Loteprednol etabonate suspension eye drops
A technology of carteprednol and eye drops, which is applied in the direction of liquid delivery, emulsion delivery, organic active ingredients, etc., can solve the problems of drug concentration inconsistent with the nominal value, poor redispersibility, etc., and achieve good re-dispersion The effect of dispersion properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
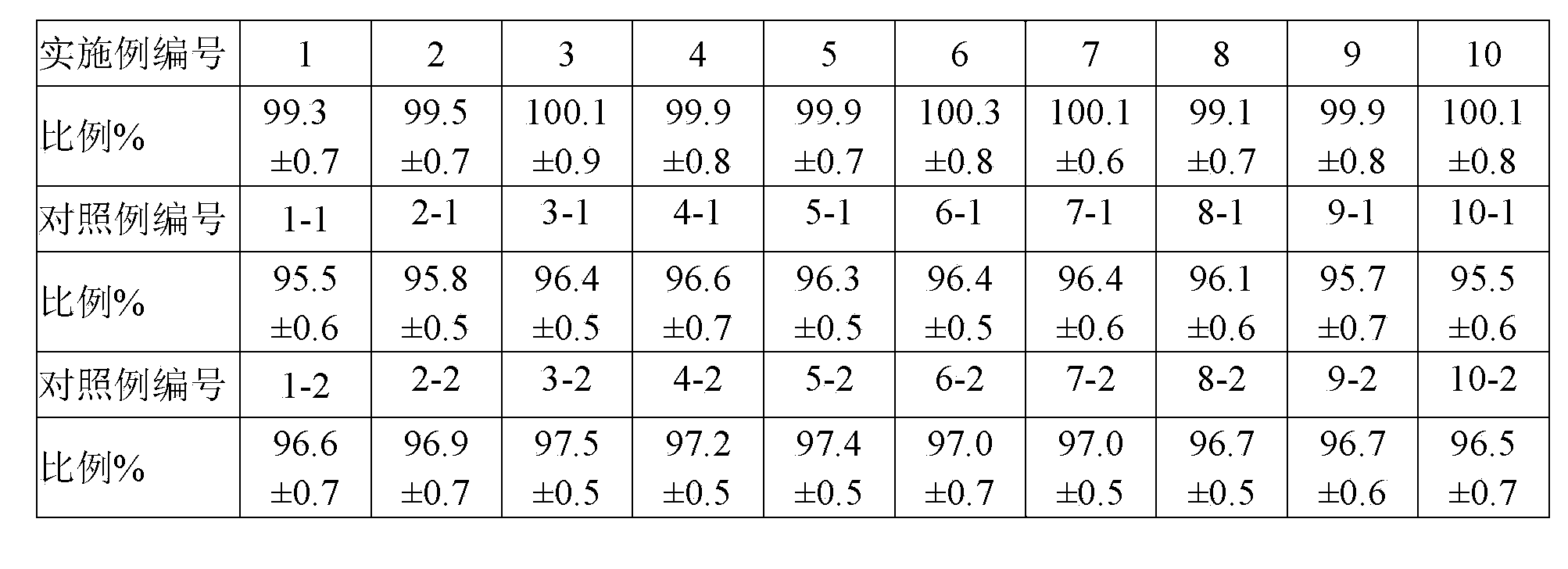
Examples
Embodiment 2
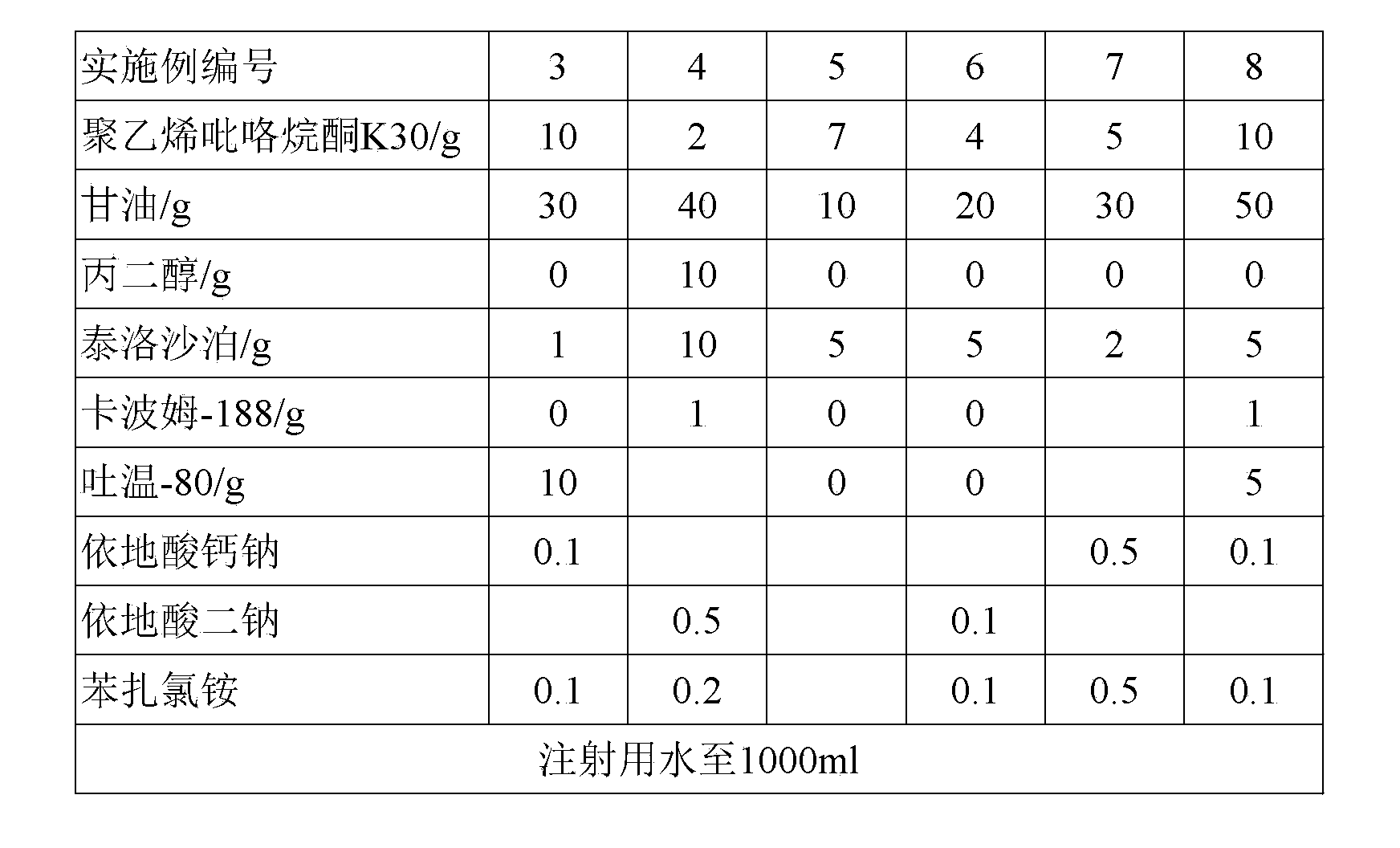
[0039] Component 2 in Example 2 is tobramycin, component 2 in Example 6 is levofloxacin hydrochloride, and component 2 in Example 10 is gatifloxacin hydrochloride.
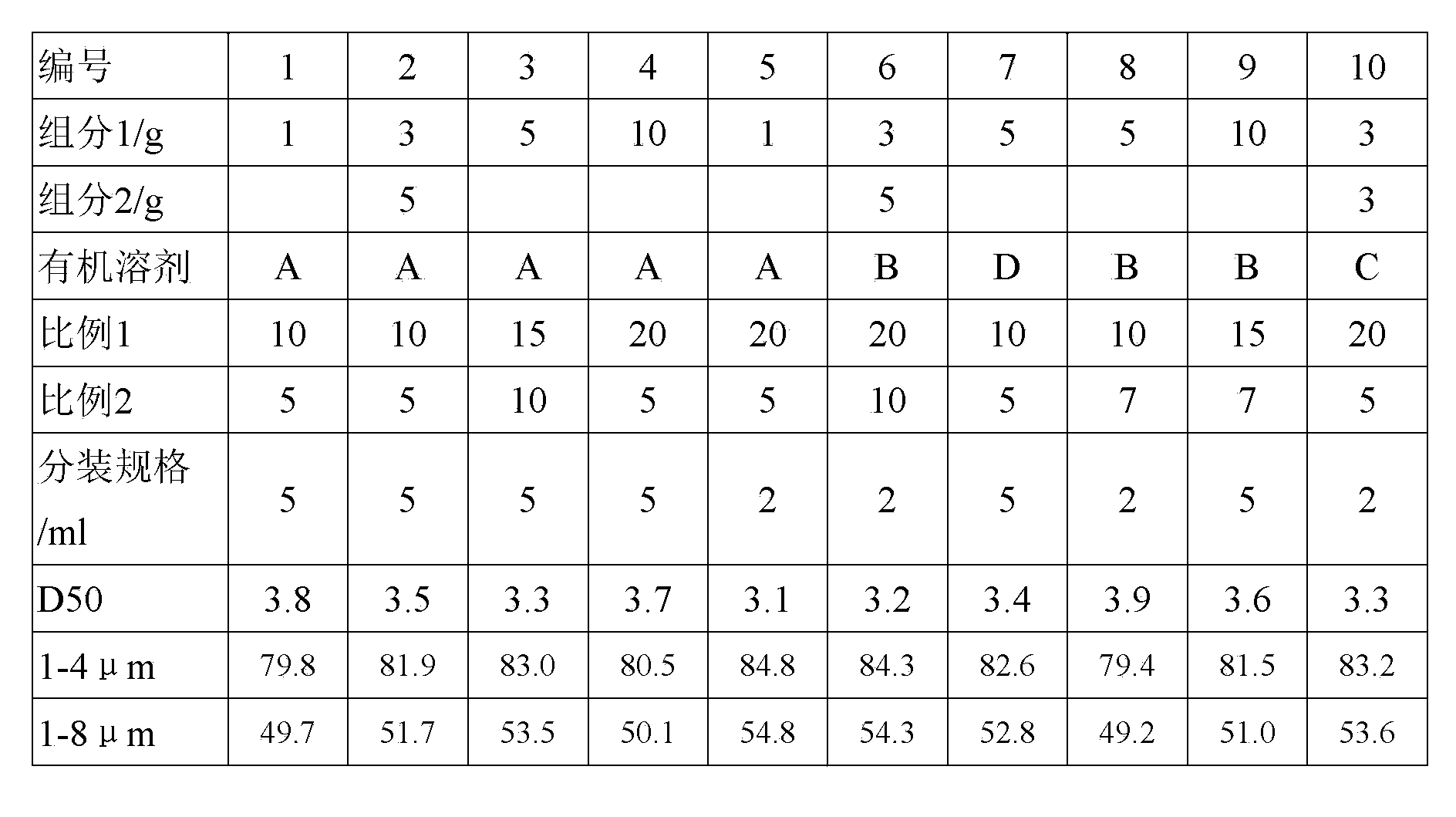
[0040] The preparation method of the etabaclotepdnol micropowder is as follows: dissolve the drug raw material in an organic solvent (heating if necessary), pass through a 0.45 μm microporous membrane, stir with water for injection, and keep stirring with 0.5- Slowly add the organic solvent in which the drug is dissolved into the water at a rate of 1 ml / s, stop stirring after the addition, leave it to stand for 1 hour, filter and dry to obtain the drug micropowder. The particle size distribution of the drug micropowder meets the following conditions simultaneously:
[0041] D50 is 3.0-4.0μm, the maximum particle size is less than 30μm,
[0042] For fine powder with a particle size of 1-8μm, the particle percentage is 79%-85%, of which
[0043] For fine powder with a particle size of 1-4μm, the particle percentage ...
Embodiment 1
[0048] The auxiliary material formula is hydroxypropyl methylcellulose 10g
[0049] Tween-80 0.5g
[0050] Polyoxyethylene hydrogenated castor oil 60 0.5g
[0051] Propylparaben 0.5g
[0052] Methylparaben 0.5g
[0053] Edetate Calcium Sodium 1g
[0055] Water for injection up to 1000ml
[0056] Example 2
[0057] The auxiliary material formula is hydroxyethyl methylcellulose 20g
[0058] Tween-80 0.5g
[0059] Glycerin 10g
[0060] Sodium Hyaluronate 1g
[0061] Polyoxyethylene hydrogenated castor oil 60 0.5g
[0062] Benzalkonium chloride
[0063] Edetate Calcium Sodium 1g
[0064] Water for injection up to 1000ml
[0065] Add tobramycin to solution during preparation and adjust the pH to 5-6 with sulfuric acid.
[0066] Embodiment 3.-8 auxiliary material formula sees the following table (based on 1000ml suspension)
[0067]
Embodiment 9
[0069] The auxiliary material formula is methyl cellulose 20g
[0070] Tween-80 3g
[0071] Propylparaben 0.5g
[0072] Methylparaben 0.5g
[0073] Edetate Calcium Sodium 1g
[0074] Sodium chloride 7g
[0075] Water for injection up to 1000ml
PUM
| Property | Measurement | Unit |
|---|---|---|
| Maximum particle size | aaaaa | aaaaa |
| Maximum particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com