Preparation method for acyclovir emulsifiable paste
A technology of acyclovir cream and total amount, which is applied in the direction of ointment delivery, emulsion delivery, skin diseases, etc., to achieve good patient tolerance, convenient medication, and good quality stability
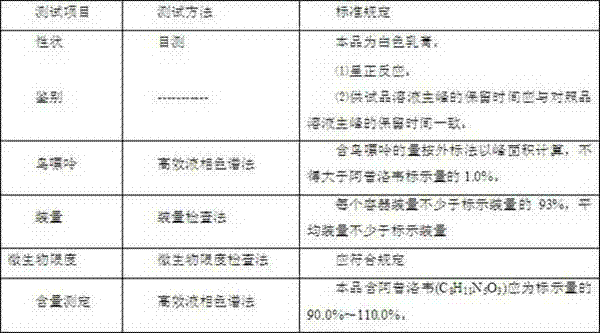
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
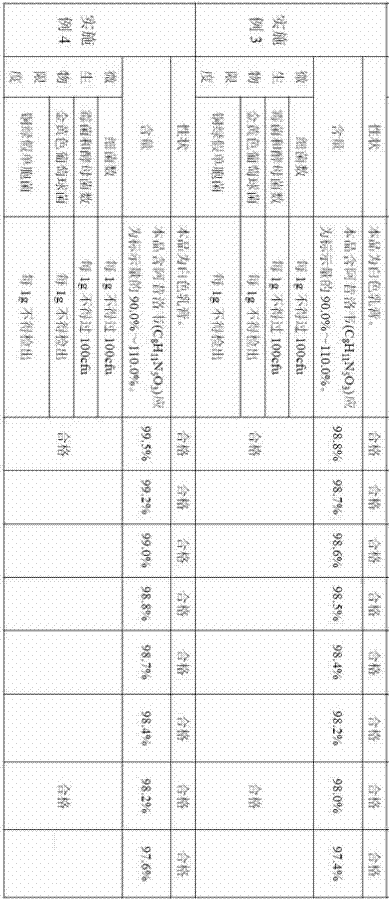
Embodiment 1
[0017] A preparation method of acyclovir cream, comprising the steps of:
[0018] 1) Prepare a total of 100g of the following raw materials: 2g of acyclovir, 4g of cetostearyl alcohol, 2g of glyceryl monostearate, 4g of white petrolatum, 4g of glycerin, 0.5g of sodium lauryl sulfate, hydroxybenzene 0.2 g of ethyl ester, 0.5 g of dimethyl sulfoxide, 0.2 g of 5 wt % benzalkonium bromide solution, and the balance is purified water.
[0019] 2) Prepare the water phase: Put the water phase materials glycerin, sodium lauryl sulfate, ethylparaben, dimethyl sulfoxide, purified water, 5wt% benzalkonium bromide solution and acyclovir into the water phase in proportion In the tank, stir at 90°C for 20 minutes until uniform, then set aside;
[0020] 3) Prepare the oil phase: add cetostearyl alcohol, glyceryl monostearate and white petrolatum into the oil phase tank in proportion to the oil phase materials, stir at 82°C for 20 minutes until uniform, and set aside;
[0021] 4) Preparation...
Embodiment 2
[0023] A preparation method of acyclovir cream, comprising the steps of:
[0024] 1) Prepare a total of 100g of the following raw materials: 3g of acyclovir, 6g of cetostearyl alcohol, 5g of glyceryl monostearate, 6g of white petrolatum, 6g of glycerin, 1g of sodium lauryl sulfate, ethyl hydroxybenzoate 0.3 g of ester, 1 g of dimethyl sulfoxide, 0.5 g of 5 wt % benzalkonium bromide solution, and the balance is purified water.
[0025] 2) Prepare the water phase: Put the water phase materials glycerin, sodium lauryl sulfate, ethylparaben, dimethyl sulfoxide, purified water, 5wt% benzalkonium bromide solution and acyclovir into the water phase in proportion In the tank, stir at 95°C for 25 minutes until uniform, then set aside;
[0026] 3) Prepare the oil phase: add cetostearyl alcohol, glyceryl monostearate and white petrolatum into the oil phase tank in proportion to the oil phase materials, stir at 85°C for 25 minutes until uniform, and set aside;
[0027] 4) Preparation of...
Embodiment 3
[0029] A preparation method of acyclovir cream, comprising the steps of:
[0030] 1) Prepare a total of 100g of the following raw materials: 4g of acyclovir, 8g of cetostearyl alcohol, 8g of glyceryl monostearate, 8g of white petrolatum, 8g of glycerin, 1.5g of sodium lauryl sulfate, hydroxybenzene 0.4 g of ethyl ester, 1.5 g of dimethyl sulfoxide, 0.8 g of 5 wt % benzalkonium bromide solution, and the balance is purified water.
[0031] 2) Prepare the water phase: Put the water phase materials glycerin, sodium lauryl sulfate, ethylparaben, dimethyl sulfoxide, purified water, 5wt% benzalkonium bromide solution and acyclovir into the water phase in proportion In the tank, stir at 95°C for 20 minutes until uniform, then set aside;
[0032] 3) Prepare the oil phase: add cetostearyl alcohol, glyceryl monostearate and white petrolatum into the oil phase tank in proportion to the oil phase materials, stir at 87°C for 20 minutes until uniform, and set aside;
[0033] 4) Preparation...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com