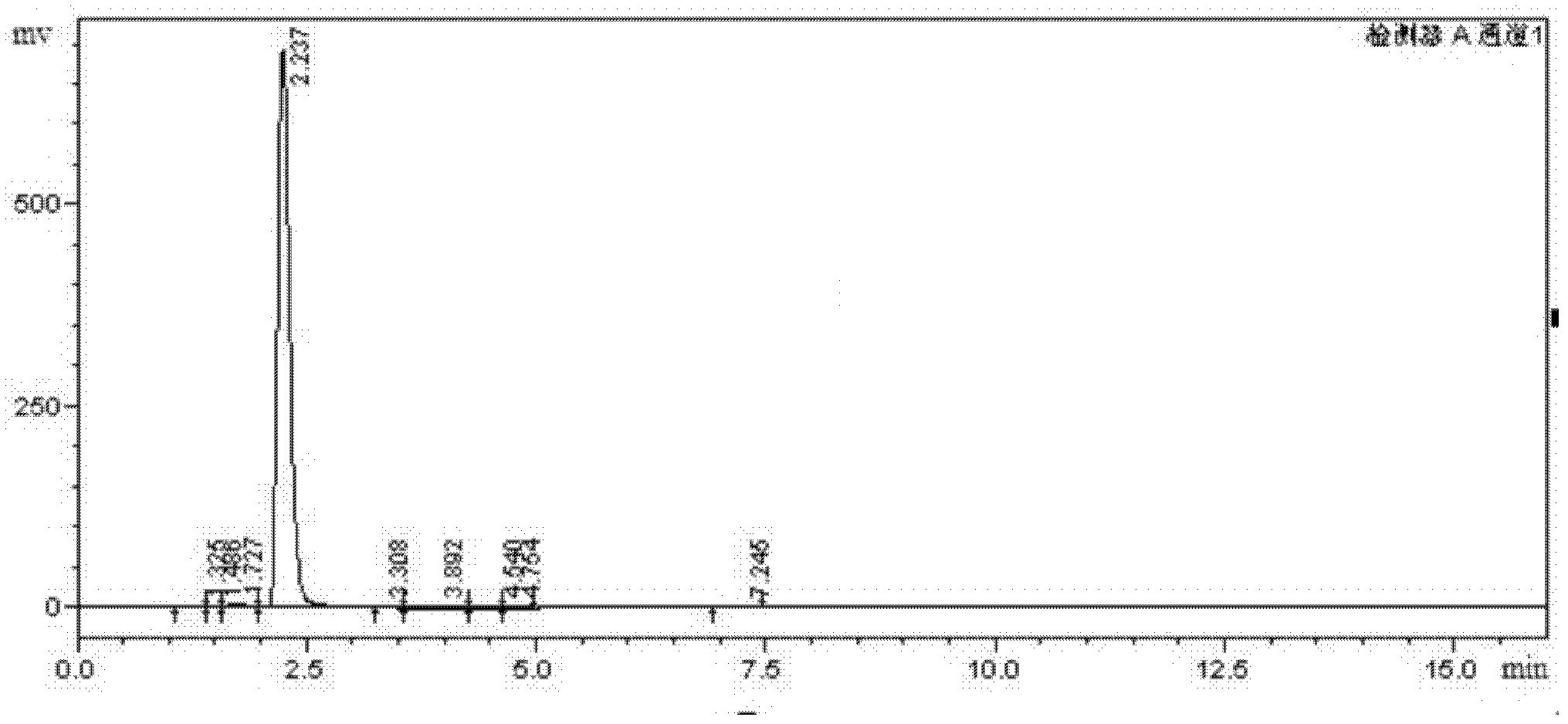
Image
Examples
Embodiment 1
[0027] In the present embodiment, 5-bromo-2-furancarbaldehyde is prepared according to the following steps:
[0028] (1) Preparation of Bromination Reagent
[0029] Put 12.3g of N-methylimidazole in a 500mL three-necked flask, and add 22.5g of n-bromobutane dropwise under stirring. The n-bromobutane has been distilled in advance to remove water. , heat up to the reaction system to generate reflux (105-140°C), keep the temperature for 9-10h, cool, and rotate to evaporate excess n-bromobutane for recovery, the reaction mixture is washed 3 times with ethyl acetate, each time with ethyl acetate The amount of ester was 40mL, and then dried in a vacuum oven at 60°C to constant weight to obtain a golden yellow viscous liquid, namely 1-butyl-3-methylimidazole bromide; the obtained 1-butyl bromide Base-3-methylimidazole was placed in a 500mL three-necked flask, and 4.8g of bromine was added dropwise with stirring at 10-15°C. The dropping time was controlled at 1h. After the drop was c...
Embodiment 2
[0033] In the present embodiment, 5-bromo-2-furancarbaldehyde is prepared according to the following steps:
[0034] (1) Preparation of Bromination Reagent
[0035] Put 12.3g of N-methylimidazole in a 500mL three-necked flask, and add 25.7g of brominated n-butane dropwise under stirring. The brominated n-butane has been distilled in advance to remove water. , heat up to the reaction system to generate reflux (105-140°C), keep the temperature for 9-10h, cool, and rotate to evaporate excess n-bromobutane for recovery, the reaction mixture is washed 3 times with ethyl acetate, each time with ethyl acetate The amount of ester was 40mL, and then dried in a vacuum oven at 70°C to constant weight to obtain a golden yellow viscous liquid, that is, 1-butyl-3-methylimidazole bromide; the obtained 1-butyl bromide Base-3-methylimidazole was placed in a 500mL three-necked flask, and 4.8g of bromine was added dropwise with stirring at 10-15°C. The dropping time was controlled at 1h. After ...
Embodiment 3
[0039] In the present embodiment, 5-bromo-2-furancarbaldehyde is prepared according to the following steps:
[0040] (1) Preparation of Bromination Reagent
[0041] Put 12.3g of N-methylimidazole in a 500mL three-necked flask, and add 25.7g of brominated n-butane dropwise under stirring. The brominated n-butane has been distilled in advance to remove water. , heat up to the reaction system to generate reflux (105-140°C), keep the temperature for 9-10h, cool, and rotate to evaporate excess n-bromobutane for recovery, the reaction mixture is washed 3 times with ethyl acetate, each time with ethyl acetate The amount of ester was 40mL, and then dried in a vacuum oven at 70°C to constant weight to obtain a golden yellow viscous liquid, that is, 1-butyl-3-methylimidazole bromide; the obtained 1-butyl bromide Base-3-methylimidazole was placed in a 500mL three-necked flask, stirred and added dropwise with 5.4g of bromine at 10-15°C, the dropping time was controlled at 1h, and reacted...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| flash point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com