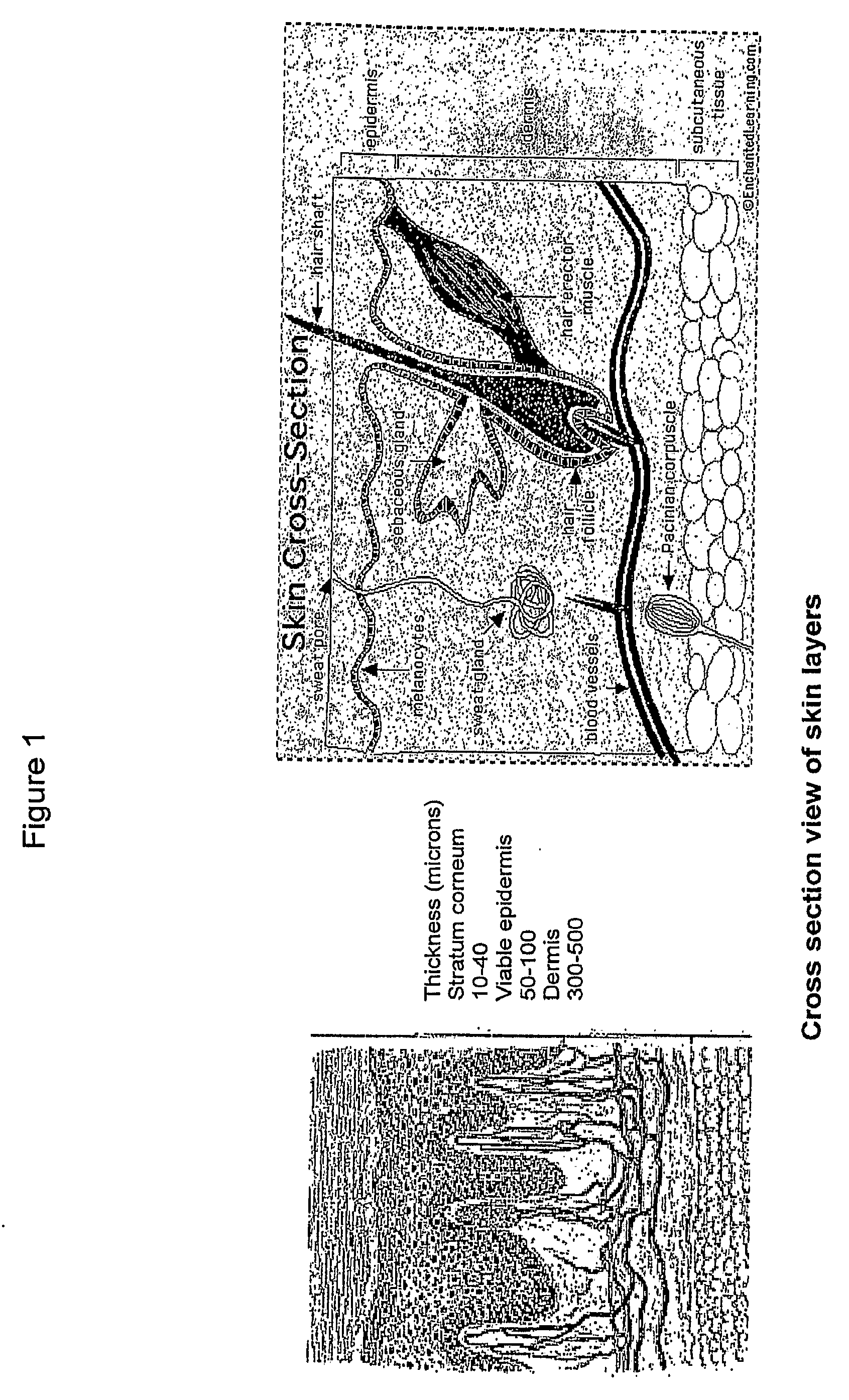
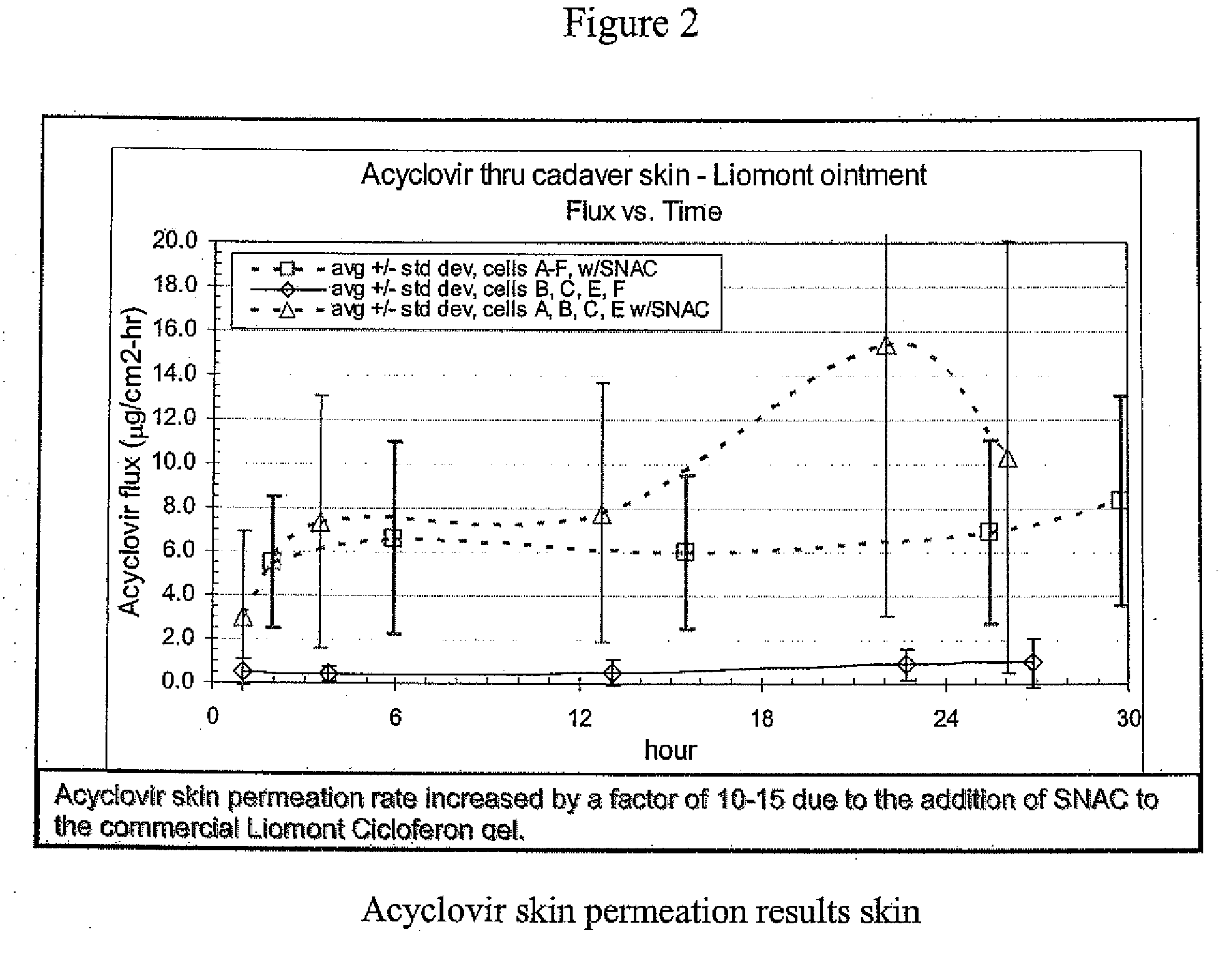
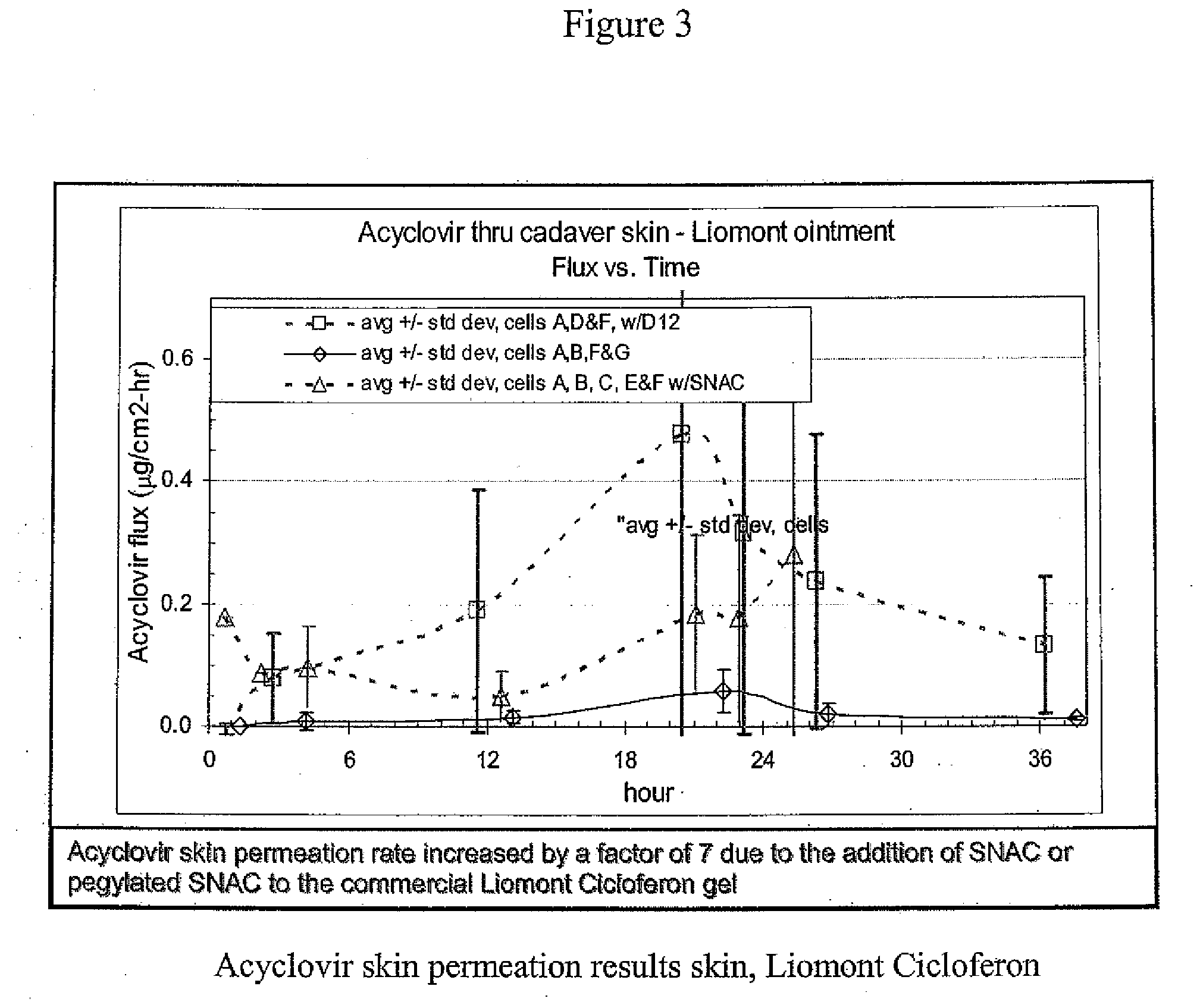
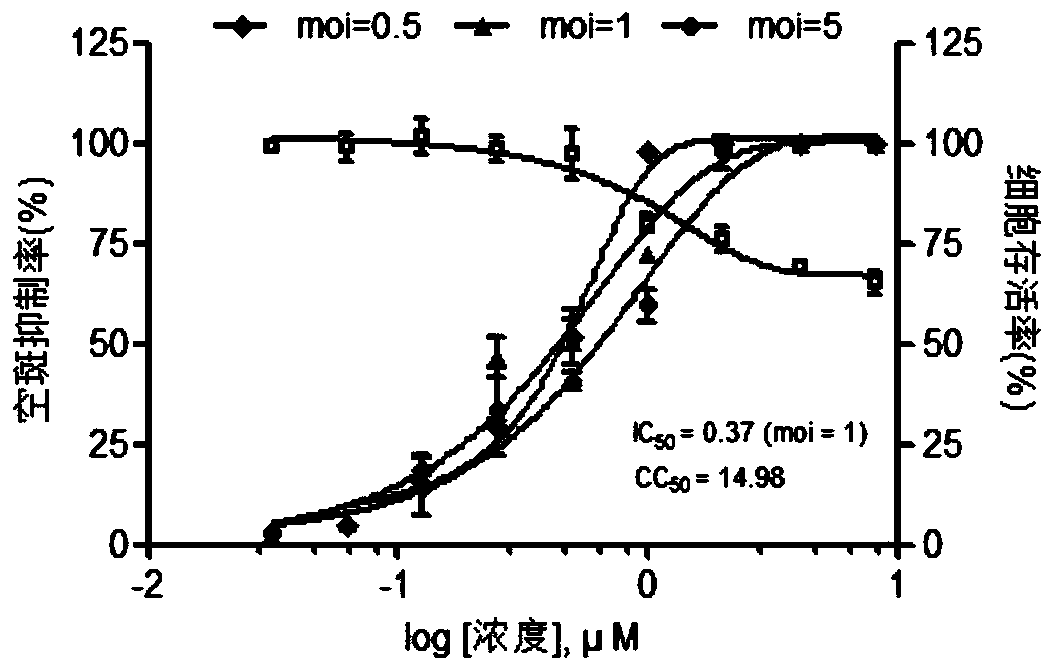
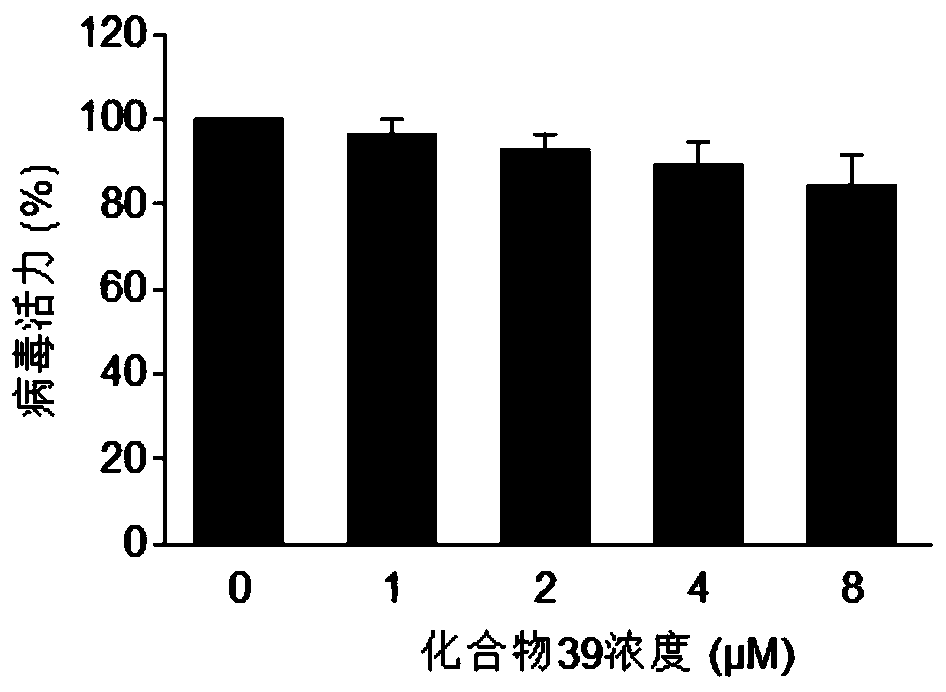
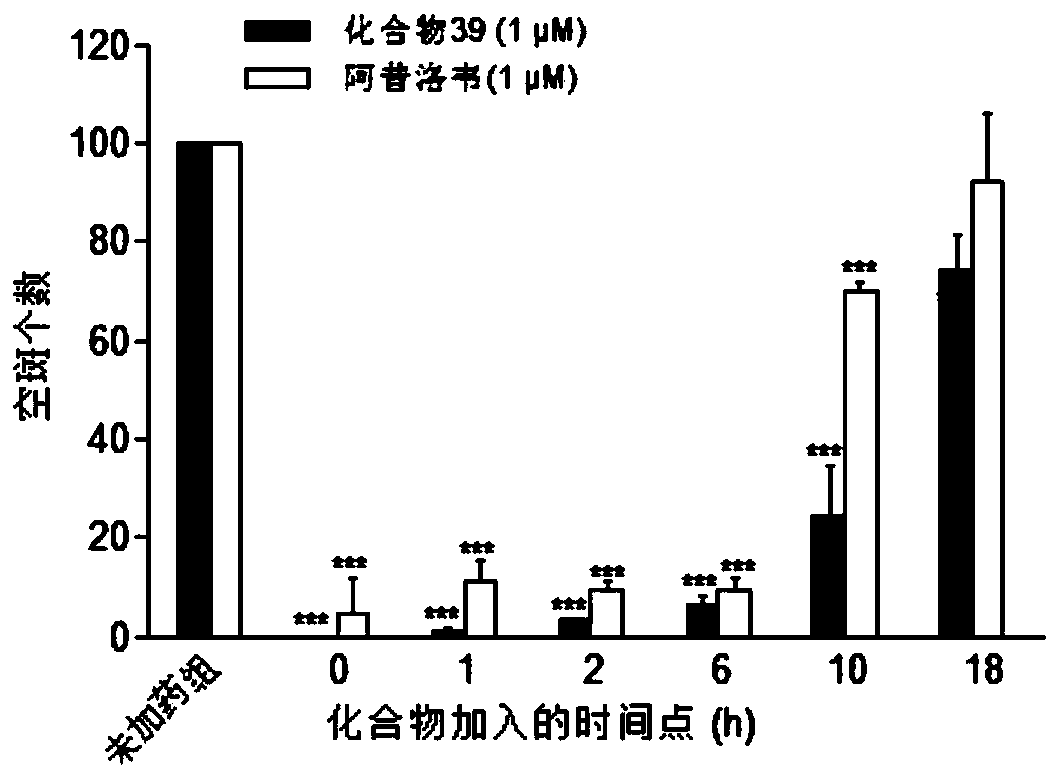
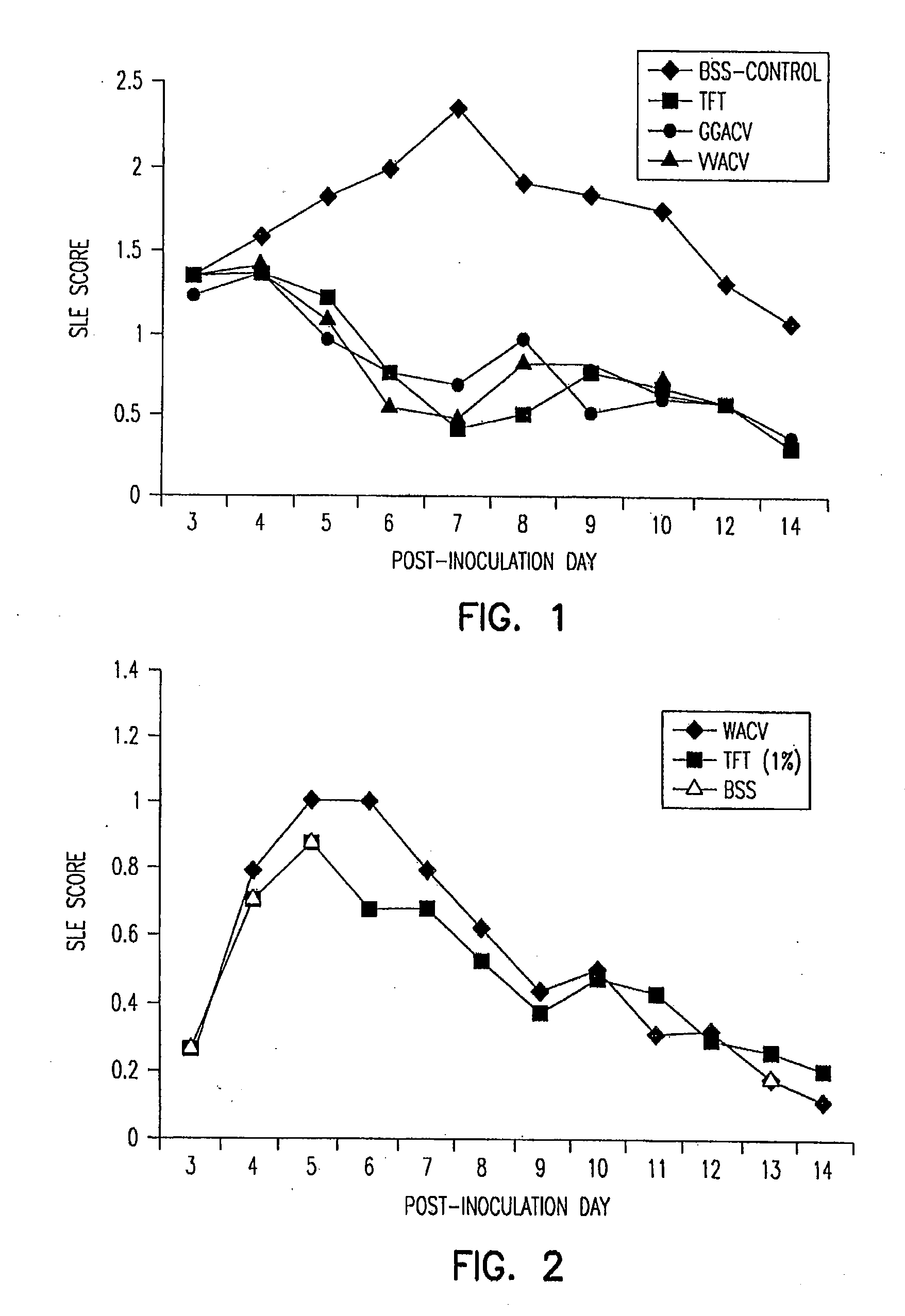
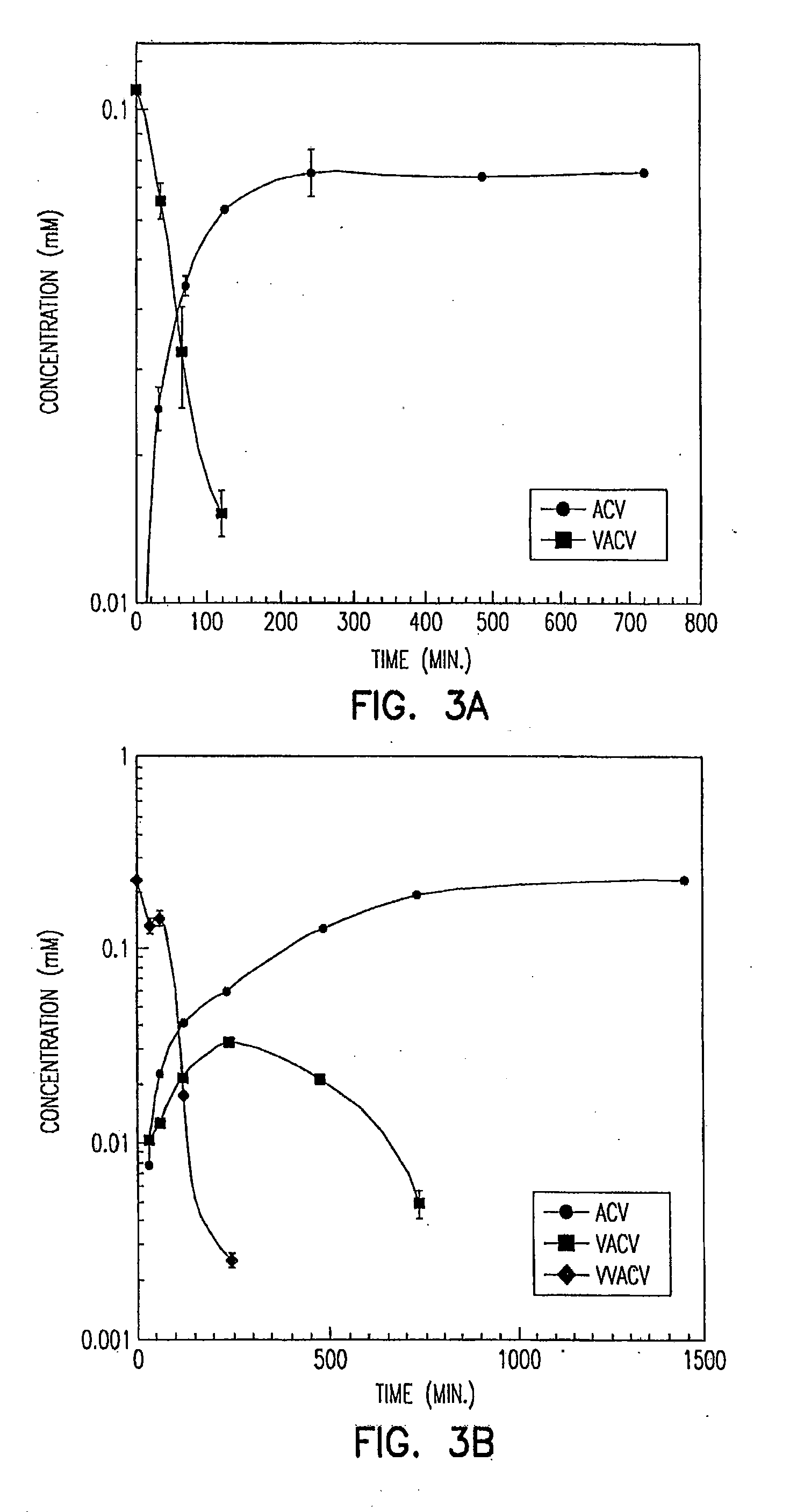
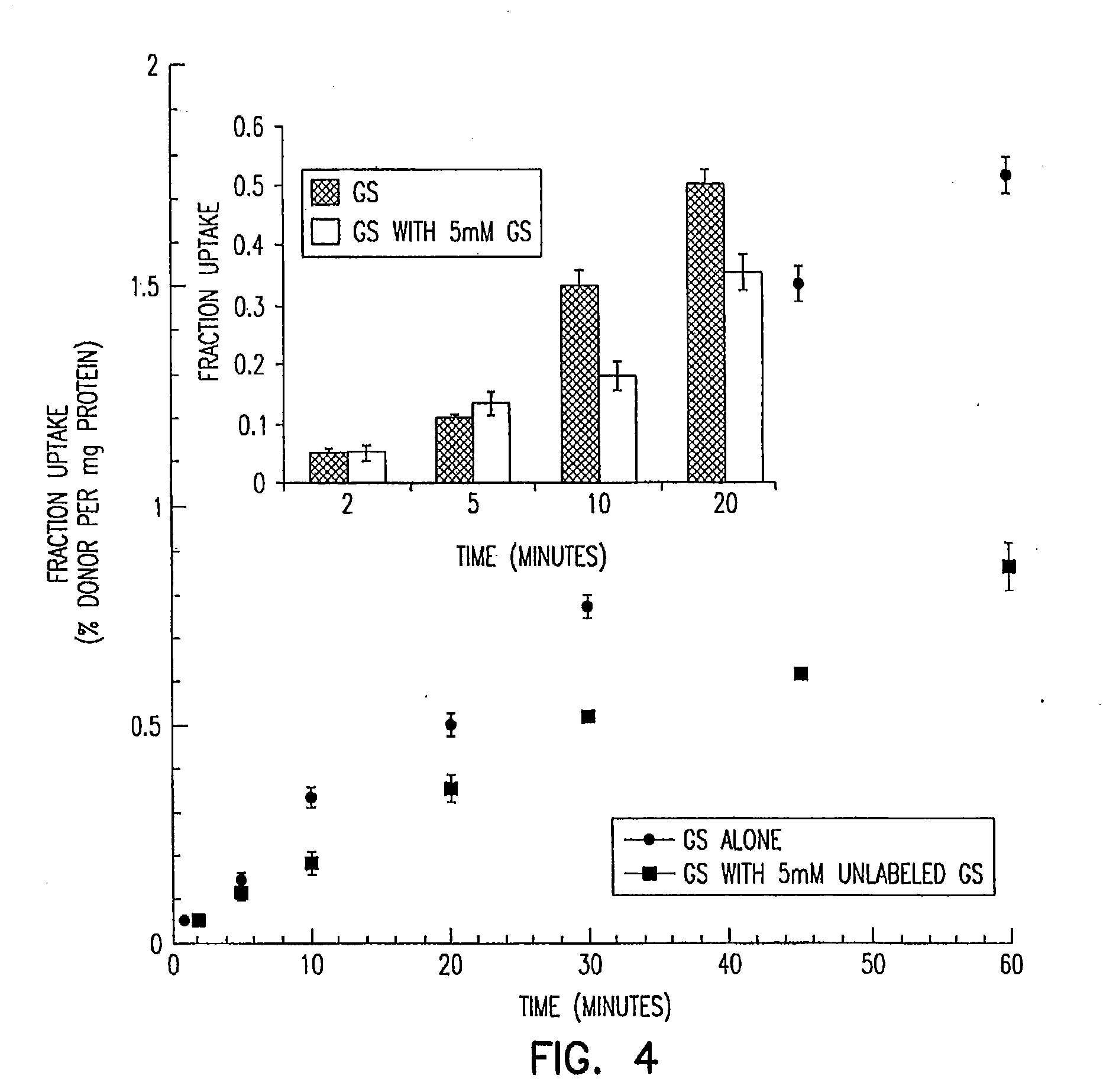
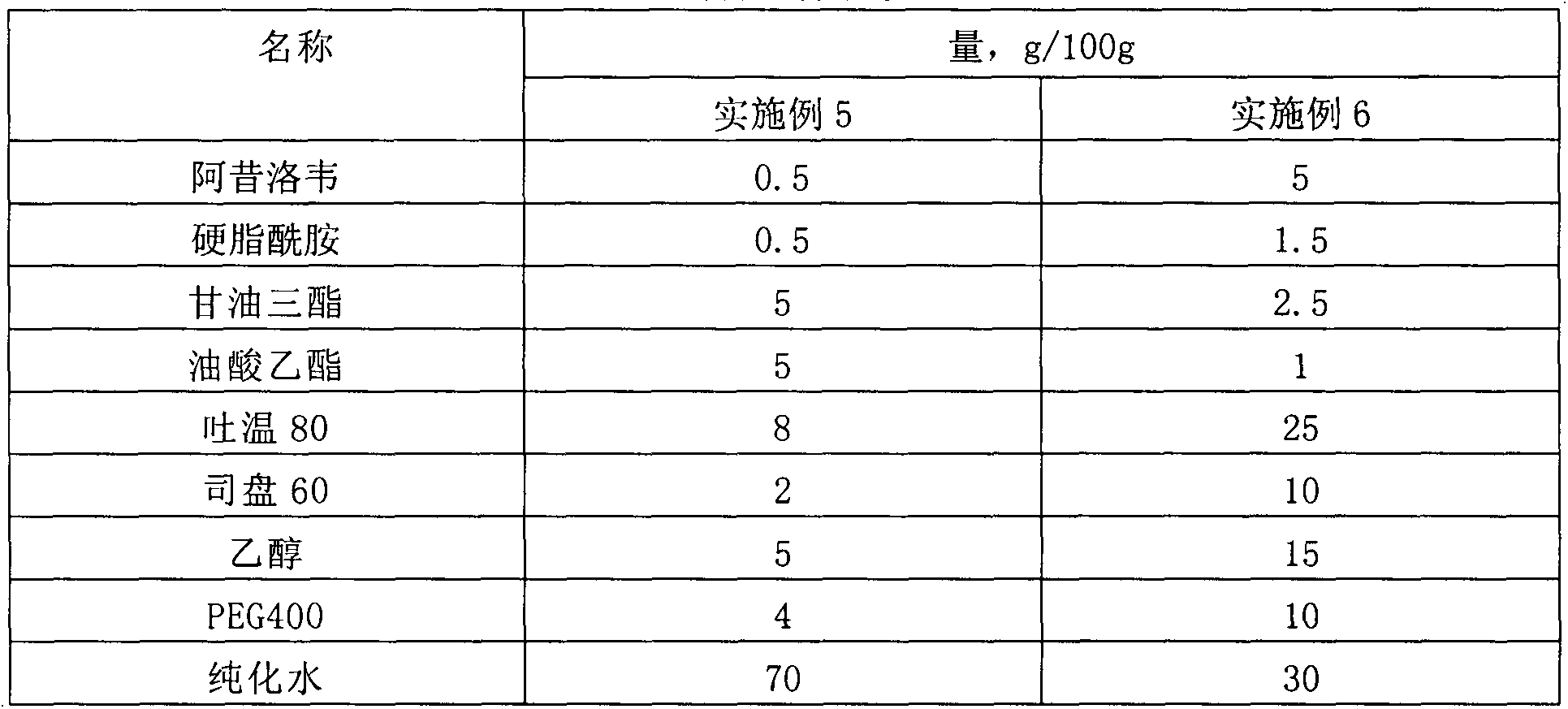
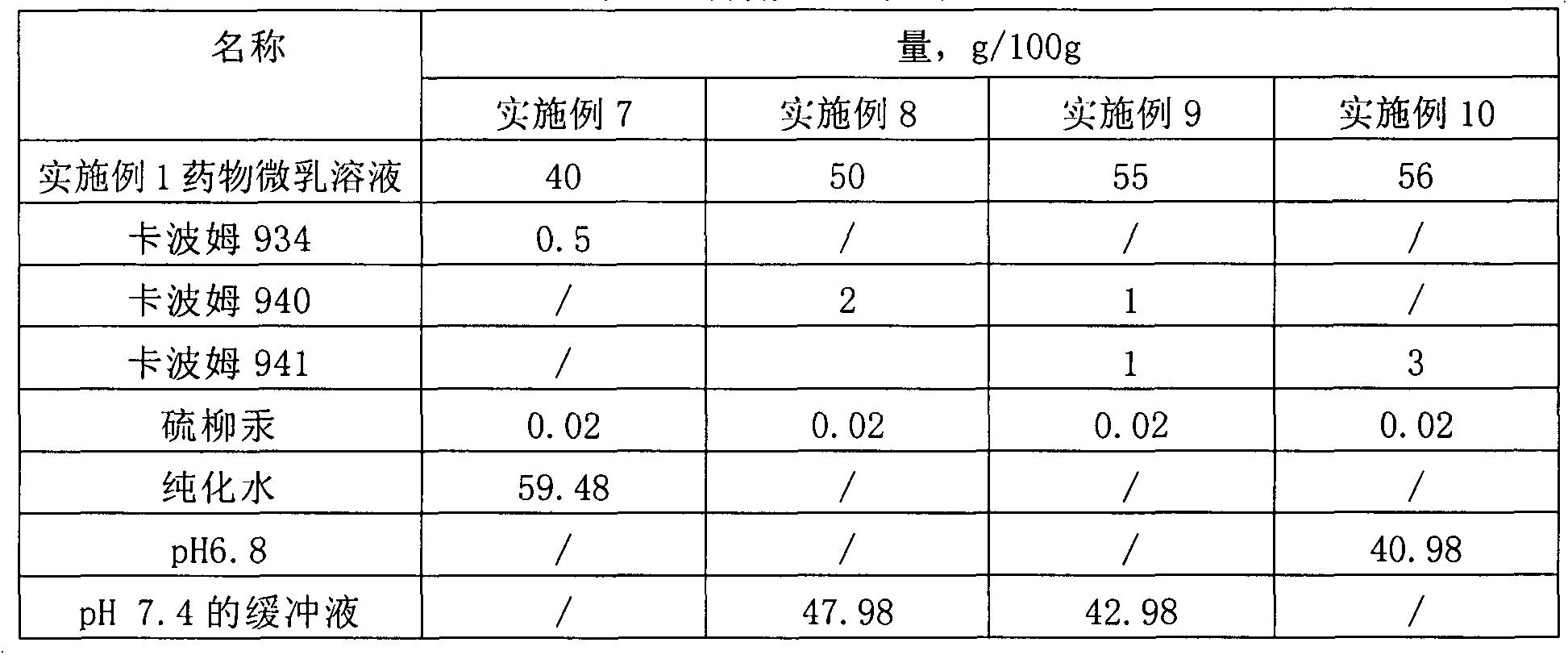
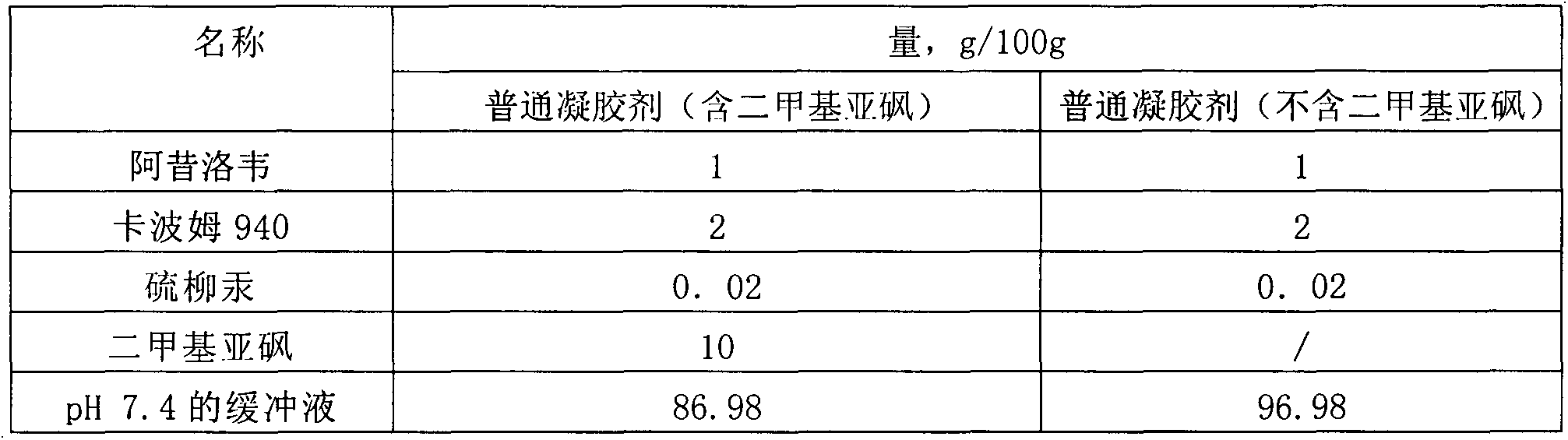
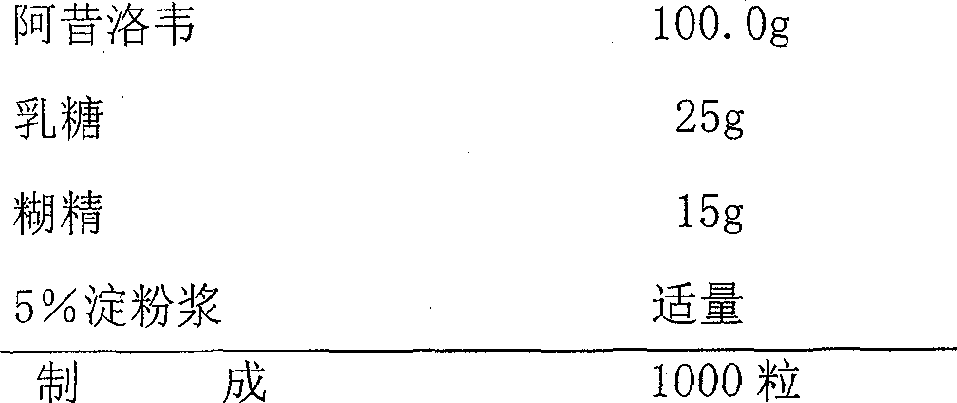Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
46 results about "Aciclovir" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
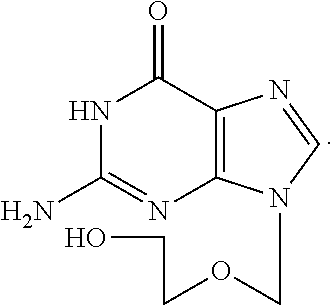
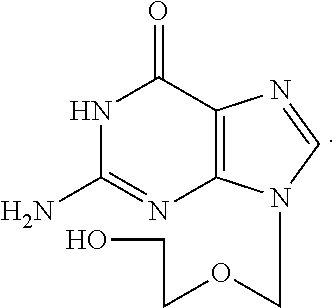
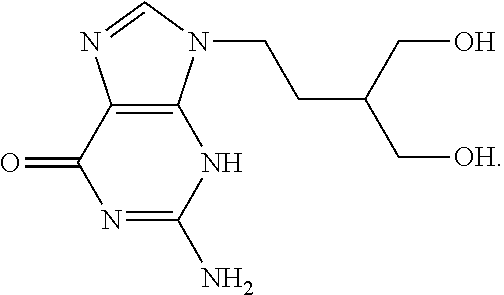
Acyclovir is used to treat infections caused by certain types of viruses. It treats cold sores around the mouth (caused by herpes simplex), shingles (caused by herpes zoster), and chickenpox. This medication is also used to treat outbreaks of genital herpes. In people with frequent outbreaks, acyclovir is used to help reduce the number of future episodes.
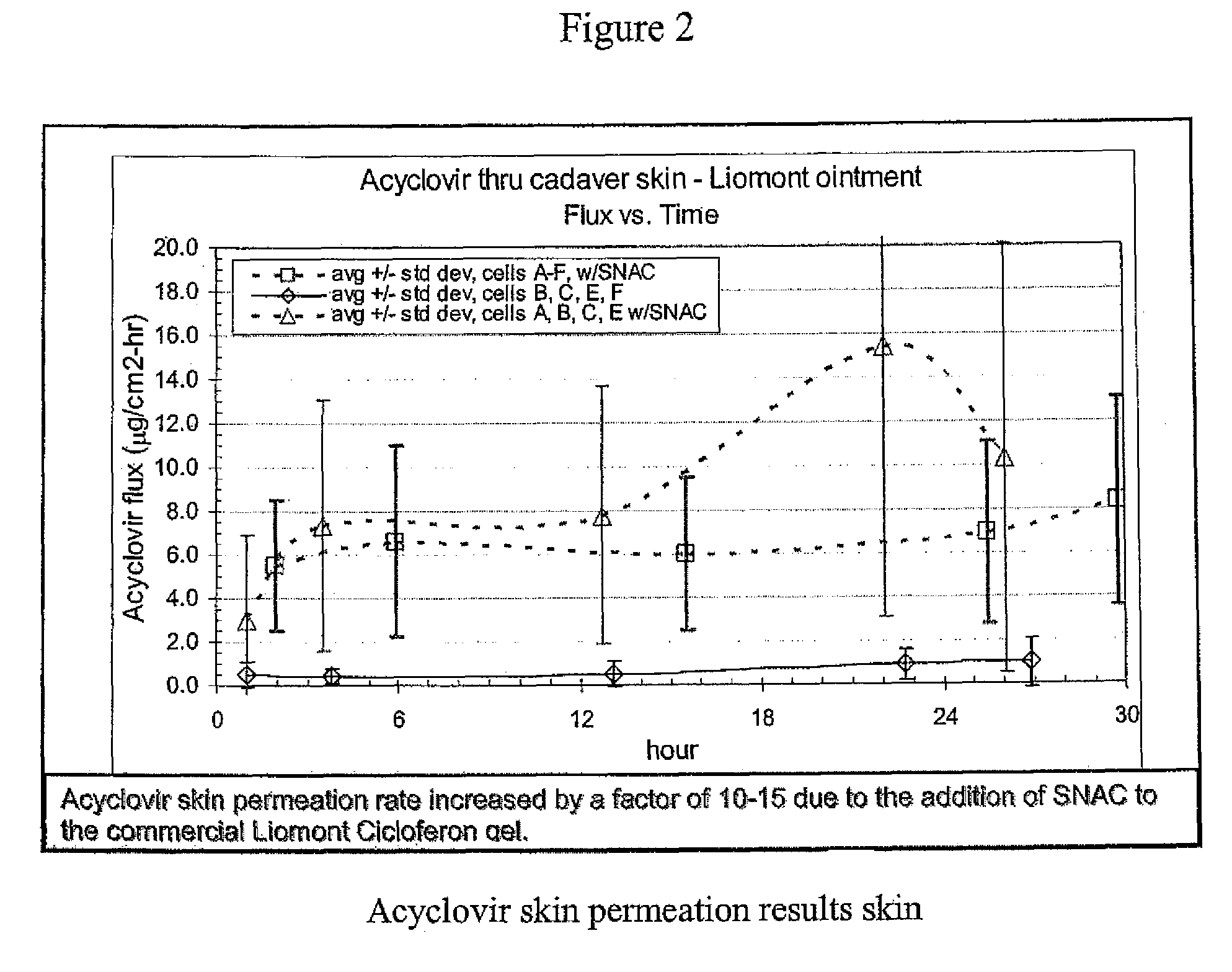
Topical adminstration of acyclovir
InactiveUS20090118168A1Reduce deliveryIncrease concentrationBiocidePharmaceutical delivery mechanismDermatologyAciclovir
Owner:NOVO NORDISK NORTH AMERICA OPERATIONS AS
Application of polycyclic polyketides in preparation of anti-HV (herpes virus) drug
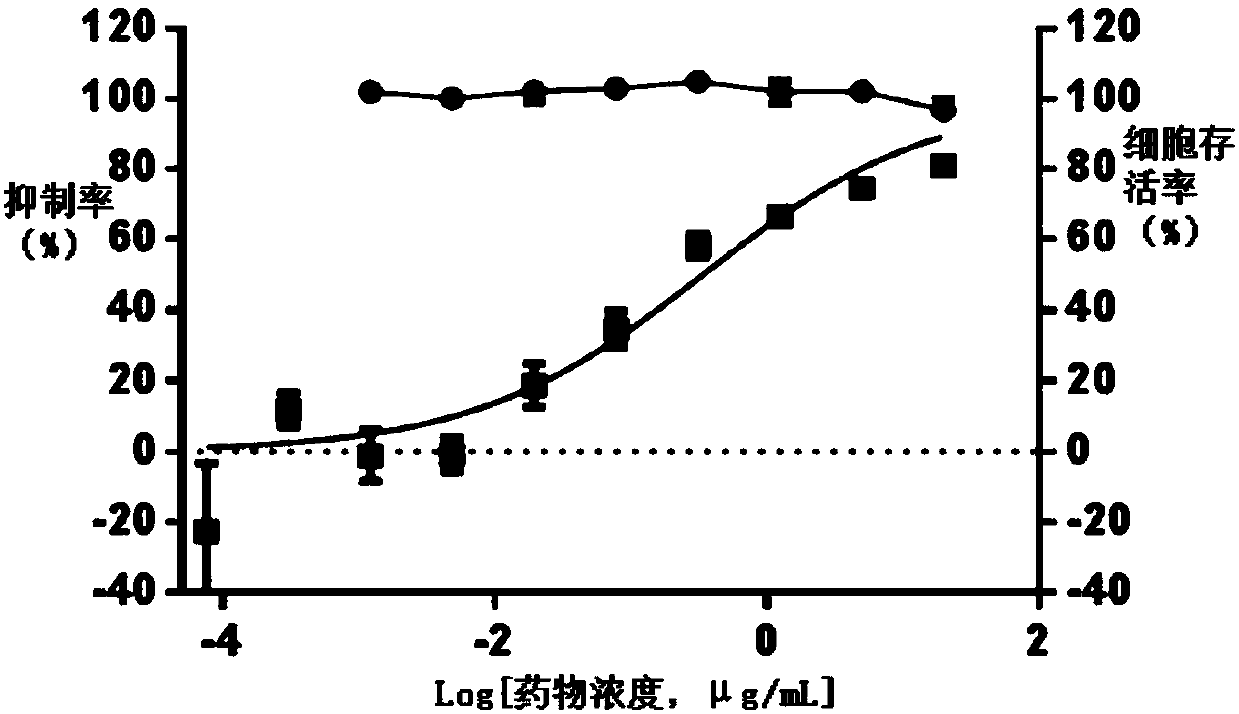
The invention discloses an application of polycyclic polyketides in preparation of an anti-HV (herpes virus) drug. It is found that the polyketides can inhibit diseases caused by infection of four HVs including HSV-1 (herpes simplex virus-1), HSV-2 (herpes simplex virus-2), VZV (varicella zoster virus) and CMV (cytomegalo virus). The compounds show equivalent activity but have different acting mechanisms as compared with commercial drugs such as acyclovir and can overcome drug resistance of existing commercial drugs. Therefore, the compounds have good application prospects in treatment of related diseases caused by infection of HVs including HSV-1, HSV-2, VZV and CMV.
Owner:JINAN UNIVERSITY
Stereochemically defined dipeptide esters of antiviral agents for enhanced ocular treatment
InactiveUS20090149482A1Enhanced enzymatic stabilitySufficient hydrophilicityBiocideSenses disorderDipeptideMedicine
Stereochemically defined dipeptide esters of nucleoside-analogous antiviral agents including acyclovir and ganciclovir are provided. Certain of these stereochemically defined dipeptide esters are found to have unexpectedly enhanced delivery to and uptake by ocular tissues, crossing the blood-ocular barrier more effectively than other stereochemically defined dipeptide esters. For example, (L-Val)-(D-Val)-acyclovir was found to be taken up more effectively into corneal tissue than were underivatized acyclovir, monoesters (L-Val)-acyclovir or (D-Val)-acyclovir, or diester (L-Val)-(L-Val)-acyclovir.
Owner:UNIVERSITY OF MISSOURI
Microemulsion-based gel pharmaceutical composition and preparation method thereof
ActiveCN104107162AThrough highPromote penetration through the stratum corneum of the skin and enterAerosol deliveryOintment deliveryGel preparationDisease
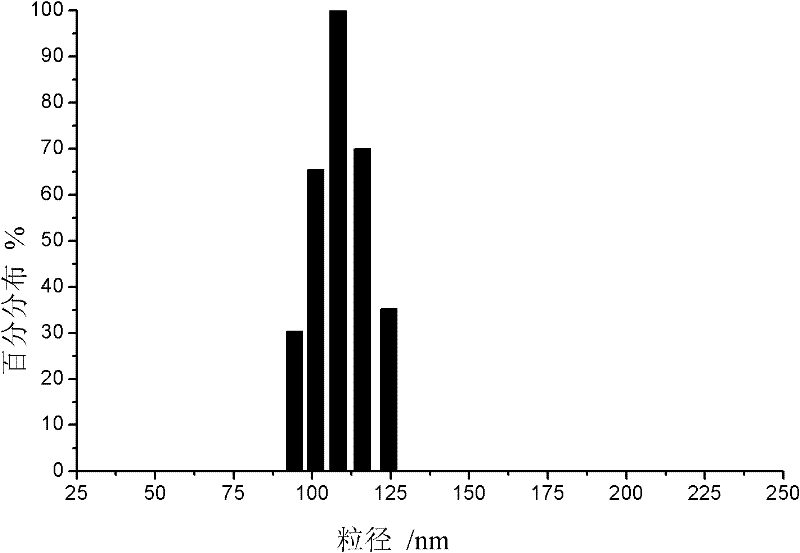
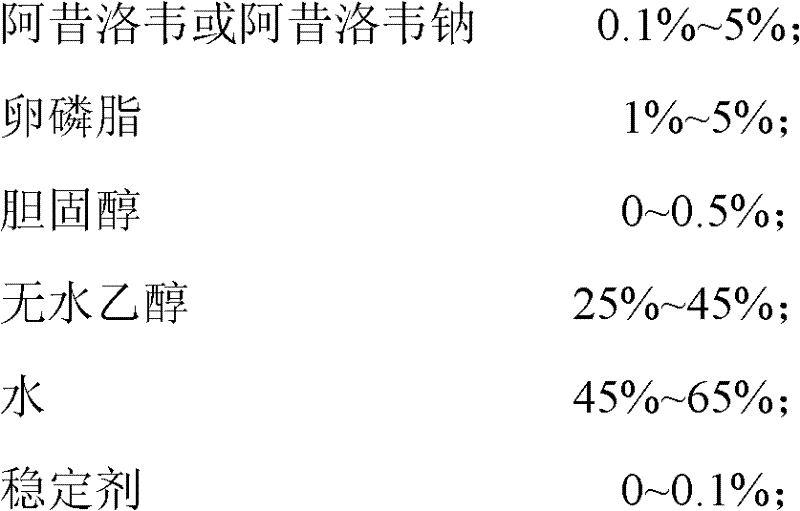
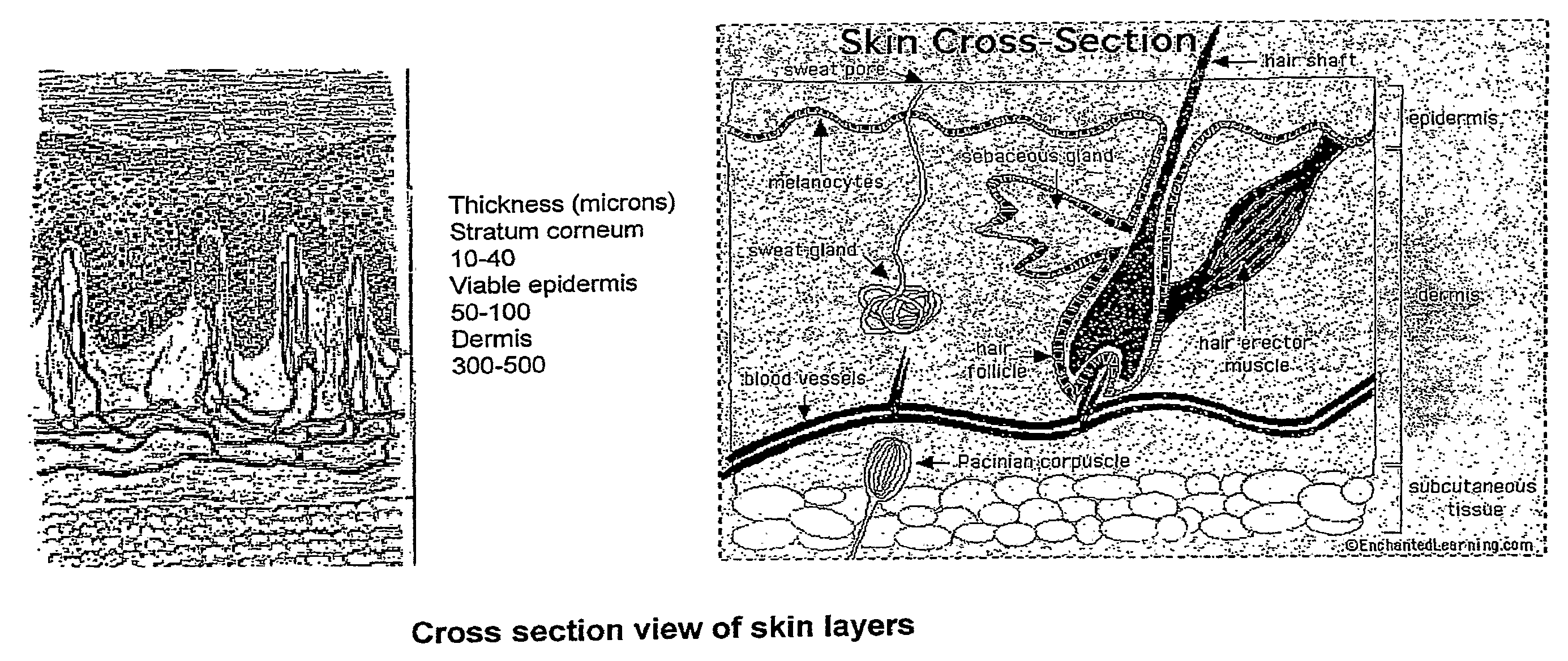
The invention provides acyclovir microemulsion-based gel. The acyclovir microemulsion-based gel is a clear and transparent O / W acyclovir microemulsion prepared from an oil phase, a water phase, a surfactant and a cosurfactant. Through use of carbomer as a hydrophilic gel matrix, the acyclovir microemulsion-based gel is prepared. The acyclovir microemulsion-based gel has surface positive charge, small and uniform particles and average granularity in 50nm, and can carry a drug into skin minimal structures such as hair follicles, sebaceous gland and sweat gland opening, keep them in the minimal structures, improve a drug retention amount of the skin and realize continuous drug release in the skin. Compared with the common gel preparations, the acyclovir microemulsion-based gel has good skin targeting effects and can be used for treating diseases related to virus infection.
Owner:JIANGSU SEMPOLL PHARMA
Therapeutic composition to treat lesions caused by herpes simplex virus
InactiveUS20110065655A1Shorten the construction periodAvoid problemsBiocidePharmaceutical delivery mechanismPenciclovirBULK ACTIVE INGREDIENT
The present invention is generally directed toward therapeutic compositions for treating infections caused by Herpes Simplex Virus (“HSV”). The therapeutic compositions meet a long felt need in the art of providing a treatment for lesions that result from HSV that drastically reduce the duration of a cold sore when vesicles have already appeared and a treatment that will prevent the outbreak of a lesion and formation of vesicles when applied in the prodormal stage. The therapeutic compound comprises a mixture of Acyclovir (“ACV”), Penciclovir (“PCV”), and 2-Deoxy-D-Glucose (“2-DDG”). The therapeutic compositions of the present invention include multiple formulations of the three active ingredients and may also include inactive ingredients.
Owner:G2L TOUCH
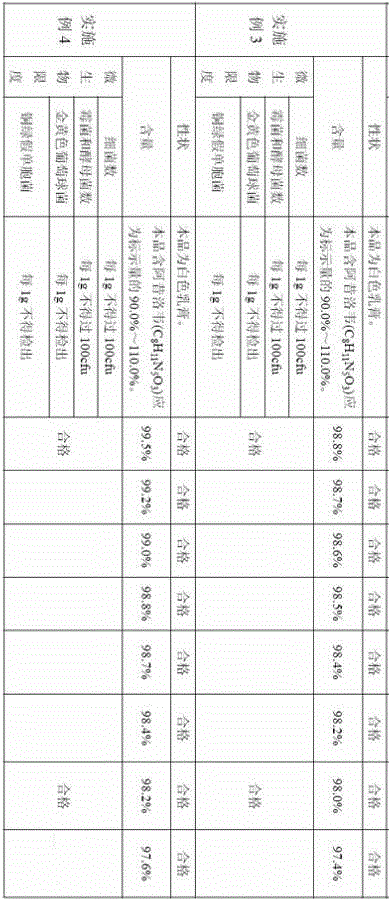
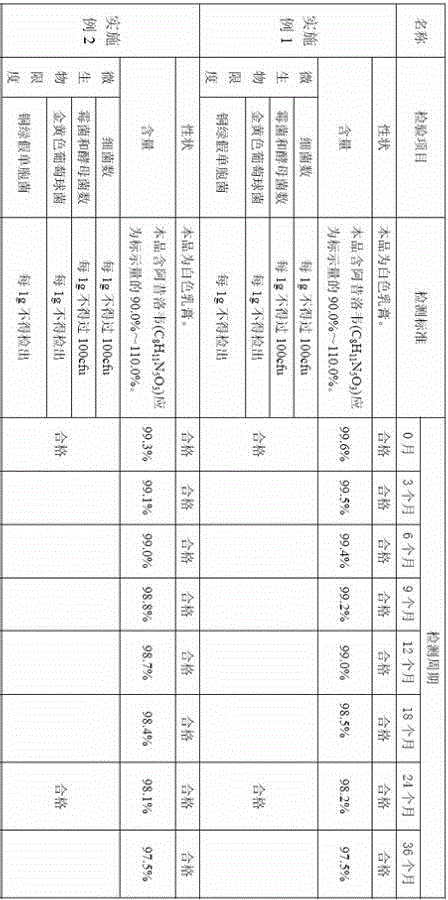
Aciclovir high-molecular hydrogel dressing and its preparation method
InactiveCN1970091AProlong the duration of actionIncrease contact timeAbsorbent padsHeterocyclic compound active ingredientsPotassium persulfateAqueous sodium hydroxide
The invention discloses a making method of Asiluowei macromolecular gel dressing, which comprises the following steps: dissolving N, N'-methylene diacrylamide into acroleic acid completely to obtain solution A; dissolving Asiluowei powder into sodium hydroxide solution to obtain solution B; dissolving polyvinyl alcohol solution, glycerin, potassium persulfate solution and water completely to obtain solution C; dripping solution A into solution B to react; adding solution C; stirring evenly; pouring into mould; heating to polymerize and crosslink to obtain the product to treat herpes zoster.
Owner:SOUTH CHINA UNIV OF TECH
Medicinal resin biological adhering slow-releasing liquid prepn. contg. aciclovir, and its prepn. method
InactiveCN1618427AExtended stayIncrease concentrationPharmaceutical delivery mechanismAntiviralsAntiviral drugAcrylic resin
A slow-release antiviral medicine in the form of liquid is prepared from acyclovir (30-90 wt%) and auxiliary (10-70 wt.%) including the cationic exchange resin and ethylcellulose and / or acrylic resin for slow releasing and the hydroxypropyl cellulose for adhering. Its advantages are high curative effect and low toxic by-effect.
Owner:SHENYANG PHARMA UNIVERSITY
Use of chloride 13-hexyl berberine and chloride 13-hexyl palmatine in preparation of medicine for treating skin mucosa herpes viral infection
InactiveCN1923202AOrganic active ingredientsPharmaceutical delivery mechanismHerpesvirus infectionPalmatine
The invention relates to a method for using alcaine 13-hexyl berberine and relative alcaine 13-hexyl palmatine to prepare the drug that treats herpesvirus infection on skin mucosa. And said two compounds have strong functions to resist HSV, I, II herpesvirus. And their restrain density (IC50) of external resist HSV-1 type and HSV-2 type is 0.78-1.56 mug / ml. And the 0.5% 13-hexyl berberine or alcaine 13-hexyl palmatine, or 1% emulsion can treat bleb, stronger than 3% assili lovou.
Owner:CHUGOKU IGAKU KAGAKUIN HIFUBIYOU KENKYUSHO
Compositions For Delivering Acyclovir
InactiveUS20080132527A1Reduce deliveryImprove bioavailabilityBiocideSenses disorderBioavailabilityEfficacy
The present invention relates to an acyclovir formulation having improved bioavailability resulting in better efficacy and / or requiring less frequent administration.
Owner:NOVO NORDISK NORTH AMERICA OPERATIONS AS
Sustained release drug delivery system
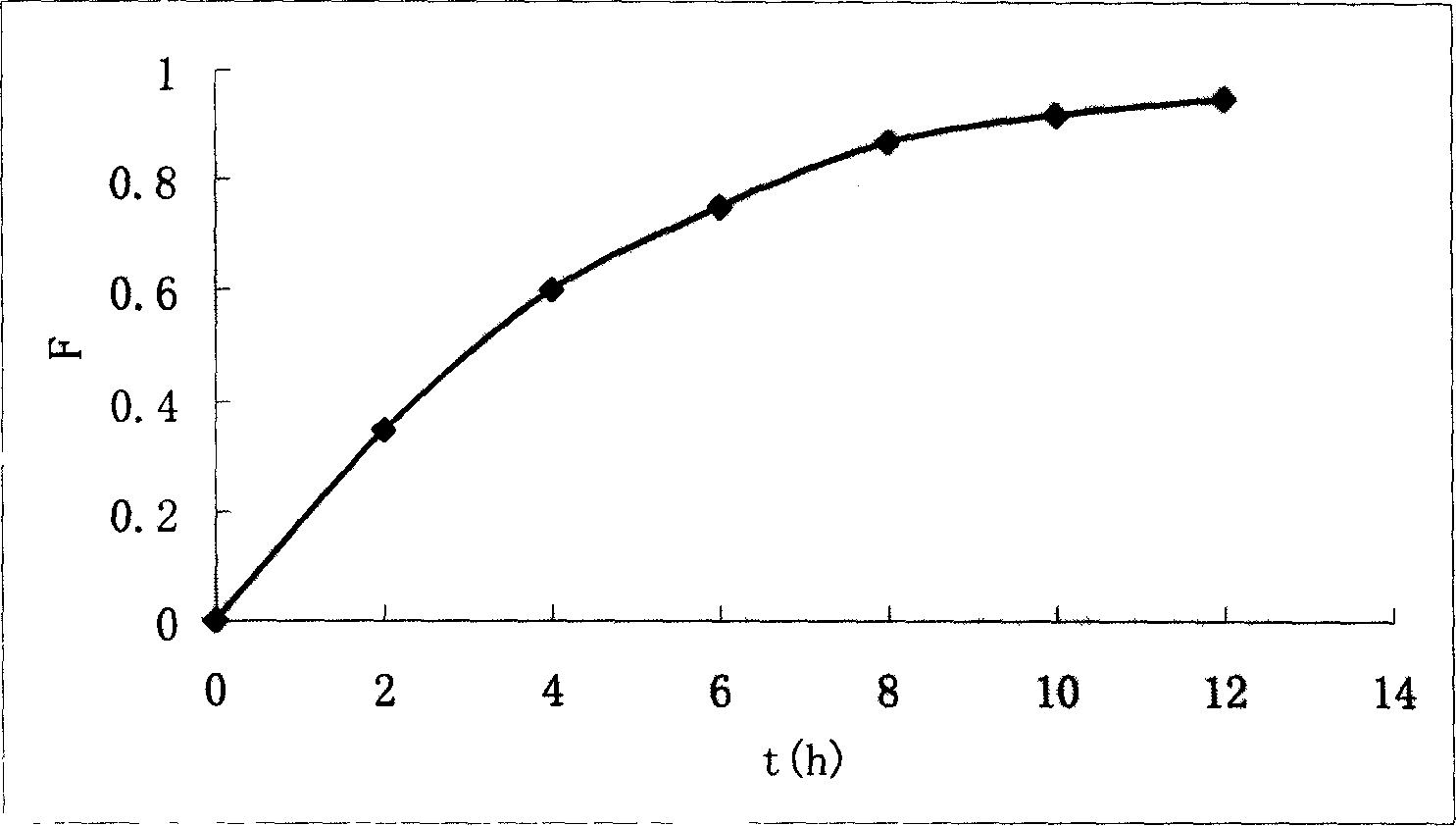
The invention discloses a controlled release dosage form comprising a therapeutically effective amount of a pharmaceutically active agent, illustrated by Acyclovir, that would release in about 12 hours not more than about 90% of the said active agent in a simulated gastric juice in a first order rate of release in a USP type 1 dissolution test, and not containing a solubilizer or a swelling enhancer or both, comprising (a) a tablet made from polymer matrix of at least two biocompatible polymers, illustrated by Carbopol 974P and polyethylene oxide, the said pharmaceutically active agent and pharmaceutically permitted excipients; the said tablet capable of rapid swelling without disintegration in the said simulated gastric juice to a size that shall result in its gastric retention in the stomach and start controlled release of the said active agent by starting controlled erosion as well as diffusion immediately after coming into contact with the said gastric juice, or (b) microspheres of ungrafted chitosan or a chitosan derivative illustrated by thiolated chitosan and trimethyl chitosan, or Carbopol incorporating the said active agent, wherein the said pharmaceutically active agent is not a polymeric molecule and after administration in stomach, the said microspheres adhare to the gastric mucosa for a long time releasing the active agent in a controlled way.
Owner:BIOPLUS LIFE SCI PVT
Acyclovir enteric-coated formulation composition and method for preparing the same
InactiveCN101502520AAvoid nauseaThe preparation process has good quality controllability and stabilityAntiviralsCapsule deliverySustained-Release PreparationsDisease
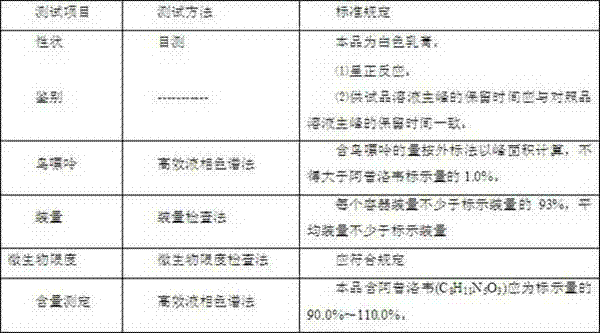
The invention discloses a group of acyclovir enteric preparation combinations and a preparation method thereof. The group of acyclovir enteric sustained-release preparation combinations are mainly prepared from acyclovir bulk drugs and other appropriate auxiliary materials. The acyclovir enteric preparation provided by the invention can prevent acyclovir from disintegrating in the stomach and causing irritation to gastric mucosa and avoid the adverse reactions caused by the administration, such as nausea, abdominal pain, diarrhea and the like. The acyclovir enteric preparation combination is particularly suitable for patients with stomach-upset diseases. The invention provides a novel form of drug featuring higher safety and better curative effect than the existing relevant acyclovir preparations and having the advantages of high quality controllability and stability of the preparation process.
Owner:山东淄博新达制药有限公司
Pien Tze Huang and new application of preparation thereof in preparing drug for treating herpes zoster
InactiveCN108042589ADefinite curative effectImprove clinical symptomsAntiviralsMammal material medical ingredientsAnti virusHerpes zoster virus
The invention belongs to the field of traditional Chinese medicine, and particularly relates to Pien Tze Huang and new application of a preparation thereof in preparing a drug for treating herpes zoster. An in-vitro experiment shows that Pien Tze Huang can dose-dependently inhibit duplication of chicken varicella-herpes zoster viruses (VZV). A clinical study finds that the combination of Pien Tzehuang and anti-virus drugs of valaciclovir and acyclovir has an obvious curative effect on alert period zoster viruses due to liver yu heat from the line, improvement of clinical symptoms of a patientcan be promoted, incrustation of herpes can be quickly promoted, new herpes can be stopped from generating, pain can be relieved, and the treatment time can also be shortened; besides, the incidenceof postherpetic neuralgia and the occurrence rate of adverse reactions occurring after treatment can also be lowered, and the clinical application has obvious advantages.
Owner:ZHANGZHOU PIEN TZE HUANG PHARM
Preparation method for acyclovir emulsifiable paste
ActiveCN103751101AGood quality and stabilityMedication convenienceAerosol deliveryOintment deliveryDrugs preparationsAciclovir
The invention discloses a preparation method for an acyclovir emulsifiable paste, and belongs to the field of chemical medicinal preparations. The method comprises four steps of raw material preparation, water phase preparation, oil phase preparation and emulsifiable paste preparation. The acyclovir emulsifiable paste prepared by employing the preparation method is good in quality stability, is capable of rapidly permeating skin and being reserved for a relatively long time, is convenient to use and good in patient tolerance, causes no adverse reactions, and is worth for clinic popularization and application.
Owner:ZHENGZHOU HANDU PHARMA GROUP
Acyclovir pharmaceutical composition
ActiveCN104644585AReduce hardnessHigh hardnessAntiviralsPill deliveryDrugs preparationsPharmaceutical drug
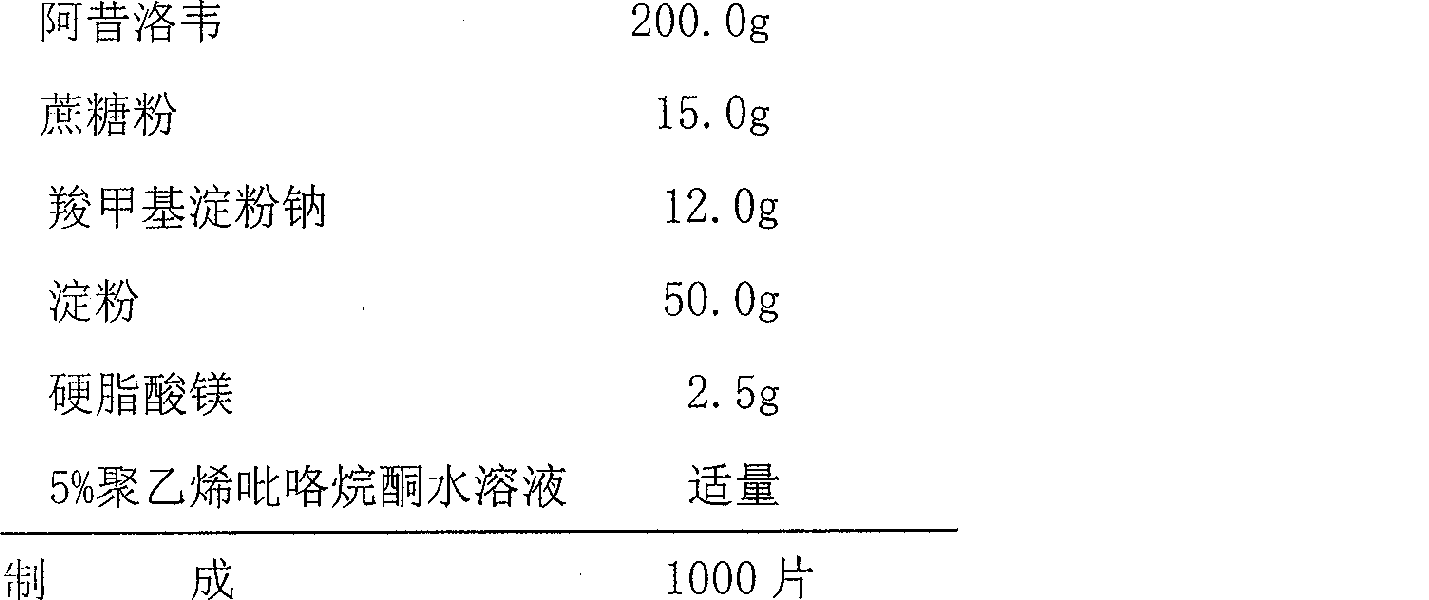
The invention relates to the field of pharmaceutical preparations, and in particular relates to an acyclovir pharmaceutical composition and a preparation method thereof. The acyclovir pharmaceutical composition disclosed by the invention is a mixture comprising the following components in percentage by mass: 28.2-60.9% of acyclovir, 10-50% of a filling agent, 5-15% of an adhesion agent, 1-5% of a disintegrating agent and 0.1-1.8% of a lubricating agent. The acyclovir pharmaceutical composition disclosed by the invention is scientific in formula, simple in preparation process, easy to prepare, low in loss rate, smooth in surface and good in colour, lustre and curative effect.
Owner:KAMP PHARMA
Therapeutic composition to treat lesions caused by herpes simplex virus
ActiveUS20120071498A1Shorten the construction periodAvoid problemsBiocideOrganic chemistryAdditive ingredientBULK ACTIVE INGREDIENT
The present invention is generally directed toward therapeutic compositions for treating infections caused by Herpes Simplex Virus (“HSV”). The therapeutic compositions meet a long felt need in the art of providing a treatment for lesions that result from HSV that drastically reduce the duration of a cold sore when vesicles have already appeared and a treatment that will prevent the outbreak of a lesion and formation of vesicles when applied in the prodromal stage. The therapeutic compound comprises a mixture of Acyclovir (“ACV”), Penciclovir (“PCV”), and dimethyl sulfoxide (“DMSO”). The therapeutic compositions of the present invention include multiple formulations of the three active ingredients and may also include inactive ingredients and / or additional active ingredients.
Owner:G2L TOUCH
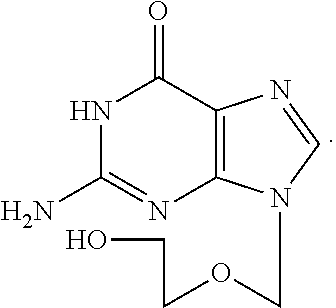
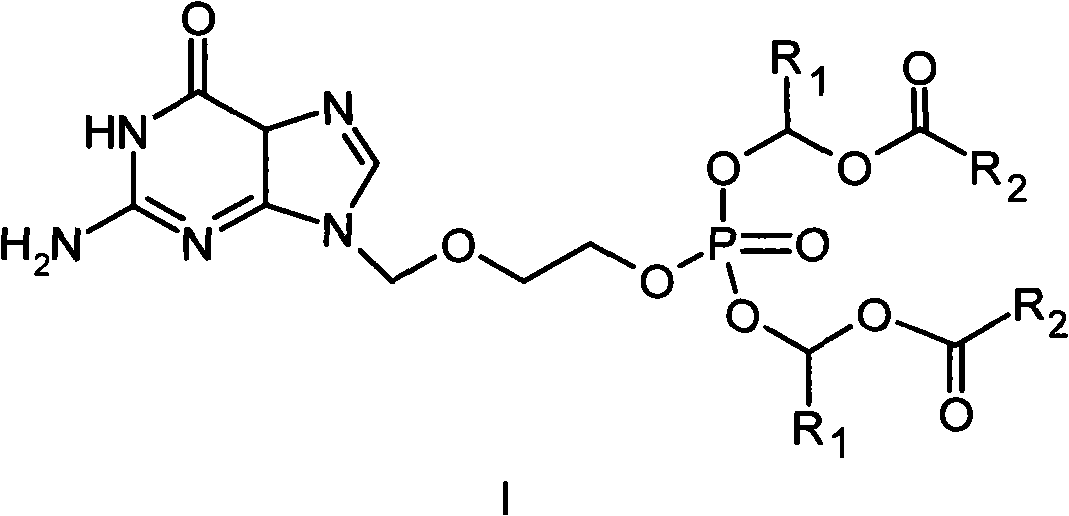
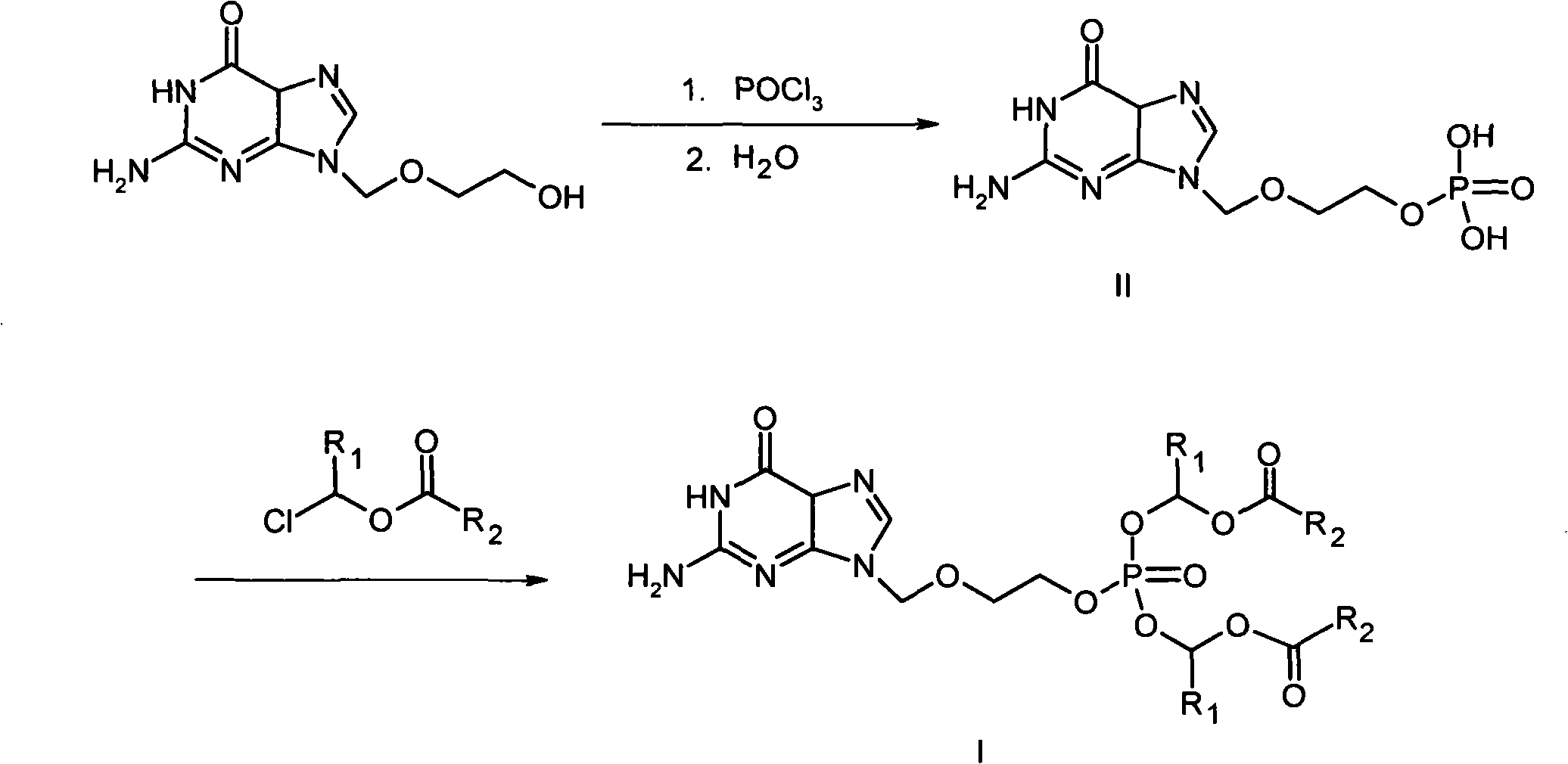
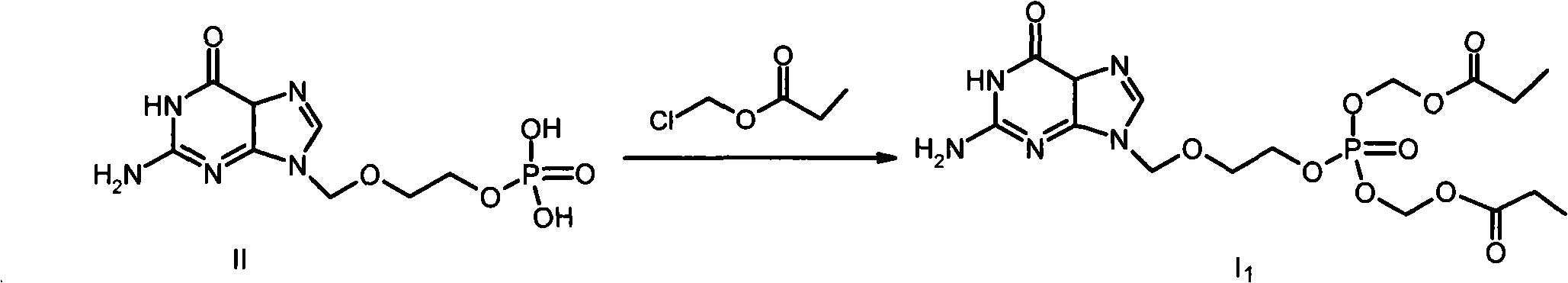
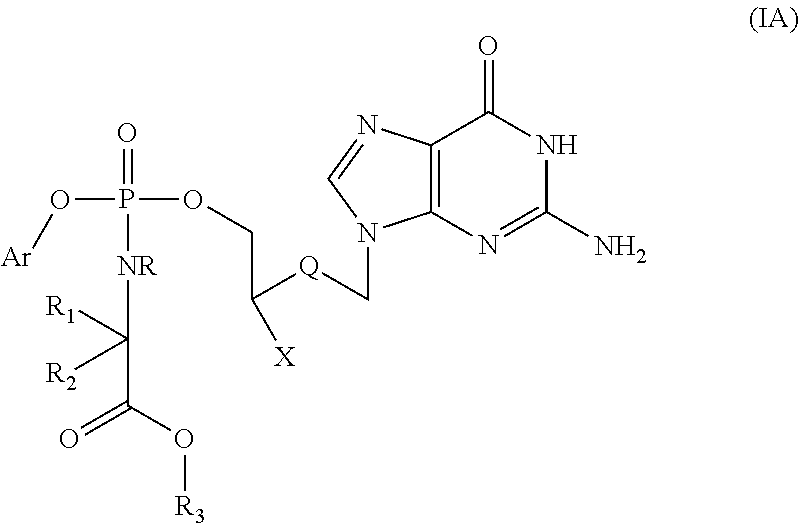
Phosphate derivative of acyclovir and medical application thereof
InactiveCN103804416AOrganic active ingredientsGroup 5/15 element organic compoundsAbsolute configurationSolvent
The invention aims to provide a ring-free nucleoside phosphate derivative having an anti-hepatitis B virus activity and represented by formula I shown in the specification and a nontoxic pharmaceutically acceptable salt, hydrate or solvate thereof, wherein R1 represents H or methyl; absolute configuration of carbon atoms connected with R1 is an R or S isomer or an RS racemate; R2 represents -R3 or -OR3; R3 represents C1-C8 alkyl or cycloalkyl, and is selected from methyl, ethyl, propyl, butyl, isopropyl, isobutyl, tert-butyl, cyclopentyl, cyclohexyl and the like.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
Acyclovir ethosome and preparation method thereof
InactiveCN102133183BChange appearanceImprove stabilityAntiviralsPharmaceutical non-active ingredientsPolyethylene glycolAciclovir
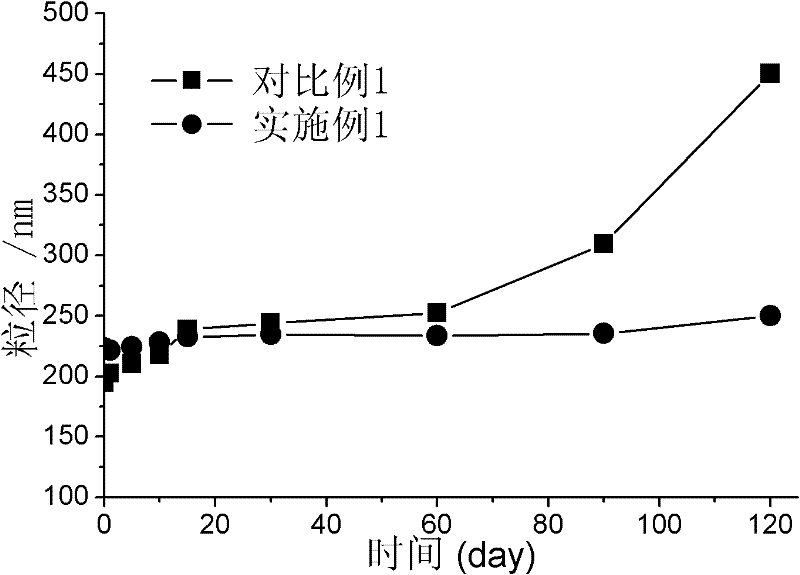
The invention discloses an acyclovir ethosome preparation with improved stability and a preparation method thereof. In the invention, polyethylene glycol (PEG) or chitosan (CS) derivatives are added to the percutaneous administration carrier ethosome to improve the stability of the ethosome. The preparation process of the preparation disclosed by the invention is simple, and the prepared acyclovir ethosome has high stability and narrow particle size distribution.
Owner:XIANGTAN UNIV
Therapeutic composition to treat lesions caused by herpes simplex virus
ActiveUS8853223B2Shorten the construction periodAvoid problemsBiocideOrganic chemistryVesicle/vacuoleBULK ACTIVE INGREDIENT
The present invention is generally directed toward therapeutic compositions for treating infections caused by Herpes Simplex Virus (“HSV”). The therapeutic compositions meet a long felt need in the art of providing a treatment for lesions that result from HSV that drastically reduce the duration of a cold sore when vesicles have already appeared and a treatment that will prevent the outbreak of a lesion and formation of vesicles when applied in the prodromal stage. The therapeutic compound comprises a mixture of Acyclovir (“ACV”), Penciclovir (“PCV”), and dimethyl sulfoxide (“DMSO”). The therapeutic compositions of the present invention include multiple formulations of the three active ingredients and may also include inactive ingredients and / or additional active ingredients.
Owner:G2L TOUCH
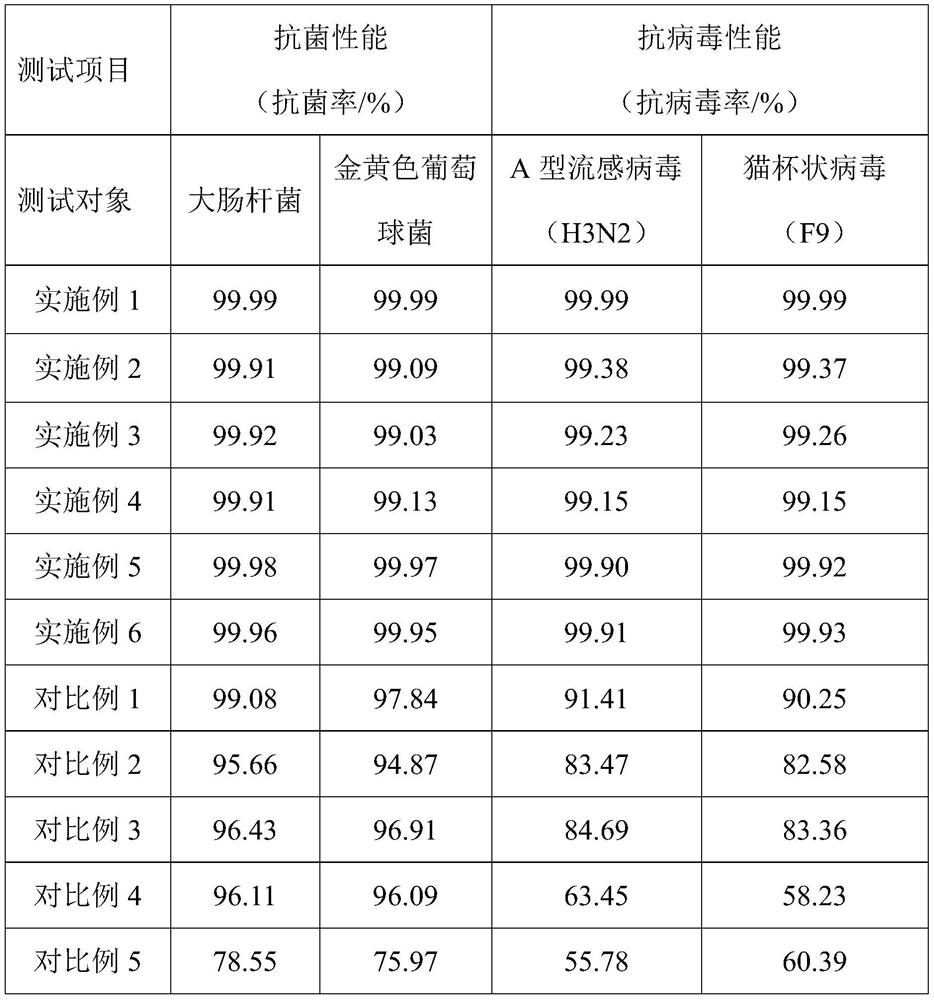
Antibacterial antiviral stainless steel and preparing method thereof
ActiveCN111763980AGood and long-lasting antibacterial and antiviral propertiesEasy to operateBiocideAnodisationSS - Stainless steelAciclovir
The invention relates to antibacterial antiviral stainless steel and a preparing method thereof. The preparing method comprises the following steps that firstly, stainless steel is subjected to anodicoxidation, and an anodic oxidation film with micro holes is formed on the surface of the stainless steel; secondly, soaking and primary heating are carried out in sol A; and the thirdly, soaking andsecondary heating are carried out in sol B, and the antibacterial antiviral stainless steel is obtained, wherein the sol A comprises butyl titanate, and the sol B comprises valaciclovir hydrochlorideand acyclovir. The preparing method mainly decomposes the butyl titanate in the sol A into nanometer titania through primary heating, through two times of sol soaking and heating treatment, the nanometer titania and valaciclovir hydrochloride and acyclovir compounded antibacterial antiviral components are attached to the interiors and the surfaces of the micro holes of the anodic oxidation film, the stainless steel has the good and durable antibacterial antiviral performance, and the preparing method is easy to operate, and beneficial to large-scale popularization.
Owner:ANHUI TONGXI JINPENG ALUMINUM
Ointement for treating ringworm
InactiveCN1981771ASimple topical treatmentHigh cure rateAntimycoticsAerosol deliveryDexamethasone acetateParapsoriasis
An ointment for treating skin tinea, especially the psoriasis, is prepared from dexamethasone acetate and aciclovir in the ratio of (0.75-4.50):30.
Owner:邓思奎
Topical administration of acyclovir
InactiveUS8771712B2Reduce deliveryIncrease concentrationBiocidePeptide/protein ingredientsBuccal administrationDermatology
Owner:NOVO NORDISK NORTH AMERICA OPERATIONS AS
Compounds and methods for the treatment of viral infection
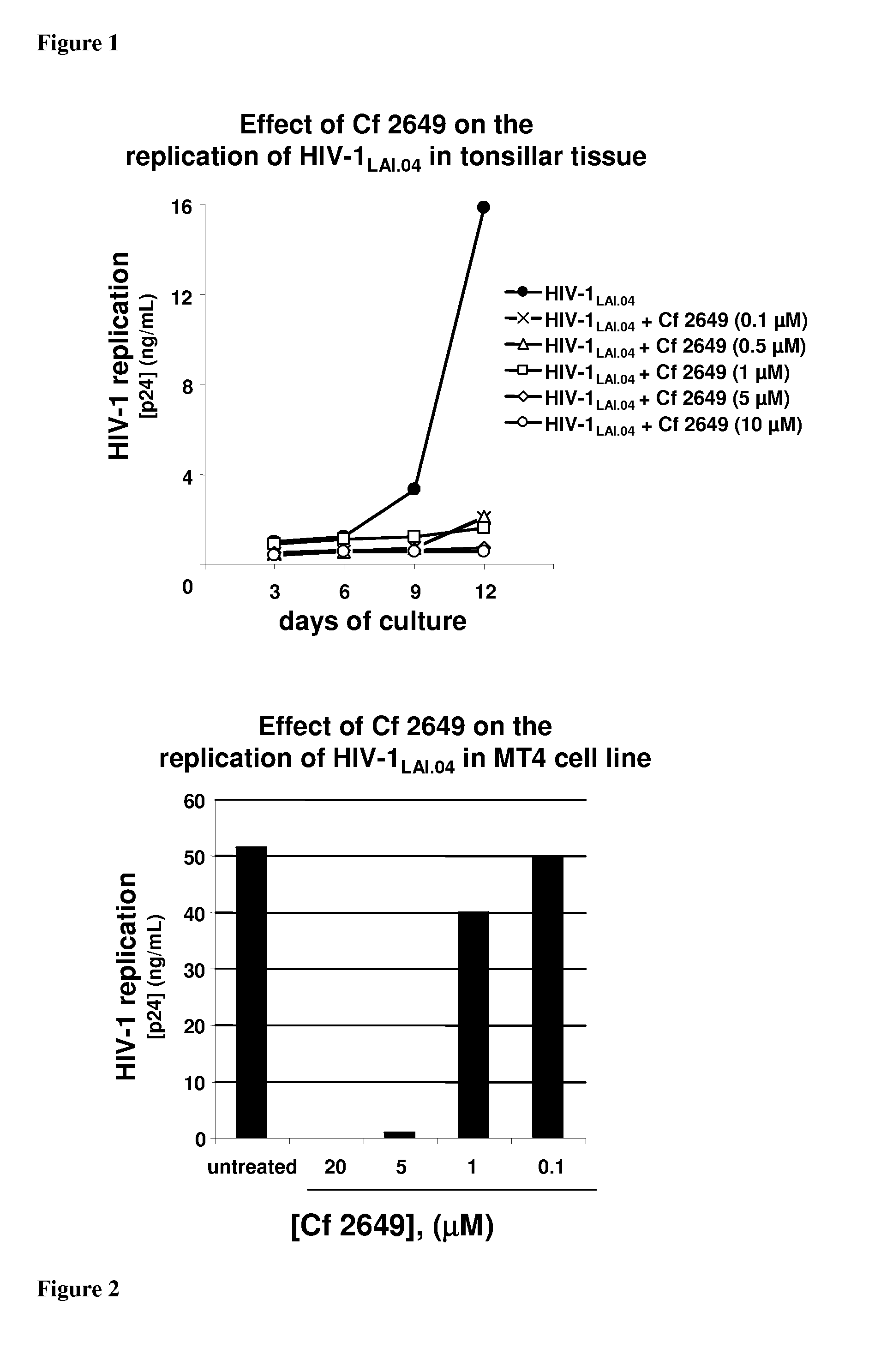
The invention relates to compounds and methods for treating or preventing a viral infection, by administering a monophosphorylated prodrug of acyclovir or monophosphorylated derivative of an acyclovir prodrug to a subject suffering from or susceptible (to a viral infection, such as HIV infection.
Owner:UNITED STATES OF AMERICA +1
Method for detoxification culture of lily by using acyclovir agent
InactiveCN105532447AHigh detoxification efficiencyShorten the timeHorticulture methodsPlant tissue cultureBiotechnologyMicrobiology
The present invention discloses a method for detoxification culture of lily by using an acyclovir agent, and belongs to the fields of plant tissue culture rapid propagation technologies and biotechnologies. The method comprises: lily seedball virus detection, preparation of culture medium to be used, explant sterilizing and inoculating, explant culture, tissue culture seedling virus detection and other steps so as to rapidly obtain a large number of detoxification tissue culture seedlings. According to the present invention, the optimal induction culture medium formula for direct differentiation of the lily into the bud and the root-free tissue culture seedling is obtained through the orthogonal method, and the acyclovir agents with a series of concentration gradients are prepared to screen the optimized detoxification concentration, wherein the detoxification efficiency can achieve 90%, the induction culture period can be shortened, the characteristics of high detoxification rate, high value-added factor and the like are provided, and the technical foundation is provided for cultivation of lily virus-free plants and promotion of research on non-toxic cultivation.
Owner:SONGTAO HONGFA MEAT FOOD CO LTD
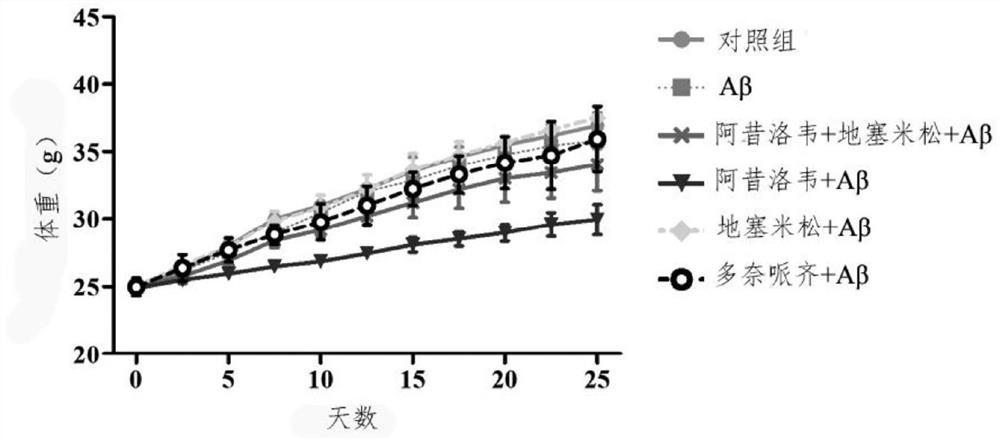
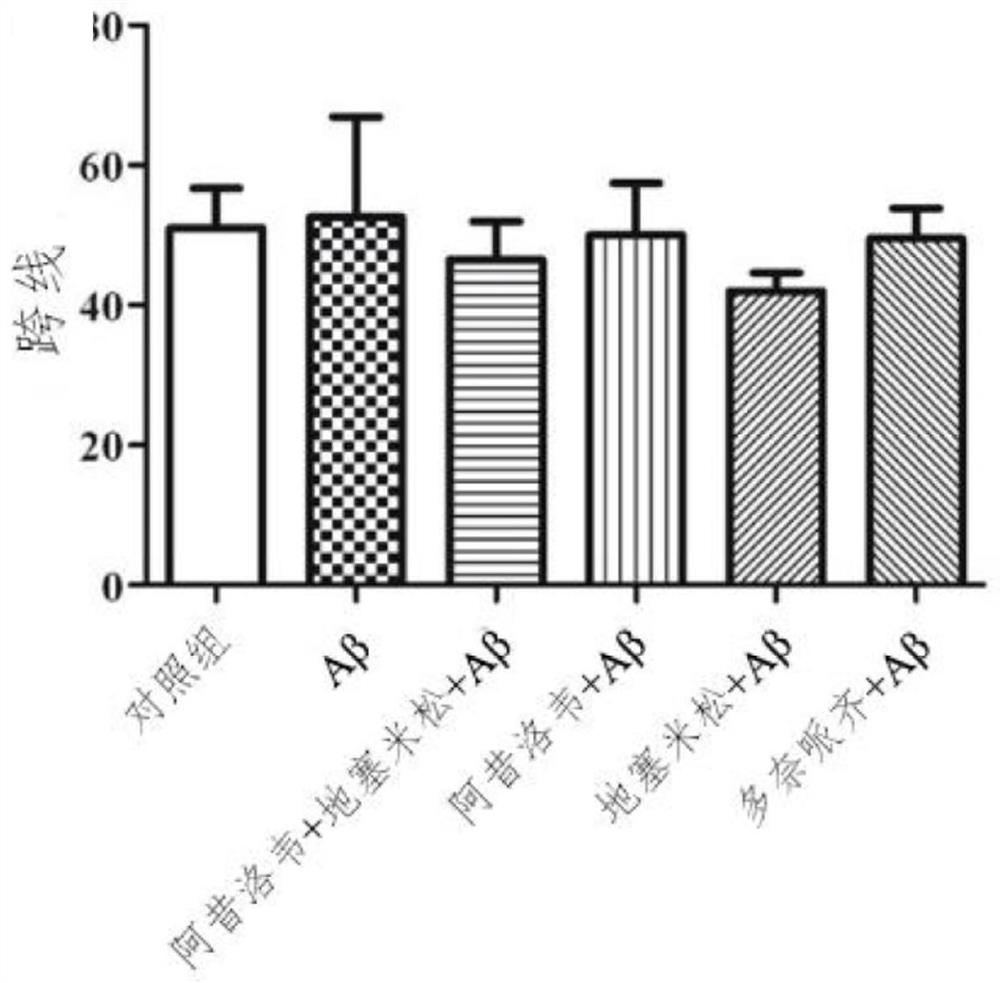
Preparation of medicine for treating Alzheimer's disease
The invention relates to an application of combination of acyclovir and dexamethasone in preparation of a drug for treating Alzheimer's disease. According to the application, for all symptoms of Alzheimer's disease patients, especially cognitive impairment and neuroinflammation, it is found that the combined application of acyclovir and dexamethasone has a synergistic effect and exerts anti-inflammatory and immunomodulatory effects at the same time to achieve the treatment effect.
Owner:PLANTARX LTD
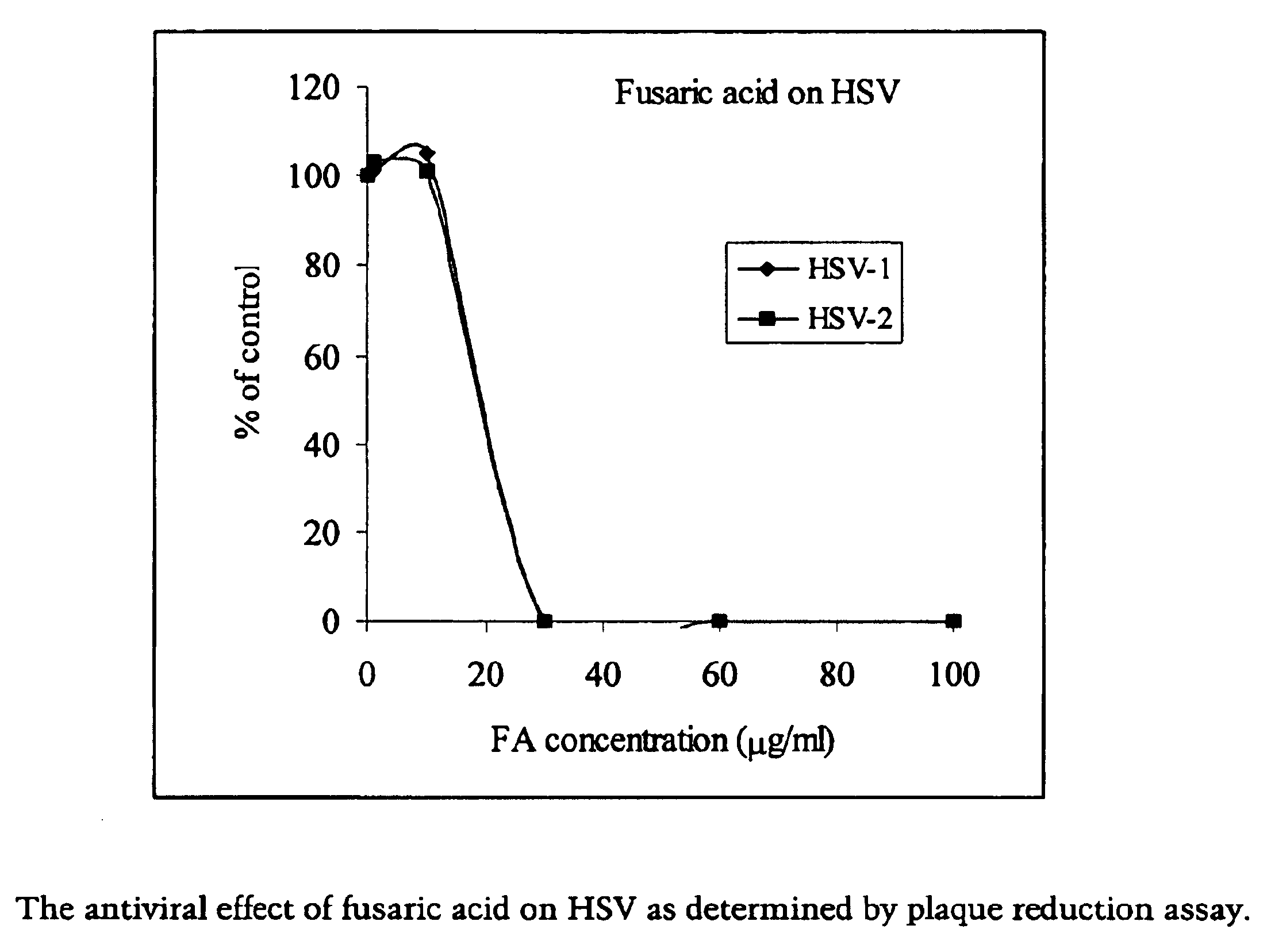
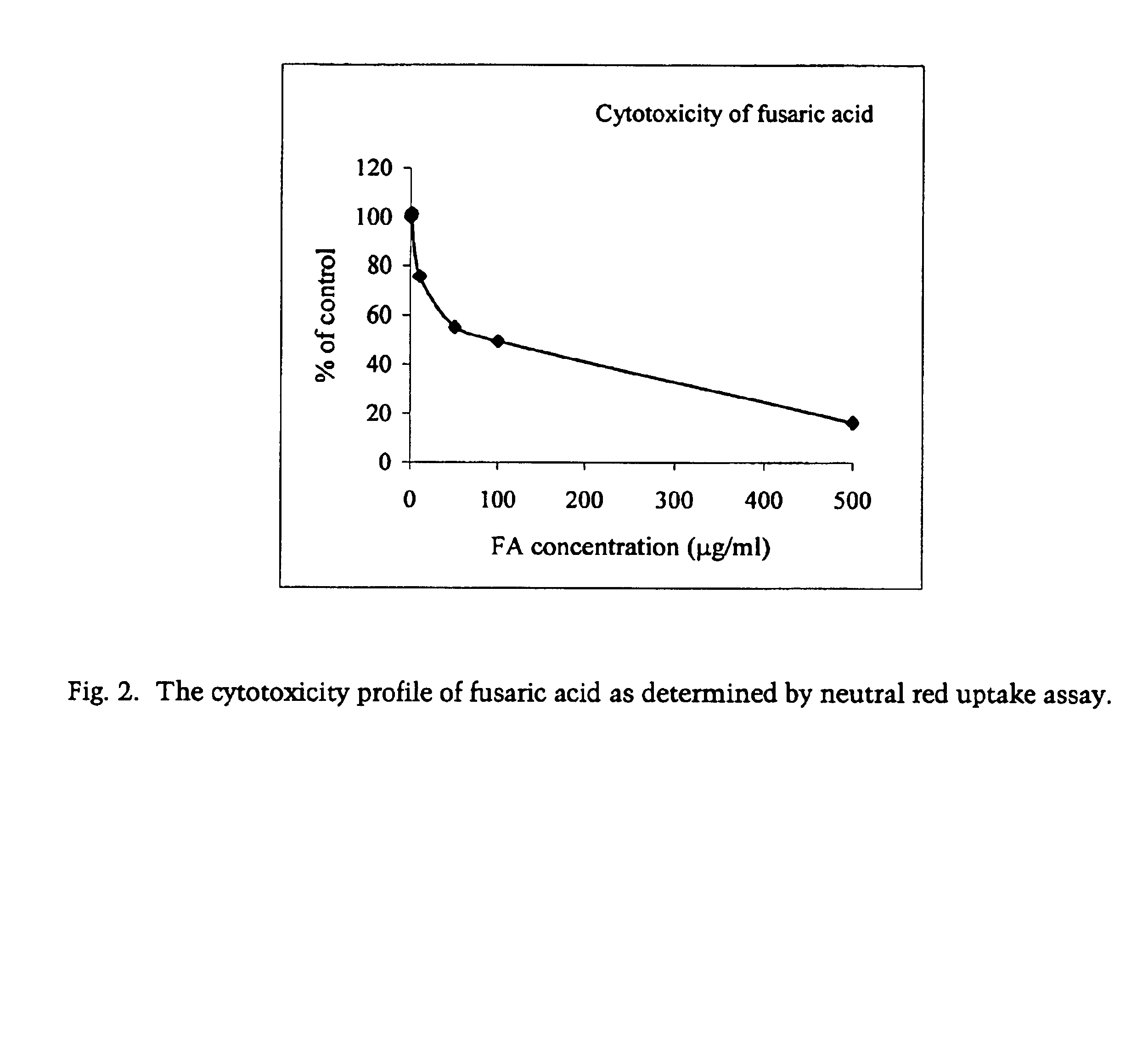
Pharmaceutical agents containing acyclovir, fusaric acid and derivatives thereof
Compositions are provided, which comprise at least one nucleoside analogue inhibitor, pharmaceutically acceptable salts, solvates, or prodrugs thereof and fusaric acid, derivatives, or pharmaceutically acceptable salts thereof. Pharmaceutical compositions comprising the above compositions are also provided that optionally further include another therapeutically effective compound, such as a carrier. Systemic and topical preparations comprising the above compositions are also provided as well as methods of treating viral diseases by the administration of the above antiviral agents to a patient.
Owner:NOVACTYL
Pharmaceutical novel applications of acyclovir in reducing toxicity of irinotecan
The invention provides applications of anti-herpes simplex virus drug acyclovir in preventing or / and treating tardive diarrhea caused by one anti-tumor drug and intestinal dysfunctions caused by diarrhea, wherein the anti-tumor drug is irinotecan or active metabolite 7-ethyl-10-hydroxycamptothecin (SN-38) of irinotecan. The drug effect of acyclovir is clear; acyclovir is capable of relieving tardive diarrhea effectively, and protecting intestinal functions.
Owner:BINZHOU MEDICAL COLLEGE
Cream for treating herpes zoster and preparation method of cream
The invention discloses an application of pulsatilla chinensis or an extract thereof to preparation of a medicine for treating herpes zoster, and a composition taking the pulsatilla chinensis extract as an active component. The composition has no toxic or side effect on human bodies, has no adverse reaction or sequelae after being used by patients, has the effect of treating herpes zoster remarkably superior to that of acyclovir cream commonly used in clinic at present, is an externally-applied good medicine for treating skin herpes zoster, and is worthy of popularization and application. The damage to the gastrointestinal function of a human body caused by oral medicines is avoided; besides, a remarkable treatment effect is achieved; and the cream has wide application value.
Owner:广西馨海药业科技有限公司
Acyclovir cream
ActiveCN103751100BGood quality and stabilityImprove toleranceAerosol deliveryOintment deliveryMonoglycerideWhite petrolatum
The invention relates to an acyclovir emulsifiable paste, and belongs to the field of chemical medicinal preparations. The emulsifiable paste is prepared from the following raw materials with the total amount of 100 parts by weight: 2-5 parts of acyclovir, 4-10 parts of cetostearyl alcohol, 2-10 parts of monoglyceride stearate, 4-10 parts of white vaseline, 4-10 parts of glycerin, 0.5-2 parts of sodium dodecyl sulfate, 0.2-0.5 part of ethylparaben, 0.5-2 parts of dimethyl sulfoxide, 0.2-1.0 part of benzalkonium bromide solution with a concentration of 5 wt%, and the balance purified water. The acyclovir emulsifiable paste provided by the invention is good in quality stability, is capable of rapidly permeating skin and being reserved for a relatively long time, is convenient to use and good in patient tolerance, causes no adverse reactions, and is worth for clinic popularization and application.
Owner:ZHENGZHOU HANDU PHARMA GROUP
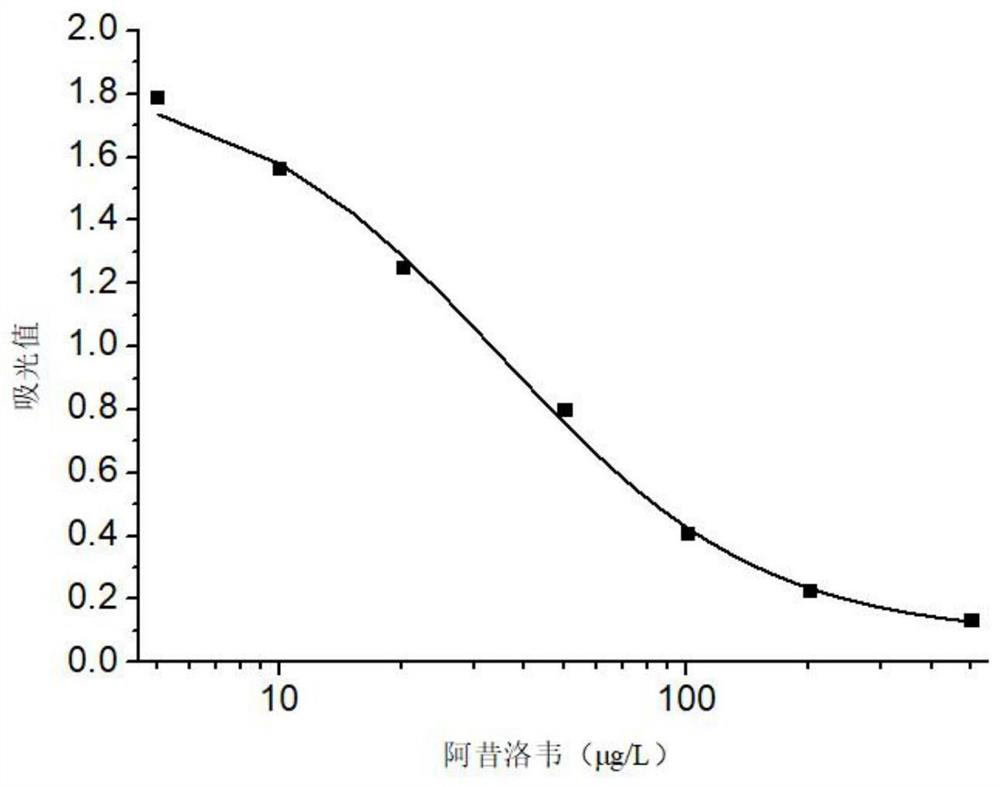
A hybridoma cell line secreting acyclovir monoclonal antibody and its preparation method
ActiveCN108330103BHigh detection sensitivityImprove featuresFused cellsImmunoglobulinsBALB/cAdjuvant
The invention relates to a hybridoma cell line secreting acyclovir monoclonal antibody and a preparation method thereof, belonging to the field of food safety immunoassay. The preservation number of the hybridoma cell line is: CGMCC No.14695. BALB / c mice were first immunized with complete Freund's adjuvant, boosted with incomplete Freund's adjuvant, and adjuvant-free adjuvant BALB / c mice were immunized by shock immunization with Lowe's complete antigen; the immunized mouse splenocytes with high titer and low IC50 were fused with mouse myeloma cells by PEG method, and were screened by indirect competition ELISA and subcloned three times , the resulting cell lines. The monoclonal antibody secreted by this cell line has good specificity and detection sensitivity to acyclovir, and can be used for the detection of acyclovir residues in food.
Owner:JIANGNAN UNIV +1
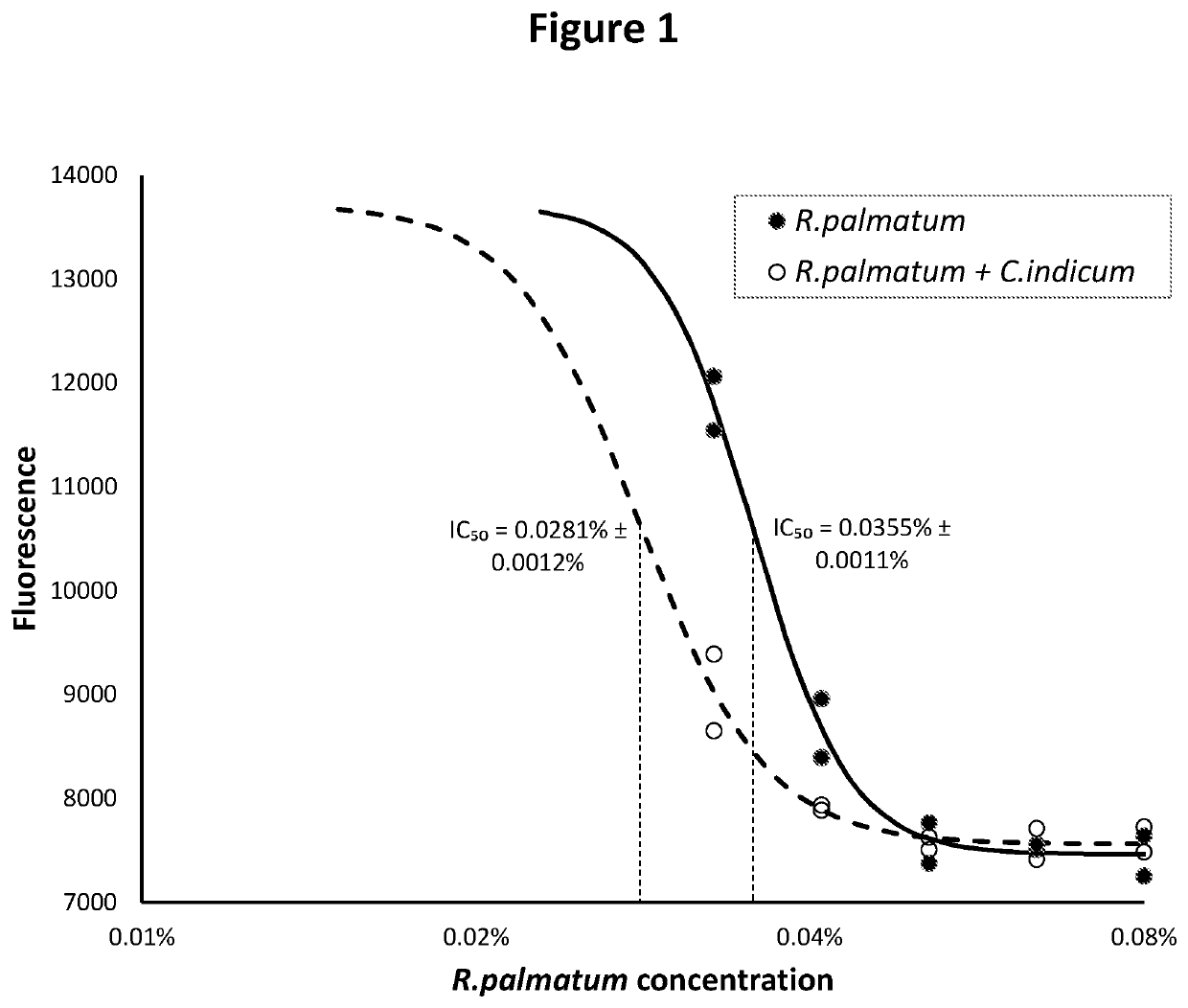
Herbal extracts for treatment of herpesvirus infections
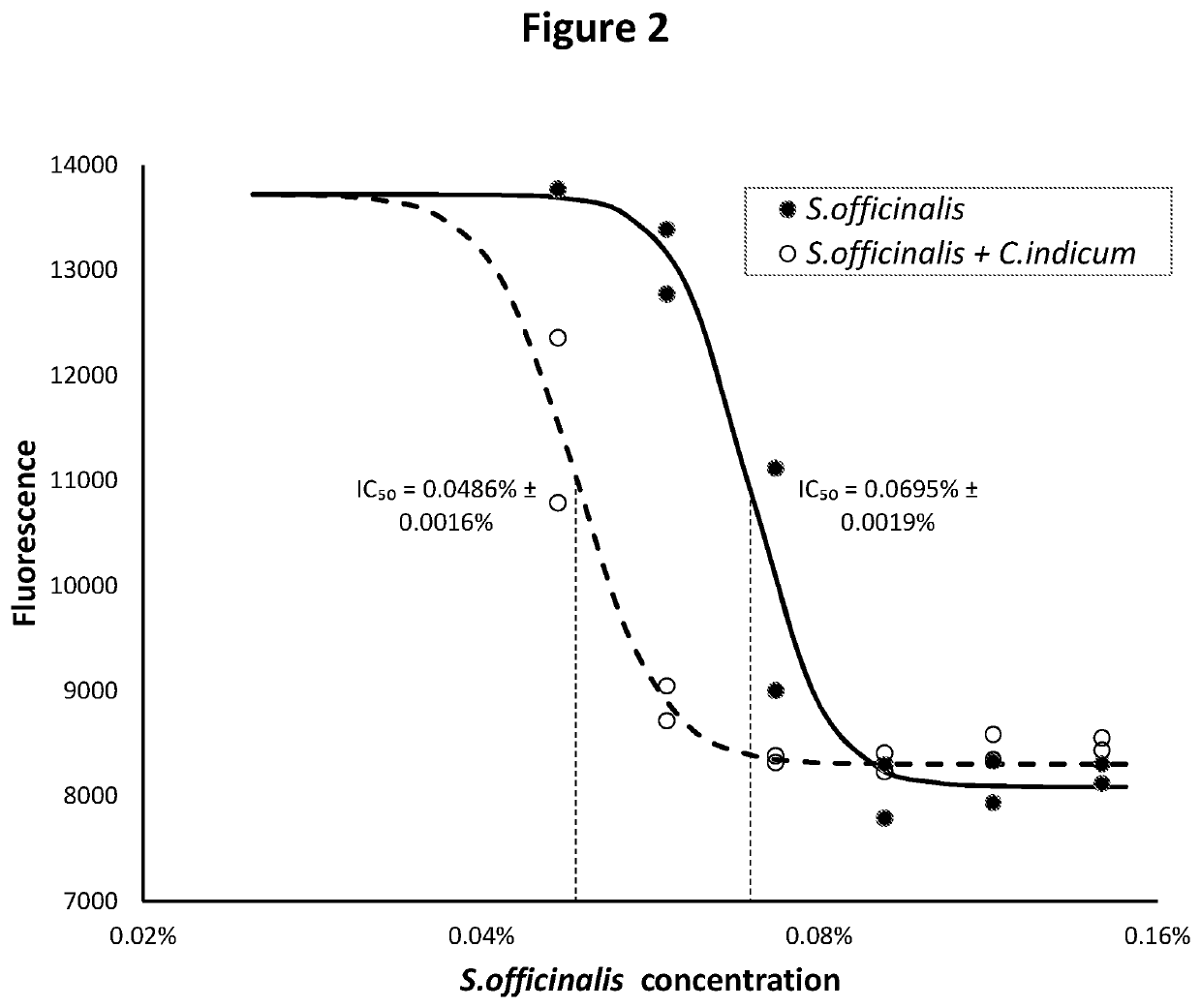
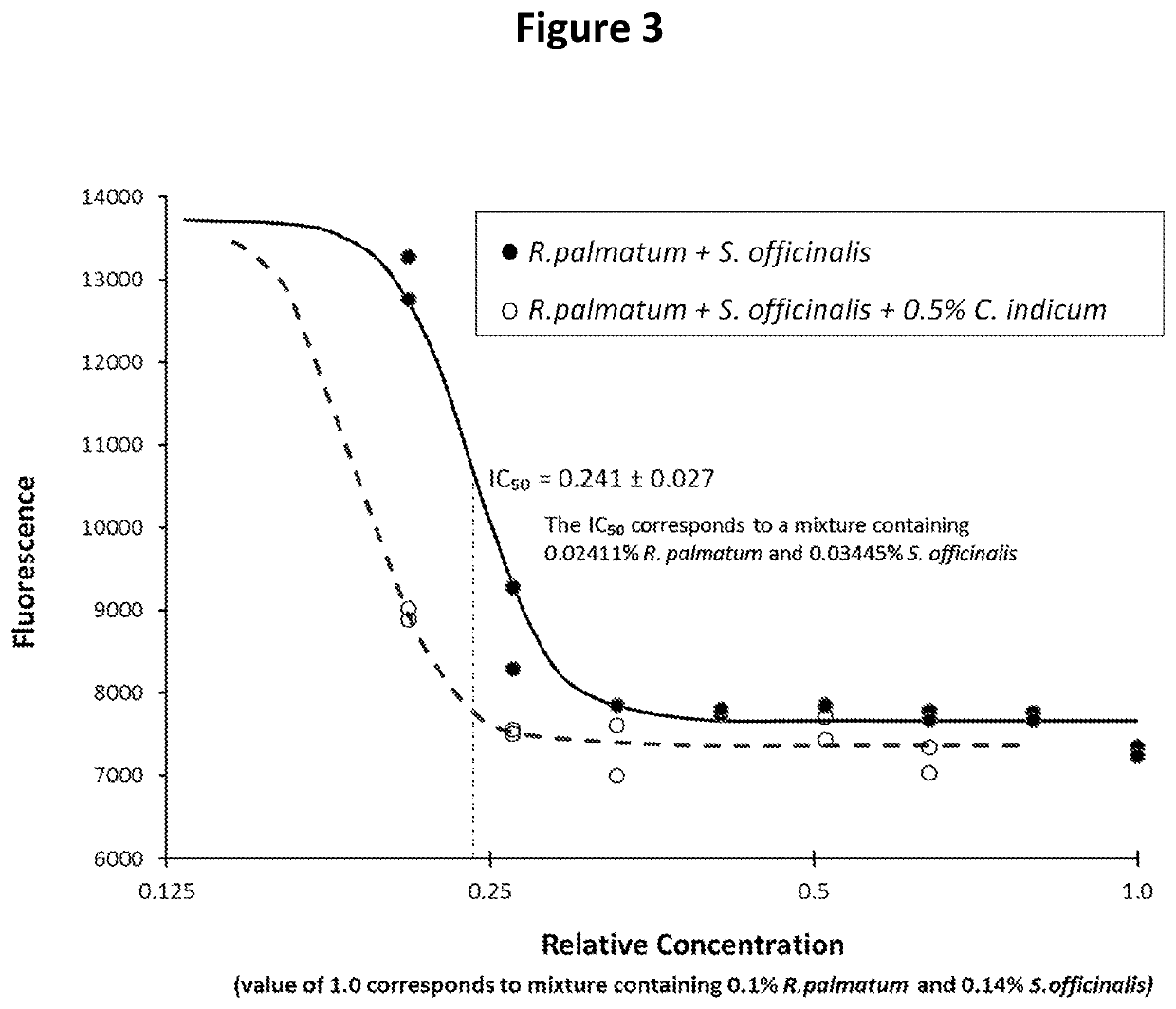
Disclosed is a formulation comprising at least two of Rheum palmatum, Chrysanthemum indicum and Sanguisorba officinalis extracts or prevalent molecules therefrom and a pharmaceutically acceptable carrier. Further disclosed is a formulation comprising one or more of Rheum palmatum, Chrysanthemum indicum and Sanguisorba officinalis extracts, or prevalent molecules therefrom in combination with acyclovir and a pharmaceutically acceptable carrier. Disclosed also is a method for treating herpesvirus infections by administering a formulation comprising extracts of Rheum palmatum, Chrysanthemum indicum or Sanguisorba officinalis extracts or prevalent molecules therefrom with or without an antiviral agent, such as acyclovir and a pharmaceutically acceptable carrier.
Owner:KAMEDIS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com