Microemulsion-based gel pharmaceutical composition and preparation method thereof
A microemulsion gel and drug technology, applied in the field of biomedicine, can solve the problems of high irritation and no targeting effect on dermal lesions, achieve high drug retention, facilitate drug efficacy, and increase drug retention Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1: the preparation of drug microemulsion solution
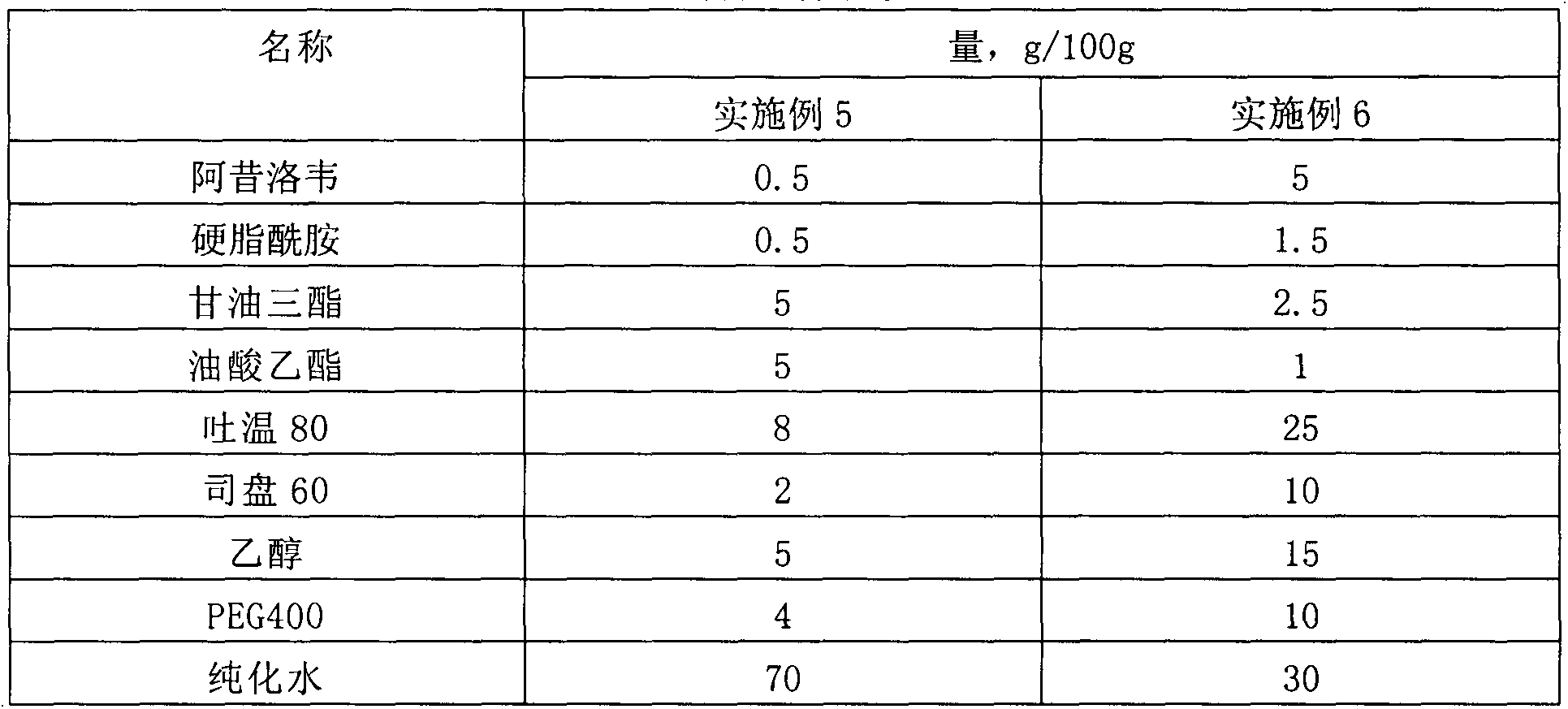
[0026] Table 1 Prescription table of drug microemulsion solution
[0027] name Quantity, g / 100g Aciclovir 2 Stearamide 1 medium chain fatty acid triglycerides 3 castor oil 4 Tween 80 20 Span 60 5 PEG400 10 glycerin 5 pH7.4 Phosphate Buffer 50
[0028] Preparation process: 1) Take medium-chain fatty acid triglycerides, castor oil, and stearamide in a water bath and heat to 60°C-80°C, add acyclovir, Tween 80, and Span 60 until dissolved; 2) Take another PEG400, Glycerin is added to pH 7.4 phosphate buffer solution to dissolve; 3) Under continuous stirring, add the water phase to the oil phase, and keep stirring until clear and transparent.
[0029] The encapsulation efficiency in the obtained drug microemulsion solution is 95.7%; the average particle size is 34.7nm.
Embodiment 2
[0030] Embodiment 2: the preparation of drug microemulsion solution
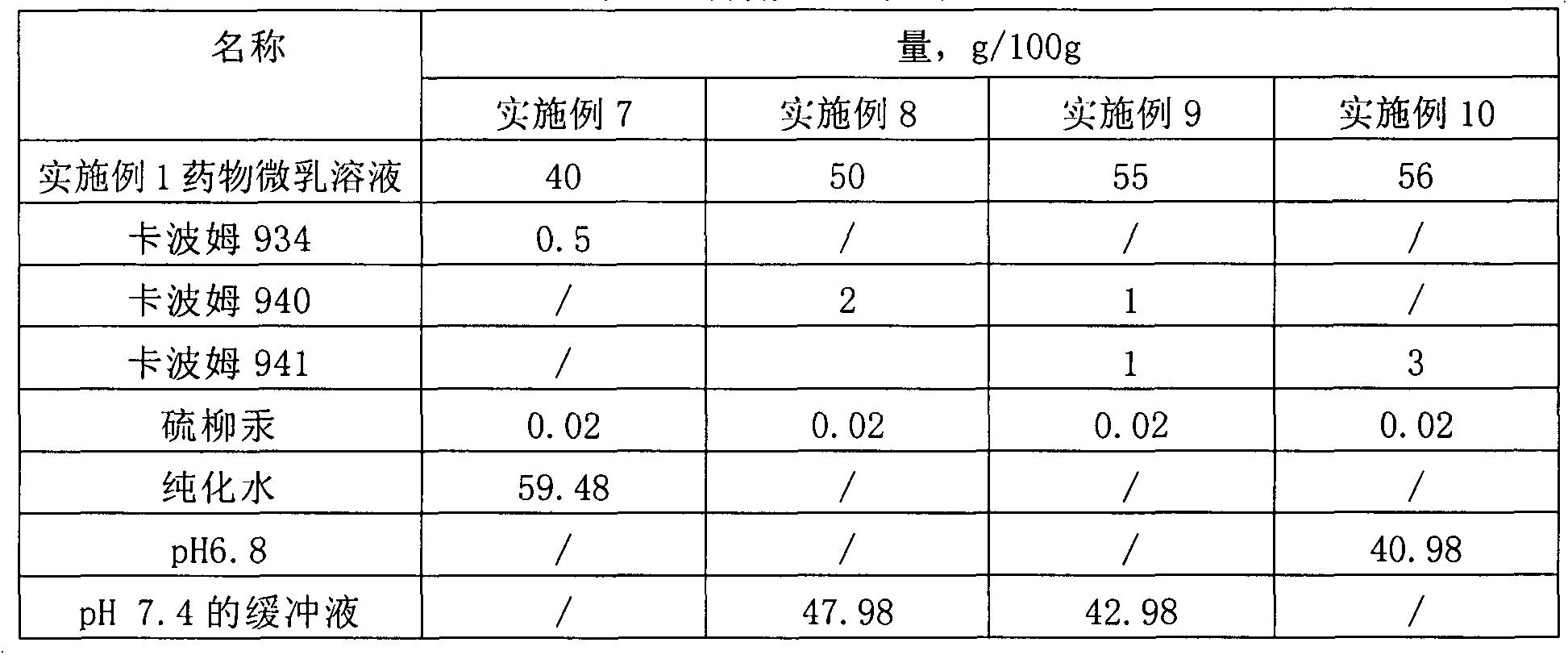
[0031] Table 2 Prescription table of drug microemulsion solution
[0032] name Quantity, g / 100g Aciclovir 2 Stearamide 1 medium chain fatty acid triglycerides 3 vegetable oil 4 Tween 80 20 Span 60 5 PEG400 10 glycerin 5 pH7.4 Phosphate Buffer 50
[0033] Preparation process: 1) Take medium-chain fatty acid triglycerides, vegetable oil, and stearamide in a water bath and heat to 60°C-80°C, add acyclovir, Tween 80, and Span 60 until dissolved; 2) Take another PEG400, Glycerin is added to pH 7.4 phosphate buffer solution to dissolve; 3) Under continuous stirring, add the water phase to the oil phase, and keep stirring until clear and transparent.
[0034] The encapsulation efficiency in the obtained drug microemulsion solution is 96.3%; the average particle size is 33.9nm.
Embodiment 3
[0035] Embodiment 3: the preparation of drug microemulsion solution
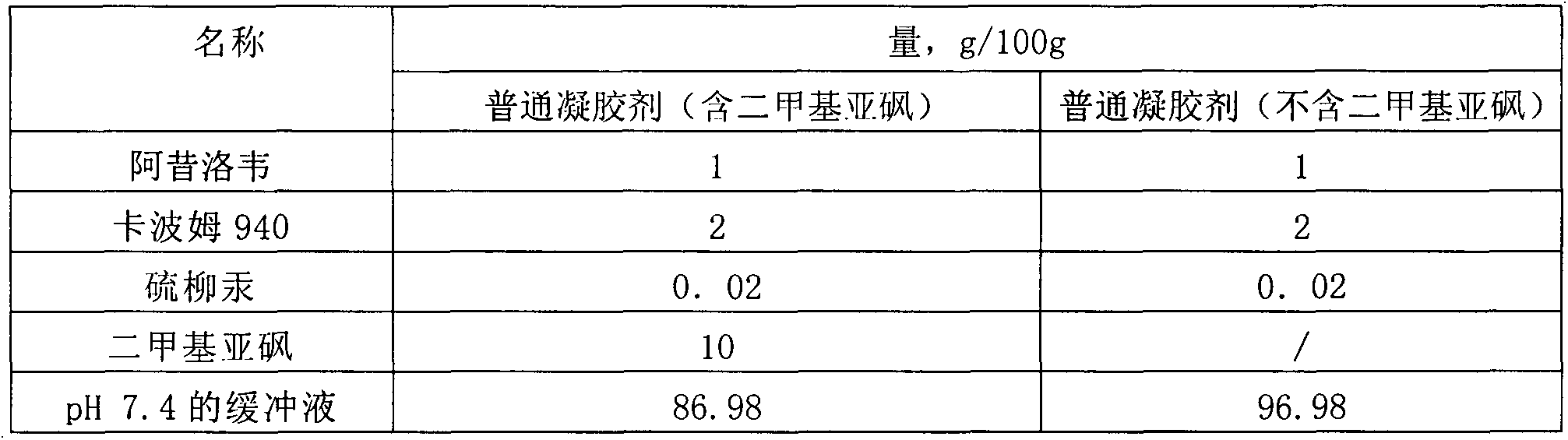
[0036] Table 3 Prescription table of drug microemulsion solution
[0037] name Quantity, g / 100g Aciclovir Palmitate 1 Stearamide 0.5 Isopropyl Laurate 3 Isopropyl myristate 2 Tween 80 10 Lecithin 10 ethanol 5 Propylene Glycol 5 pH6.8 Phosphate Buffer 63.5
[0038]Preparation process: 1) Take isopropyl laurate, isopropyl myristate, and stearamide in a water bath and heat to 60°C-80°C, add acyclovir palmitate, Tween 80, and lecithin until dissolved; 2 ) Add ethanol and propylene glycol to pH 6.8 phosphate buffer to dissolve; 3) Under continuous stirring, add the water phase to the oil phase, and keep stirring until it is clear and transparent.
[0039] The encapsulation efficiency in the obtained drug microemulsion solution is 86.5%; the average particle size is 43.2nm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Average particle size | aaaaa | aaaaa |
| Average particle size | aaaaa | aaaaa |
| Average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com