Composition for enhancing drug safety and clinical efficacy of low concentration atropines
A low-concentration atropine technology, applied in the field of compositions and preparations that enhance the safety and clinical efficacy of low-concentration atropine drugs, can solve the problems of instability of the main drug, accompanying adverse reactions and side effects, and reduce repeated medication The number of times, improve the medicinal effect, the effect of extending the shelf life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
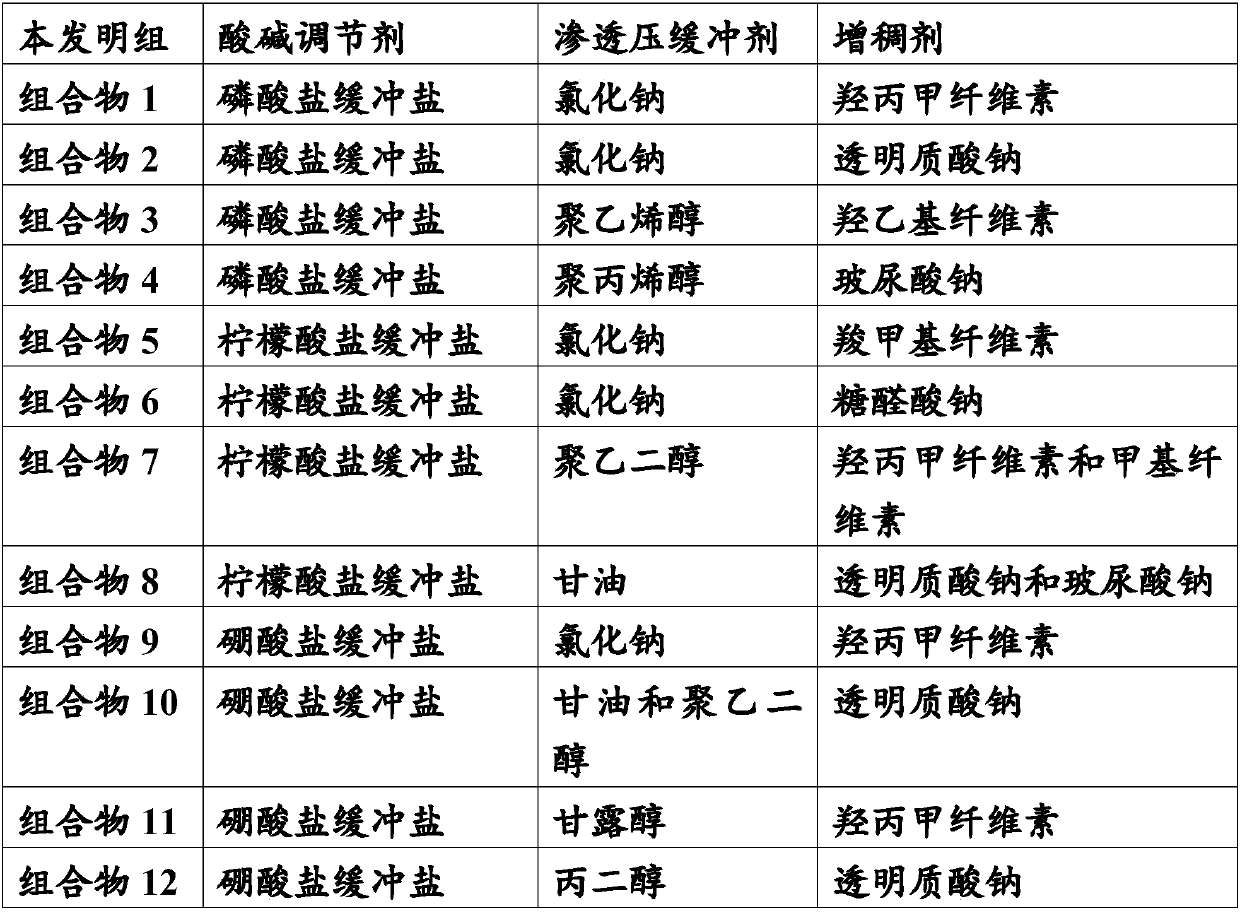
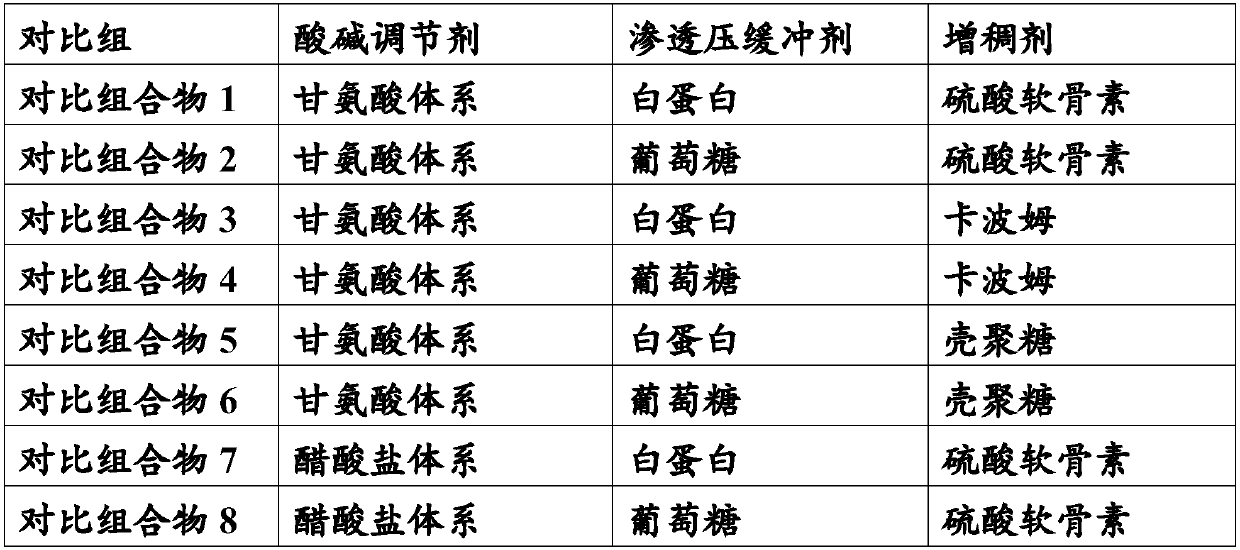
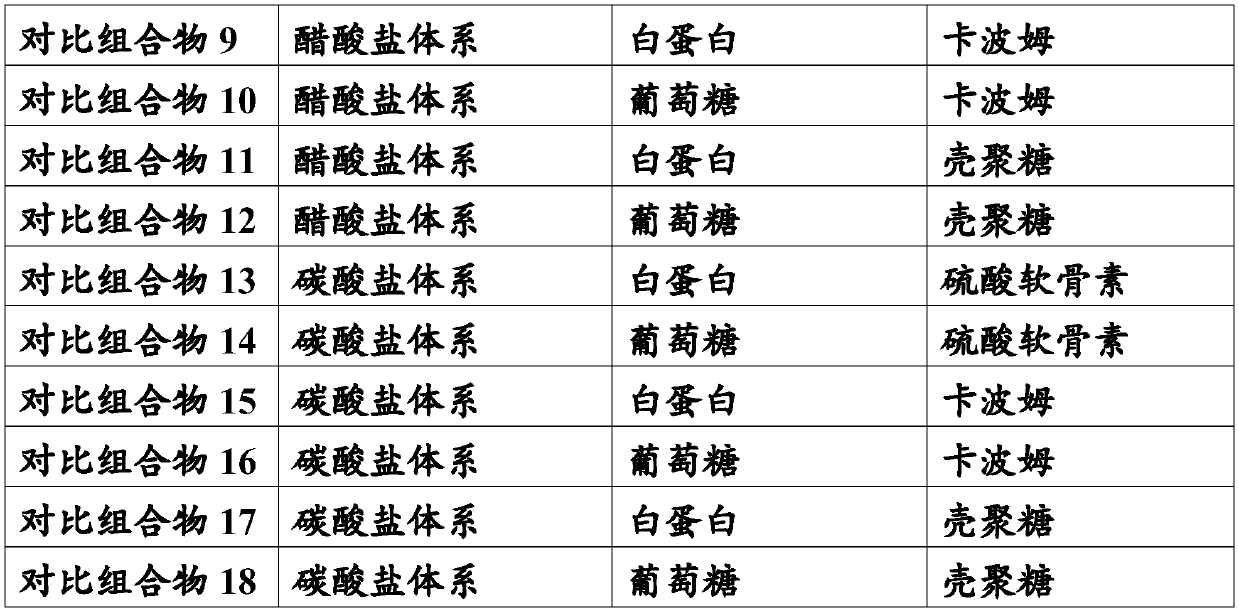
[0042] Adopt the following preparation process to prepare the stock solutions of each pharmaceutical composition of the present invention, then study respectively the influence of the composition of the pharmaceutical composition on the safety, stability and curative effect of the low concentration atropine drug eye drop preparation, and the present invention Each pharmaceutical composition was compared with a comparative pharmaceutical composition to study the differences in their influence on the safety, stability and curative effect of the low-concentration atropine drug eye drop formulation.
[0043] Stock solution preparation process: the raw material drug atropine sulfate is added to water for injection to form an aqueous solution with a concentration of 0.01% (w / v); an osmotic pressure buffer is added to the above solution to adjust the osmotic pressure to 310mOsm / L; Thickener, adjust the viscosity to 30mps; finally add acid-base regulator, the total amount is 2.5% (w / v)...
preparation Embodiment 1
[0085] The crude drug atropine sulfate is added to the water for injection, and the concentration is made into an aqueous solution of 0.001% (w / v), sodium chloride is added in the solution, the osmotic pressure is adjusted to be 250mOsm / L, and hypromellose (HPMC) is added , adjust the viscosity to 10mps, add 1.3% (v / v) of phosphate buffer system, and adjust the pH to 3.0 with dilute hydrochloric acid, after mixing evenly, stir thoroughly, according to the relevant requirements of "Chinese Pharmacopoeia 2015 Edition (Part Four)" eye drops , filtered through a 0.22 μm microporous membrane, sterilized, single-dose aseptically subpackaged, and passed the light inspection to obtain sterile low-concentration atropine drug eye drops.
preparation Embodiment 2
[0087] The crude drug atropine sulfate is added to the water for injection, and the concentration is made into an aqueous solution of 2% (w / v), boric acid is added in the solution, and the osmotic pressure is adjusted to be 360mOsm / L, and sodium hyaluronate is added to adjust the viscosity to 26mps. Add 2.3% (v / v) of citrate buffer system, and adjust the pH to 5.0 with dilute hydrochloric acid. Pore membrane filtration, sterilization, single-dose aseptic subpackaging, and light inspection pass, to obtain sterile low-concentration atropine drug eye drops.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com