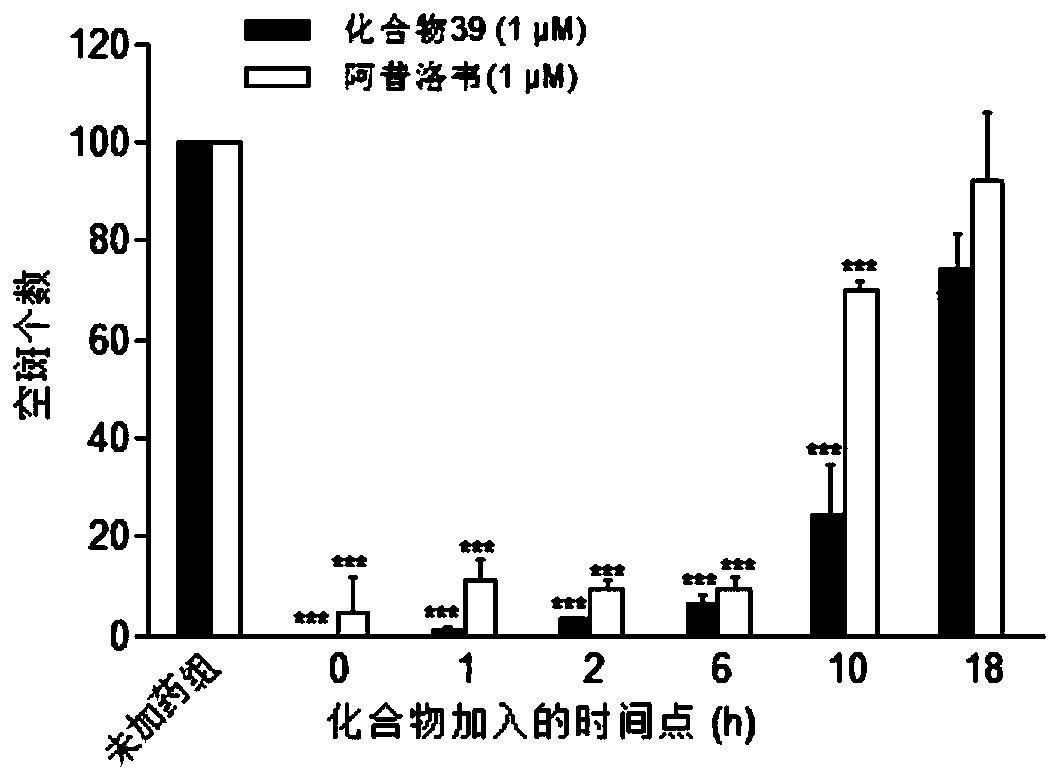
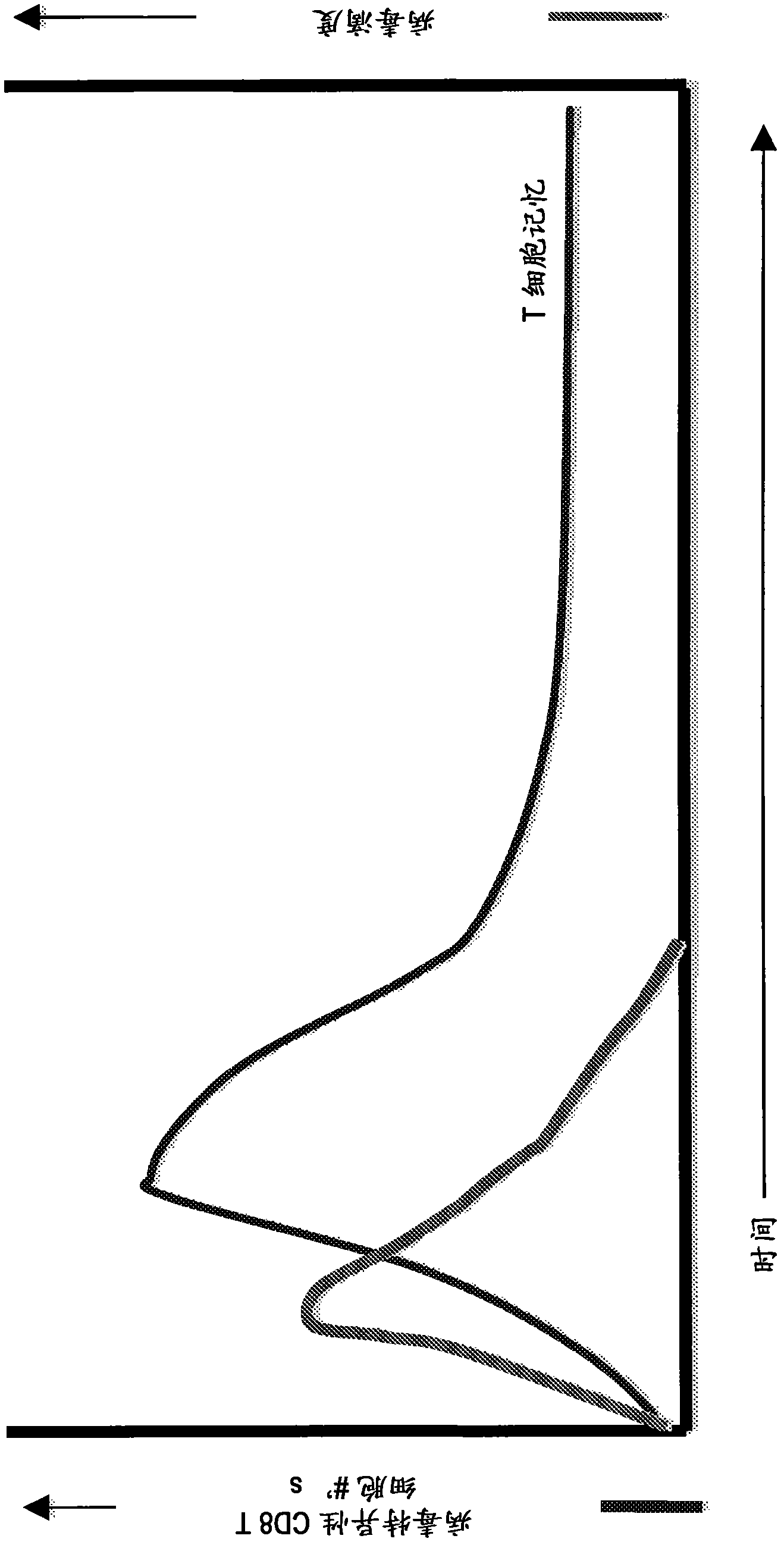
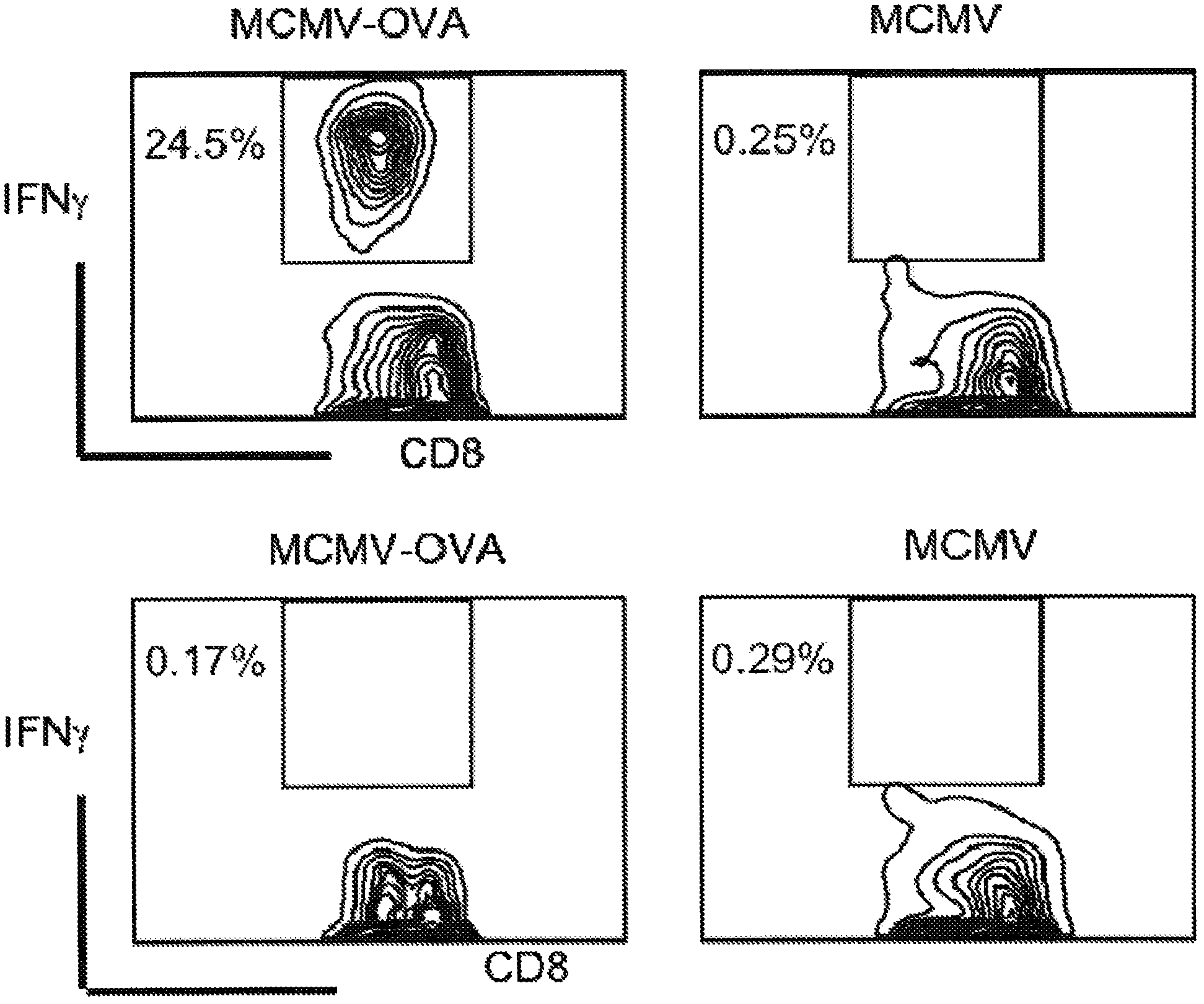
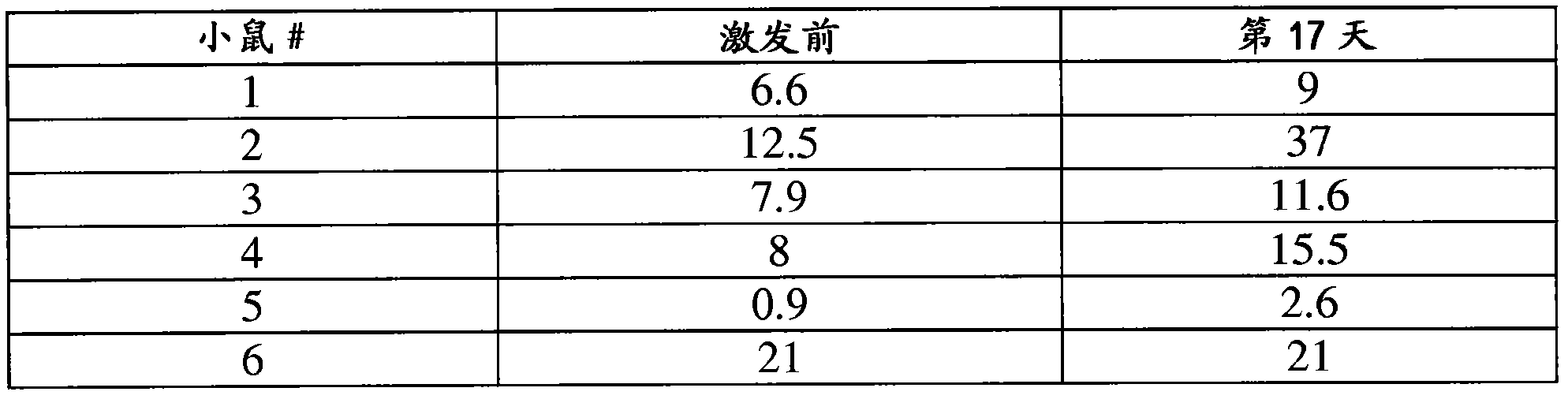
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
57 results about "Cmv cytomegalovirus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cytomegalovirus (CMV) belongs to the herpes virus family. Infection with CMV is very common. Between 50% and 80% of people in the United States have had a CMV infection by the time they are 40 years according to the Centers for Disease Control and Prevention (CDC).
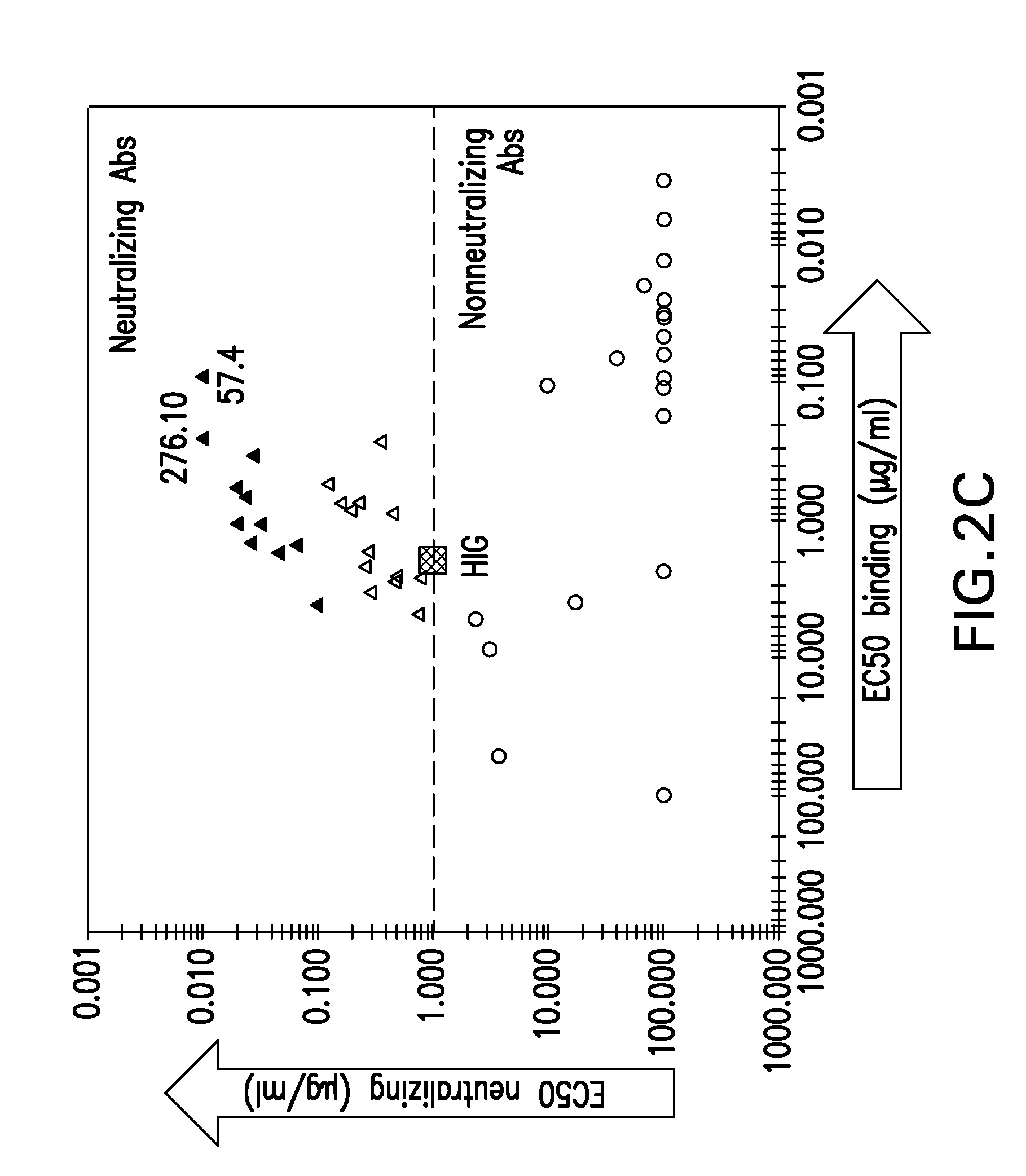
Human monoclonal antibody binding to human cytomegalovirus and its antigen binding portion
The present invention aims to provide a human monoclonal antibody and an antigen binding portion of a human monoclonal antibody with higher affinity and neutralizing capacity to the human cytomegalovirus (HCMV), a virus which causes various diseases in situations where immunodeficiencies are present. The current invention provides an anti-human cytomegalovirus (HCMV) monoclonal antibody which is a human monoclonal antibody capable of binding to HCMV and neutralizing bioactivity of the HCMV, and which may be further characterized as possessing a light chain (L chain) comprising an amino acid sequence of SEQ ID. NO. 1, and has a heavy chain (H chain) comprising an amino acid sequence of SEQ ID NO. 2.
Owner:EVEC +1
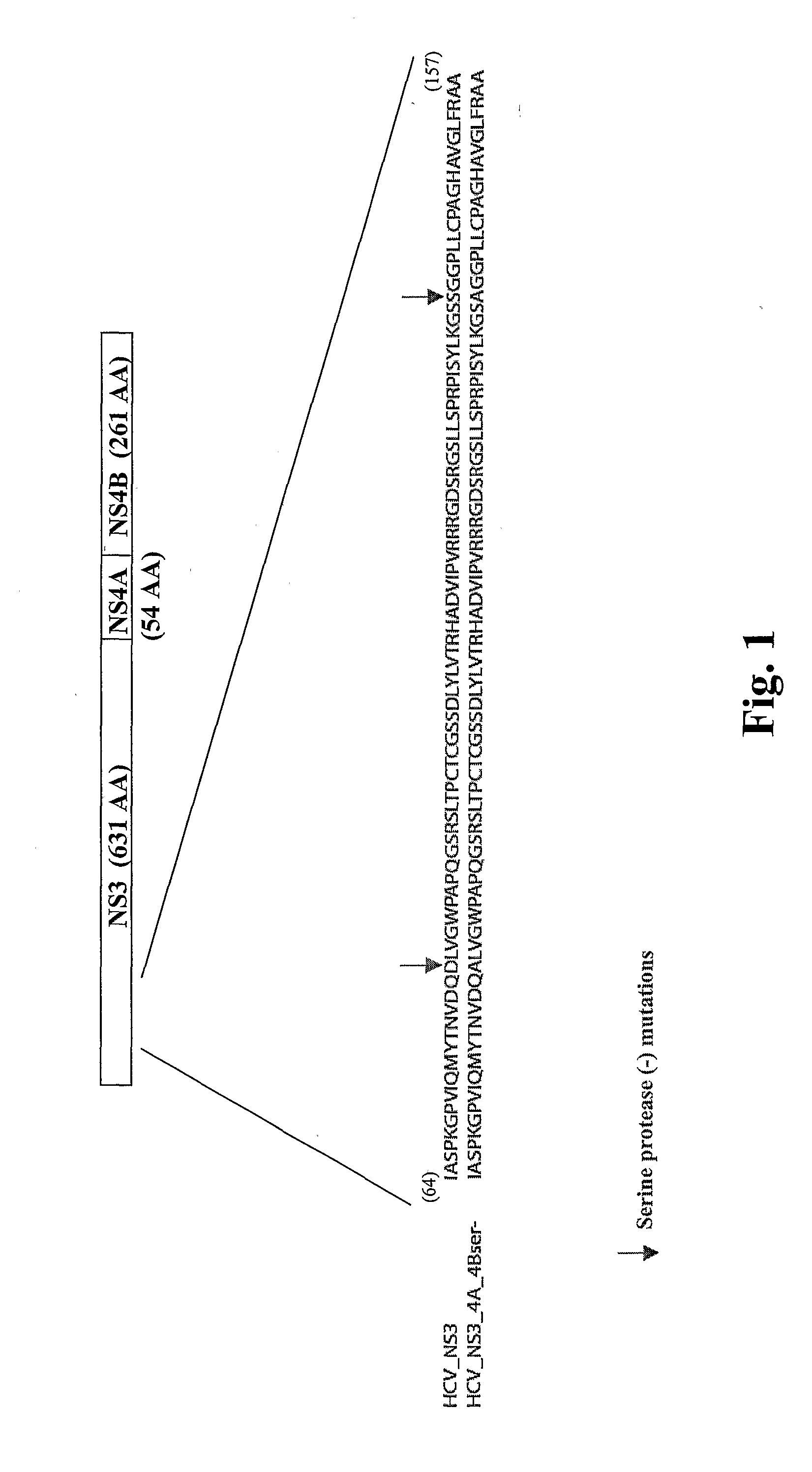
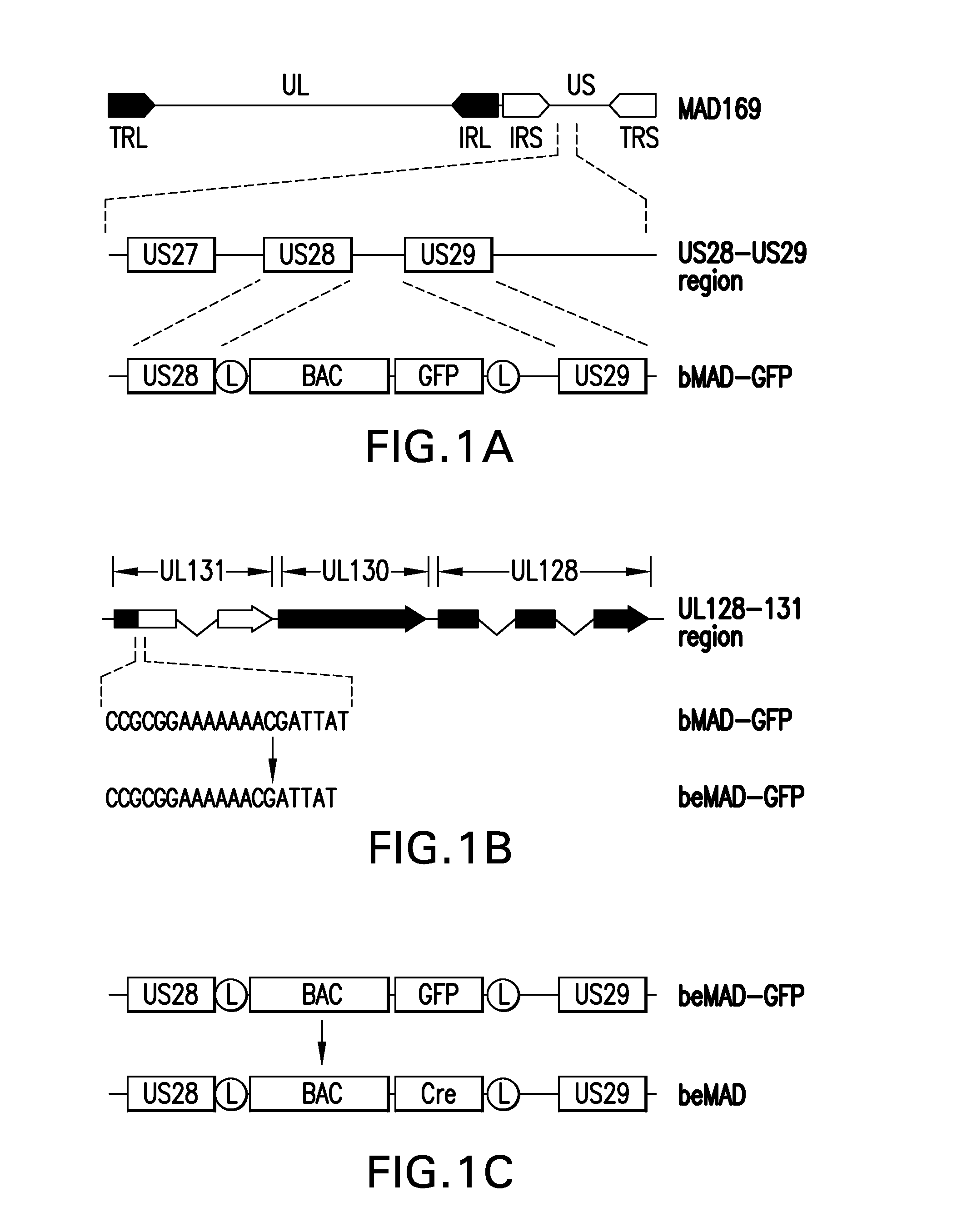
Cytomegalovirus gene function and methods for developing antivirals, anti-CMV vaccines, and CMV-based vectors
ActiveUS20050064394A1Enhance their long-term survivabilitySugar derivativesMicrobiological testing/measurementSurvivabilityORFS
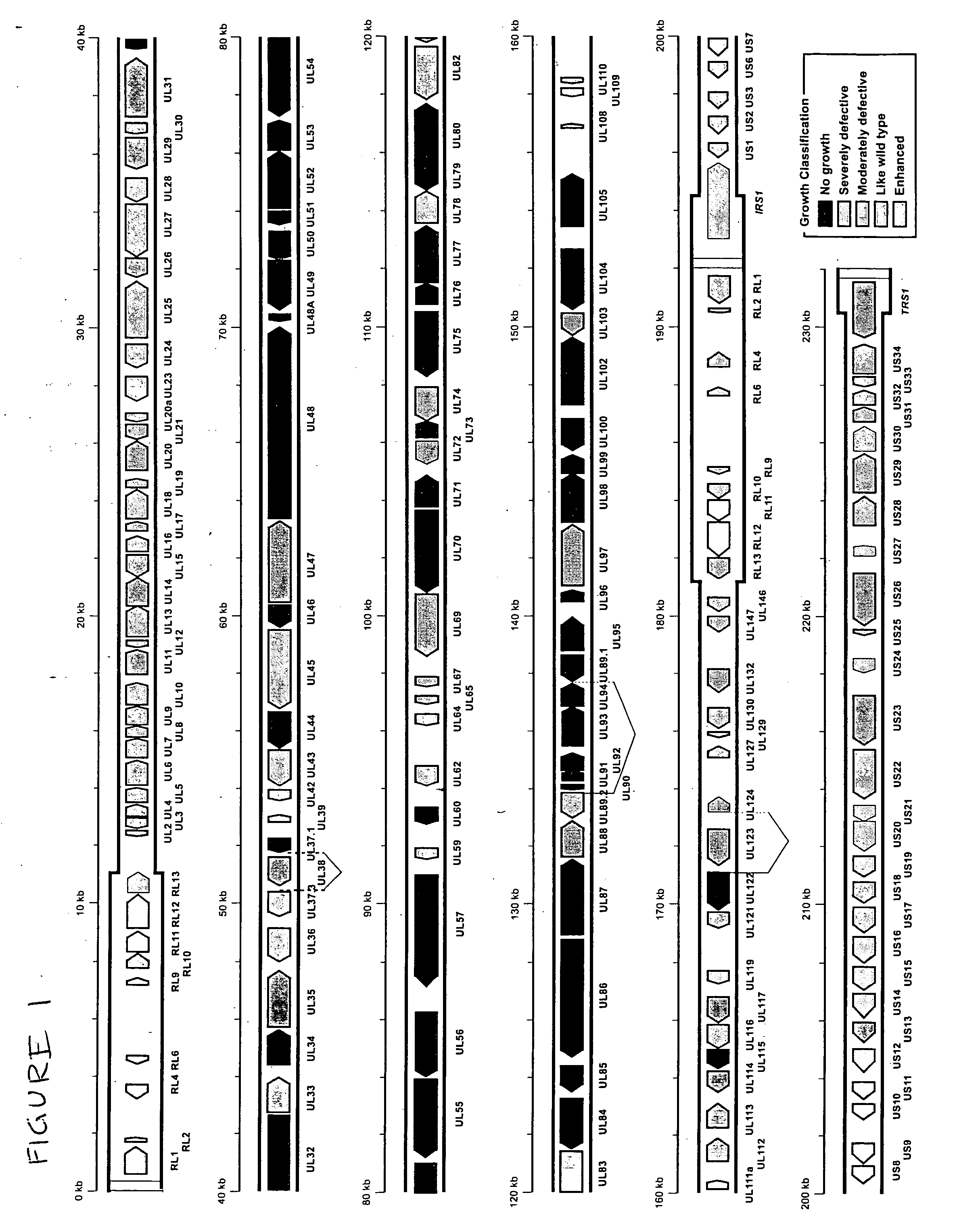
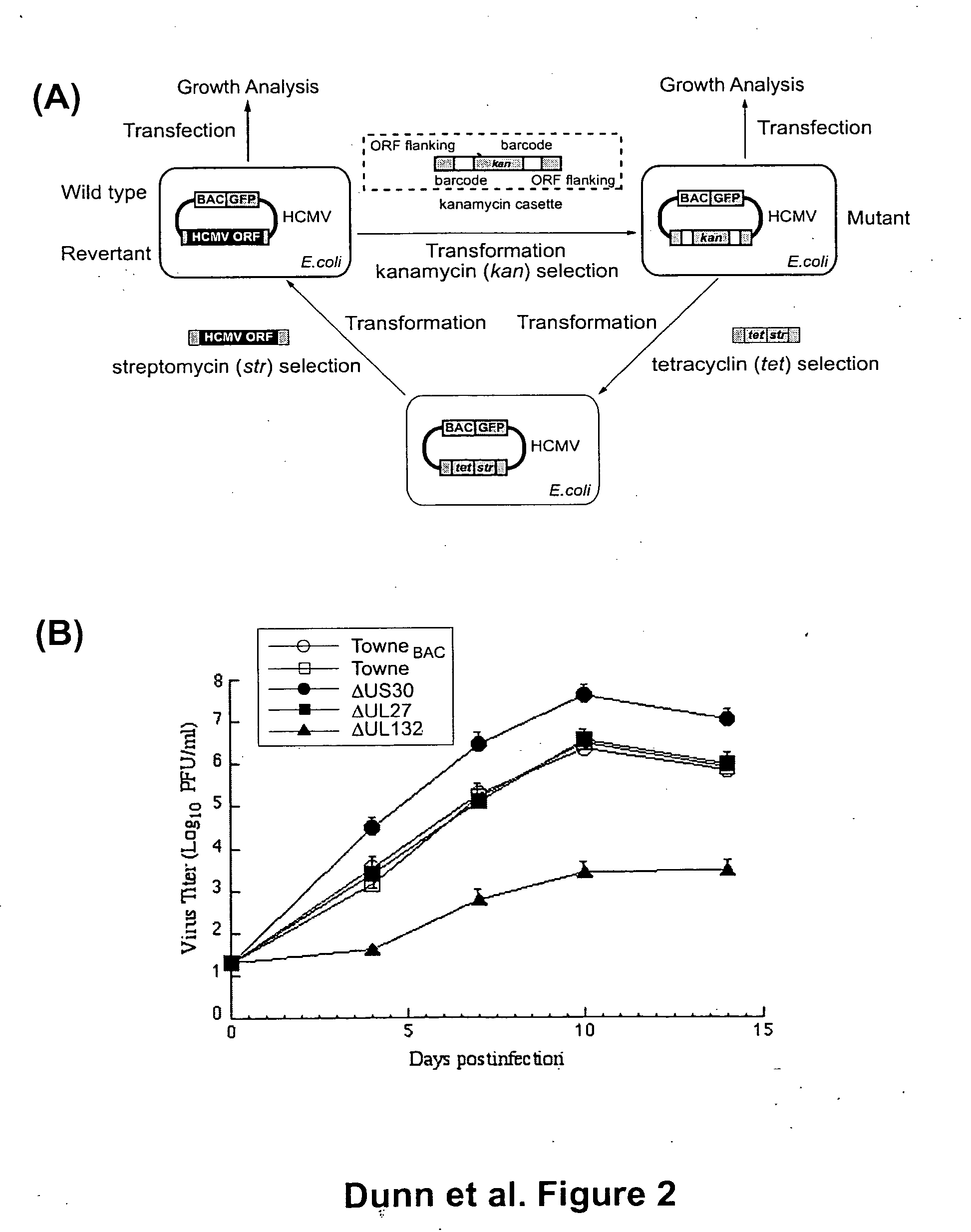
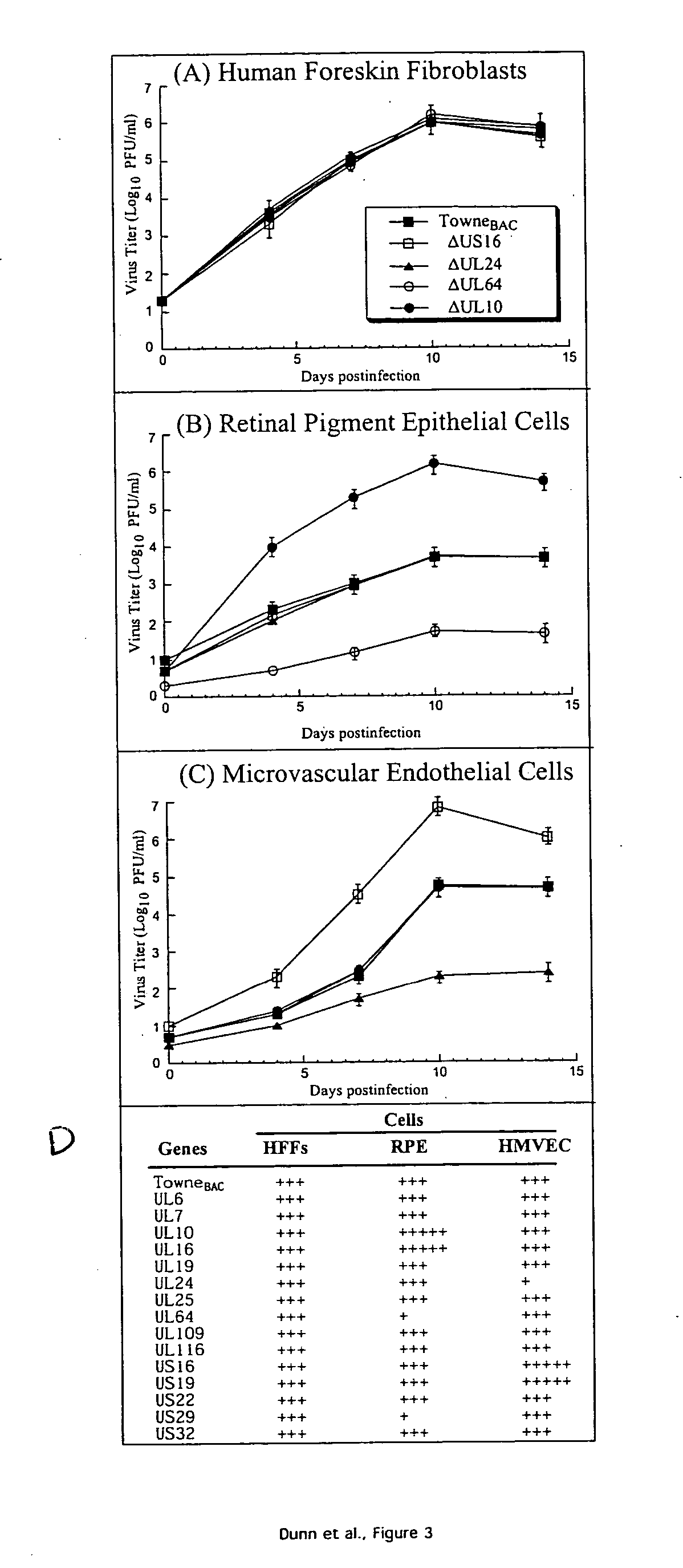
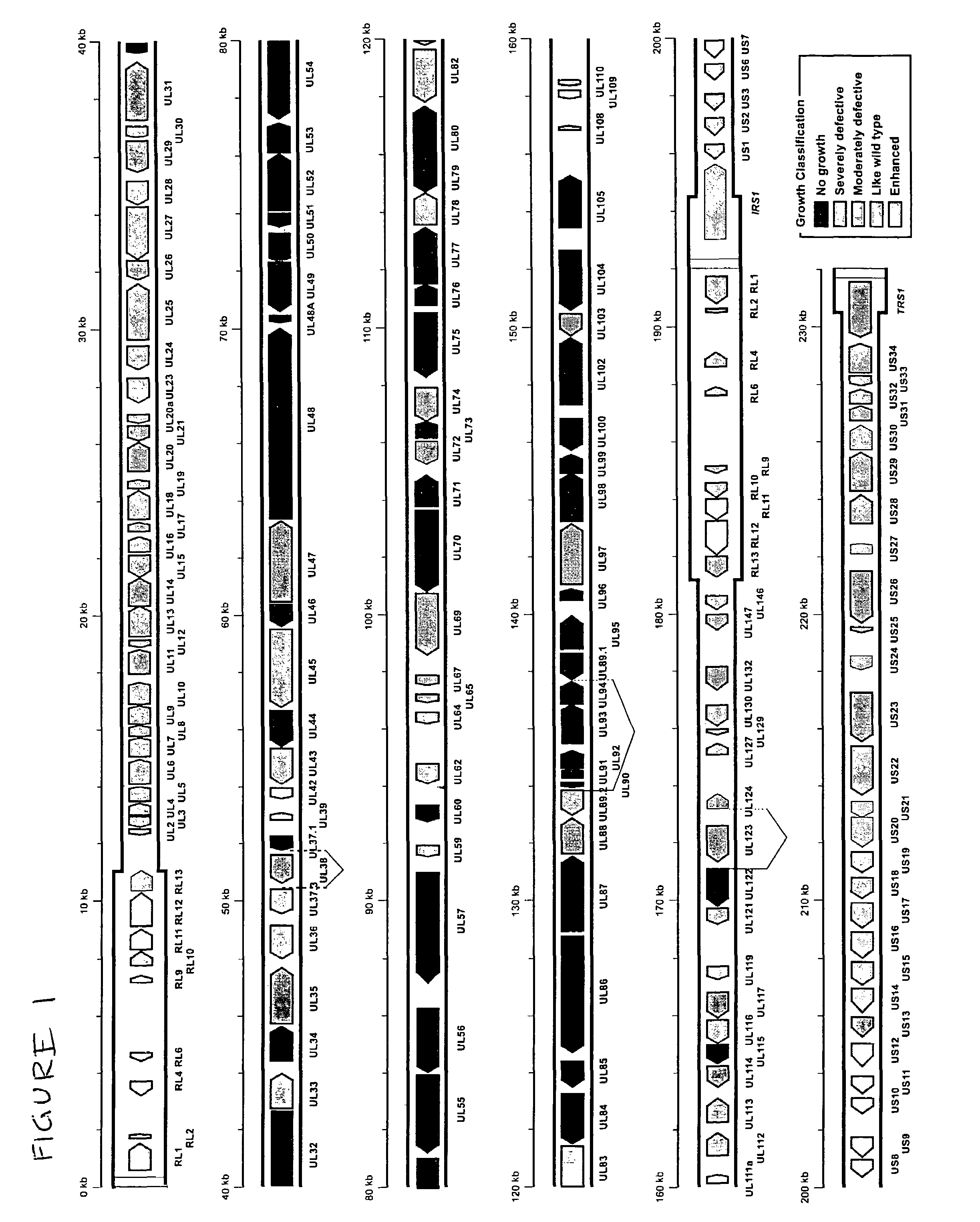
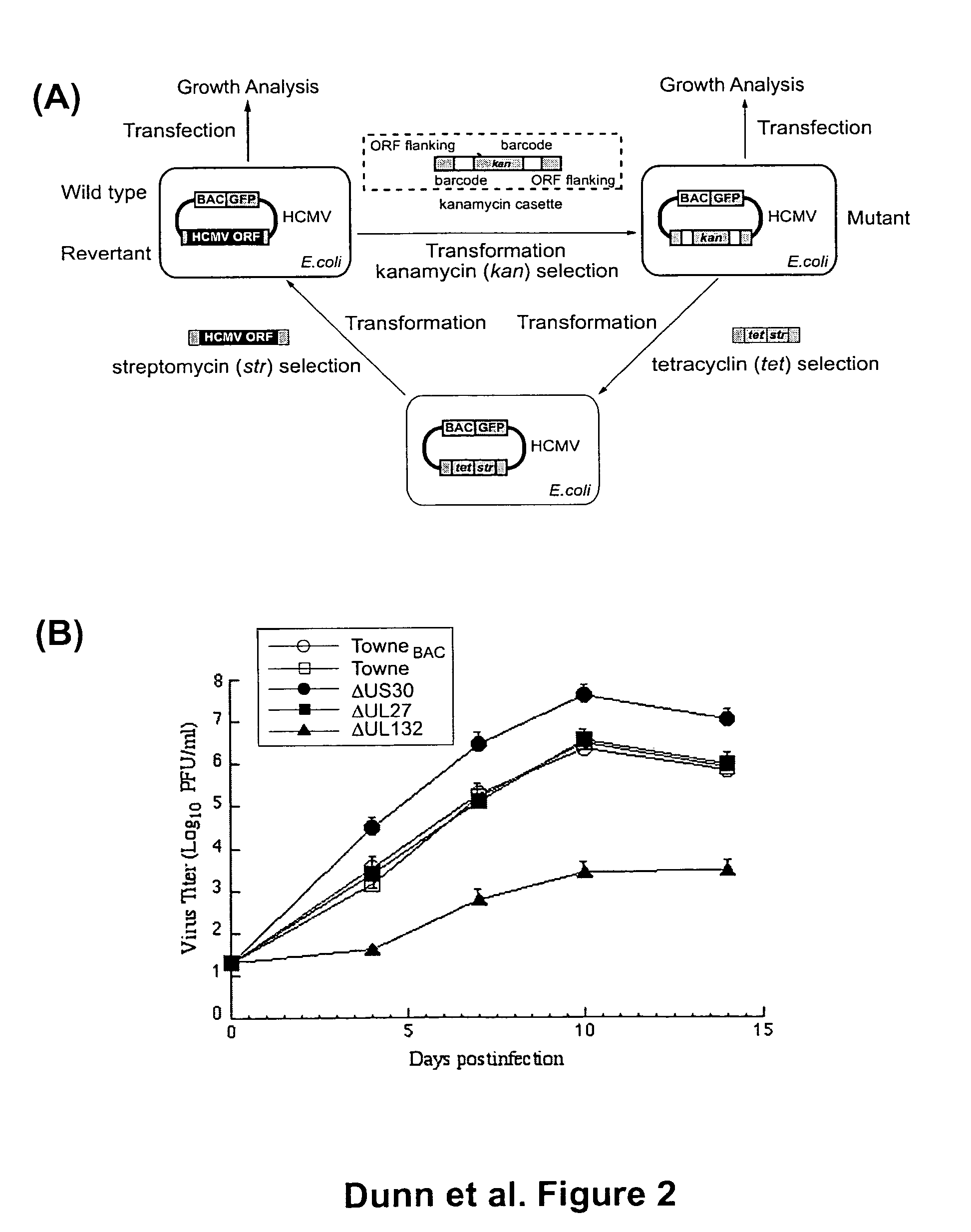
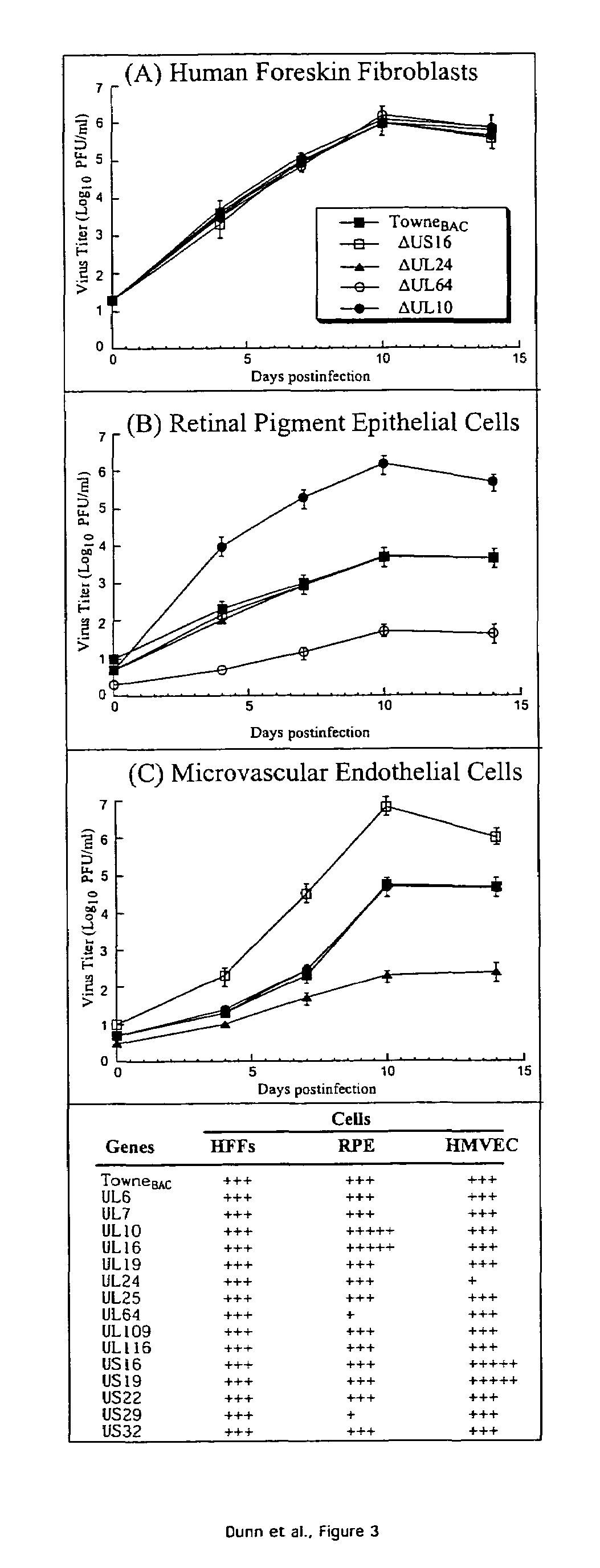
A global functional analysis of HCMV genes is performed by constructing virus gene-deletion mutants and examining their growth phenotypes in different natural HCMV host cells. This systematic analysis of the HCMV genome identified 45 viral ORFs essential for viral replication and characterizes of 115 growth-dispensable viral genes. Of particular interest is the finding that HCMV encodes genes (temperance factors) that repress its own replication on a cell type-specific basis. In addition to HCMV, pathogen temperance may be a strategy employed by other infectious agents to enhance their long-term survivability within their respective host population.
Owner:RGT UNIV OF CALIFORNIA
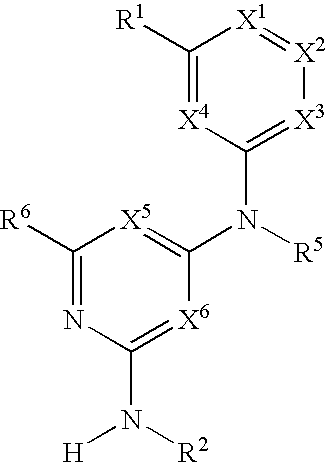
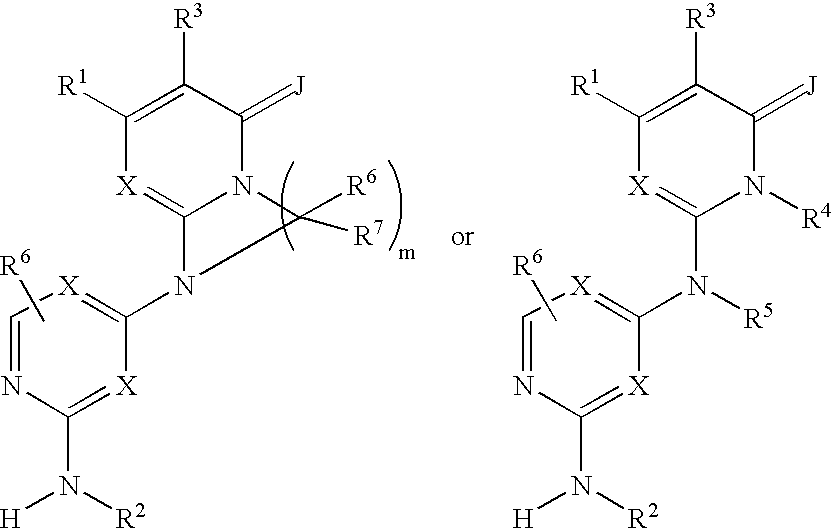
Substituted heterocyclic compounds and methods of use
The present invention relates to pyridines, pyrimidines and derivatives thereof, and pharmaceutically acceptable salts thereof. Also included is a method of treatment of inflammation, rheumatoid arthritis, Pagets disease, osteoporosis, multiple myeloma, uveititis, acute or chronic myelogenous leukemia, pancreatic β cell destruction, osteoarthritis, rheumatoid spondylitis, gouty arthritis, inflammatory bowel disease, adult respiratory distress syndrome (ARDS), psoriasis, Crohn's disease, allergic rhinitis, ulcerative colitis, anaphylaxis, contact dermatitis, asthma, muscle degeneration, cachexia, Reiter's syndrome, type I diabetes, type II diabetes, bone resorption diseases, graft vs. host reaction, Alzheimer's disease, stroke, myocardial infarction, ischemia reperfusion injury, atherosclerosis, brain trauma, multiple sclerosis, cerebral malaria, sepsis, septic shock, toxic shock syndrome, fever, myalgias due to HIV-1, HIV-2, HIV-3, cytomegalovirus (CMV), influenza, adenovirus, the herpes viruses or herpes zoster infection in a mammal comprising administering an effective amount a compound as described above.
Owner:AMGEN INC
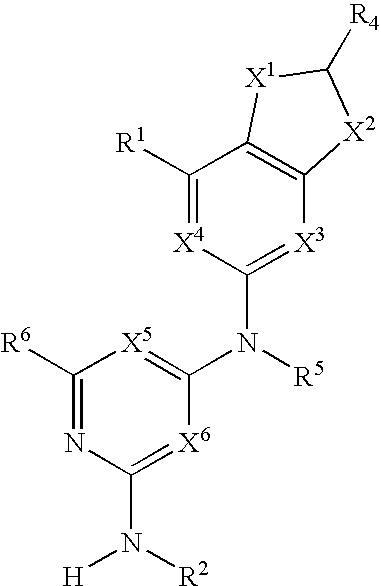
Substituted heterocyclic compounds and methods of use
The present invention relates to compounds having the general structure: and pharmaceutically acceptable salts and hydrates thereof. Also included is a method of treatment of inflammation, rheumatoid arthritis, Pagets disease, osteoporosis, multiple myeloma, uveititis, acute or chronic myelogenous leukemia, pancreatic β cell destruction, osteoarthritis, rheumatoid spondylitis, gouty arthritis, inflammatory bowel disease, adult respiratory distress syndrome (ARDS), psoriasis, Crohn's disease, allergic rhinitis, ulcerative colitis, anaphylaxis, contact dermatitis, asthma, muscle degeneration, cachexia, Reiter's syndrome, type I diabetes, type II diabetes, bone resorption diseases, graft vs. host reaction, Alzheimer's disease, stroke, myocardial infarction, ischemia reperfusion injury, atherosclerosis, brain trauma, multiple sclerosis, cerebral malaria, sepsis, septic shock, toxic shock syndrome, fever, myalgias due to HIV-1, HIV-2, HIV-3, cytomegalovirus (CMV), influenza, adenovirus, the herpes viruses or herpes zoster infection in a mammal comprising administering an effective amount a compound as described above.
Owner:AMGEN INC
HCMVPP65 antigenemia indirect immunofluorescence method detection reagent kit
InactiveCN101261272AImprove featuresIncreased sensitivityFluorescence/phosphorescenceBALB/cFluorescence
The invention discloses a HCMVPP65 antigenemia indirect immune fluorescence method detection kit, which uses cytomegalovirus-AD169 virus strain pp65 protein as the immunogen to immune a Balb / c mouse. The spleen cells of the immunized mouse and the myeloma cells of the mouse which belongs to the same type with the immune mouse are conventionally integrated, by indirect ELISA screening and finite dilution cloning, the hybridoma cell lines of the mouse cytomegalovirus pp65 protein cloning antibody are obtained, and the characteristics of the hybridoma cell lines are identified by ELISA, immune fluorescence experiment and other methods; two monoclonal antibodies that stably secrete the pp65 protein are established successfully and named respectively as 1A6 and 4A8. A monoclonal antibody which differs from the former report and aims at the pp65 protein of the cytomegalovirus (CMV) is prepared and a method used for preparing erythrocyte fast pyrolysis is established; compared with other detection kits which belongs to the same kind, the detection kit of the invention is faster, simpler and more convenient and has higher specificity and sensitivity.
Owner:天津市秀鹏生物技术开发有限公司
Alphavirus vectors for paramyxovirus vaccines
InactiveUS6475780B1Reduce doseLess timeSsRNA viruses negative-senseOrganic active ingredientsDiseaseF protein
A DNA vector comprises a first DNA sequence which is complementary to at least part of an alphavirus RNA genome and having the complement of complete alphavirus DNA genome replication regions, and a second DNA sequence encoding a paramyxovirus protein, particularly a respiratory syncytial virus fusion (RSV F) protein or a RSV F protein fragment that generates antibodies that specifically react with RSV F protein, the first and second DNA sequences being under the transcriptional control of a promoter, preferably a cytomegalovirus promoter, which may include Intron A. Such vectors also contain a further nucleotide sequence located between the promoter sequence and the alphavirus sequence to enhance the immunoprotective ability of the RSV F protein when expressed in vivo. Such DNA vectors may be used to immunize a host against disease caused by infection with RSV or other paramyxovirus, including a human host, by administration thereto, and may be formulated as immunogenic compositions with pharmaceutically-acceptable carriers for such purposes. Such vectors also may be used to produce antibodies for detection of RSV or other paramyxovirus infection in a sample.
Owner:AVENTIS PASTUER LTD
Recombinant Human Cytomegalovirus And Vaccines Comprising Heterologous Antigens
The present invention relates to recombinant HCMV (human cytomegalovirus) expressing a pp65 polypeptide or fragment thereof fused to a heterologous or non-native polypeptide, in particular immunogenic and / or antigenic polypeptides. In particular, the heterologous gene products include antigenic or immunogenic polypeptides from a variety of pathogens, cellular genes, tumor antigens, and viruses. The recombinant viruses may advantageously be used in vaccine formulations including vaccines against a broad range of pathogens and antigens.
Owner:MEDIMMUNE LLC
Application of polycyclic polyketides in preparation of anti-HV (herpes virus) drug
The invention discloses an application of polycyclic polyketides in preparation of an anti-HV (herpes virus) drug. It is found that the polyketides can inhibit diseases caused by infection of four HVs including HSV-1 (herpes simplex virus-1), HSV-2 (herpes simplex virus-2), VZV (varicella zoster virus) and CMV (cytomegalo virus). The compounds show equivalent activity but have different acting mechanisms as compared with commercial drugs such as acyclovir and can overcome drug resistance of existing commercial drugs. Therefore, the compounds have good application prospects in treatment of related diseases caused by infection of HVs including HSV-1, HSV-2, VZV and CMV.
Owner:JINAN UNIVERSITY
Cytomegalovirus gb antigen
InactiveUS20130216613A1Elicit immune responseSugar derivativesViral antigen ingredientsAntigenCytomegalovirus disease
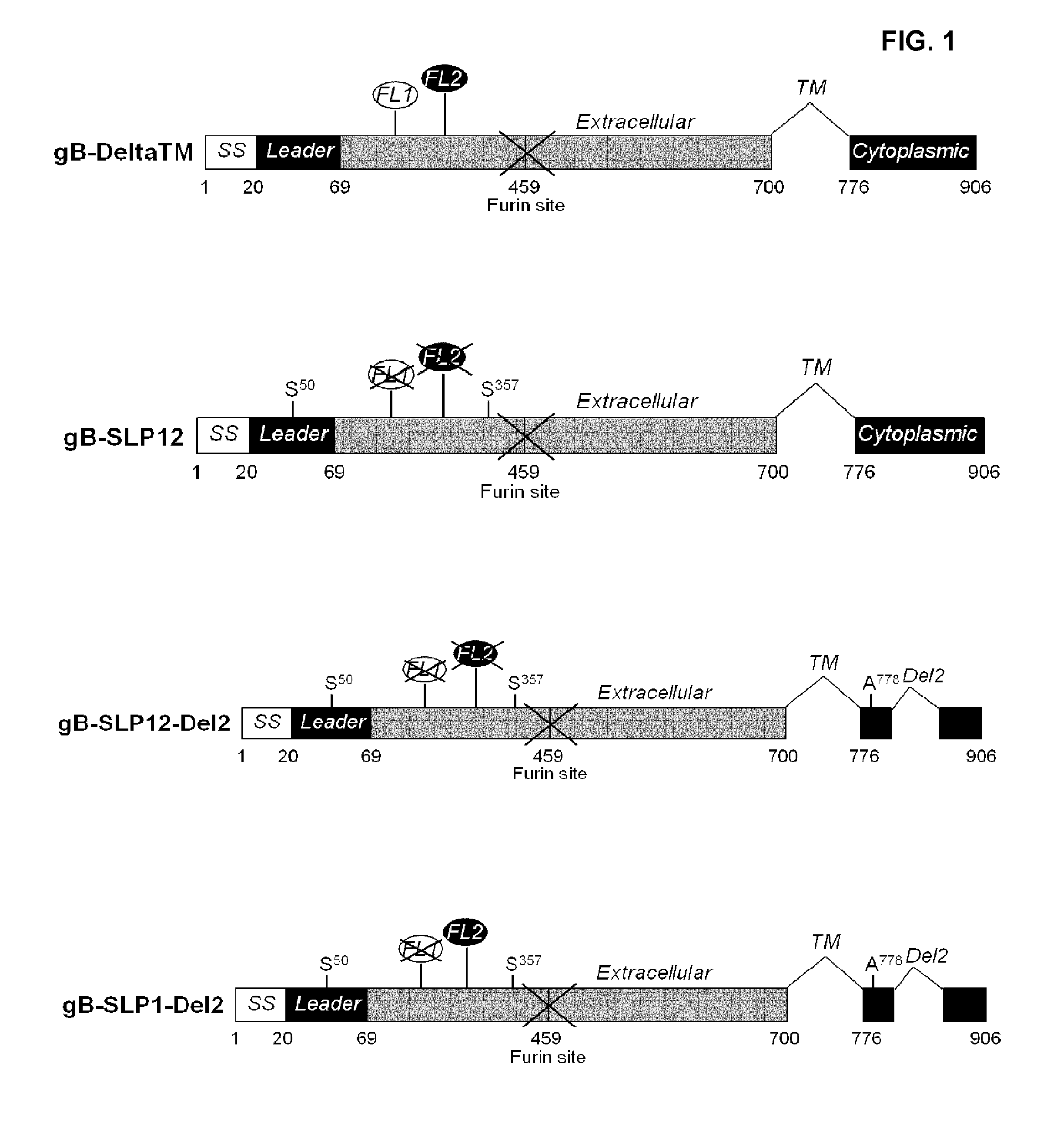
The invention relates to a cytomegalovirus (CMV) gB polypeptide comprising at least a portion of a gB protein extracellular domain comprising a fusion loop 1 (FL1) domain and a fusion loop 2 (FL2) domain, wherein at least one of the FL1 and FL2 domains comprises at least one amino acid deletion or substitution.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Cytomegalovirus gene function and methods for developing antivirals, anti-CMV vaccines, and CMV-based vectors
ActiveUS7407744B2Enhance their long-term survivabilitySugar derivativesMicrobiological testing/measurementSurvivabilityORFS
A global functional analysis of HCMV genes is performed by constructing virus gene-deletion mutants and examining their growth phenotypes in different natural HCMV host cells. This systematic analysis of the HCMV genome identified 45 viral ORFs essential for viral replication and characterizes of 115 growth-dispensable viral genes. Of particular interest is the finding that HCMV encodes genes (temperance factors) that repress its own replication on a cell type-specific basis. In addition to HCMV, pathogen temperance may be a strategy employed by other infectious agents to enhance their long-term survivability within their respective host population.
Owner:RGT UNIV OF CALIFORNIA
Cmv neutralizing antigen binding proteins
ActiveUS20160130327A1Increase activityHigh activityAnimal cellsBacteriaAntigen bindingHuman cytomegalovirus
The present invention is directed to antigen binding proteins including, but not limited to, monoclonal antibodies and antigen binding fragments thereof, that specifically bind to and preferably neutralize human cytomegalovirus (CMV). Also encompassed by the invention are antigen binding proteins that have been humanized. The antigen binding proteins of the invention are useful as a therapeutic agent for treating and / or preventing CMV infections in a patient in need thereof.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST +1
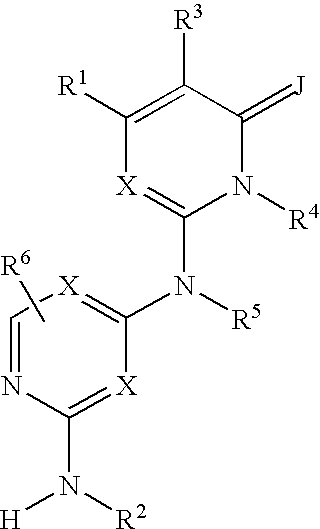
Substituted heterocyclic compounds and methods of use
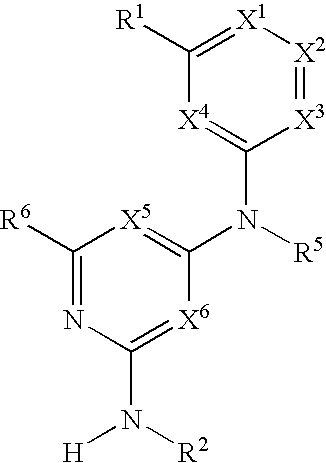
The present invention relates to compounds having the general formula or a pharmaceutically acceptable salt thereof, wherein R1 is a saturated or unsaturated 5-, 6- or 7-membered, ring containing 0, 1, 2 or 3 atoms selected from N, 0 and S, wherein the ring may be fused with a benzo group, and is substituted by 0, 1 or 2 oxo groups, and wherein R1 is additionally substituted; and R2 is a substituted C1-6alkyl. Also included is a method of prophylaxis or treatment of inflammation, rheumatoid arthritis, Pagets disease, osteoporosis, multiple myeloma, uveititis, acute or chronic myelogenous leukemia, pancreatic β cell destruction, osteoarthritis, rheumatoid spondylitis, gouty arthritis, inflammatory bowel disease, adult respiratory distress syndrome (ARDS), psoriasis, Crohn's disease, allergic rhinitis, ulcerative colitis, anaphylaxis, contact dermatitis, asthma, muscle degeneration, cachexia, Reiter's syndrome, type I diabetes, type II diabetes, bone resorption diseases, graft vs. host reaction, Alzheimer's disease, stroke, myocardial infarction, ischemia reperfusion injury, atherosclerosis, brain trauma, multiple sclerosis, cerebral malaria, sepsis, septic shock, toxic shock syndrome, fever, myalgias due to HIV-1, HIV-2, HIV-3, cytomegalovirus (CMV), influenza, adenovirus, the herpes viruses or herpes zoster infection in a mammal comprising administering an effective amount a compound as described above.
Owner:AMGEN INC
Gene recombinant human cytomegalovirus fusion protein pp150/MDBP, preparation process and application thereof
InactiveCN1763203AHigh expressionHigh yieldPeptide/protein ingredientsBiological testingAntigenC-terminus
The present invention discloses one kind of gene recombinant human cytomegalovirus fusion protein pp150 / MDBP and its preparation process and application, and relates to gene engineering technology, preventing vaccine, diagnosis reagent and other technology. The recombinant fusion protein pp150 / MDBP is fusion protein formed through connecting serially the 197th amino acid in the 495-691 amino acid segment of human cytomegalovirus pp150 and the 64th amino acid in the 538-601 amino acid segment of MDBP protein. The 197th amino acid pp150 in the N-terminal of the fusion protein and the 64th amino acid of MDBP protein in the C-terminal of the fusion protein are connected through two amino acids including one Leu and one Glu, and the fusion protein has one increased Met and has whole length of 264 amino acids. The fusion protein is used in detecting human cytomegalovirus antibody and antigen, preparing antibody and protein chip, vaccine, ELISA detecting kit and other products.
Owner:SHANDONG MEDICAL BIO TECH RES CENT
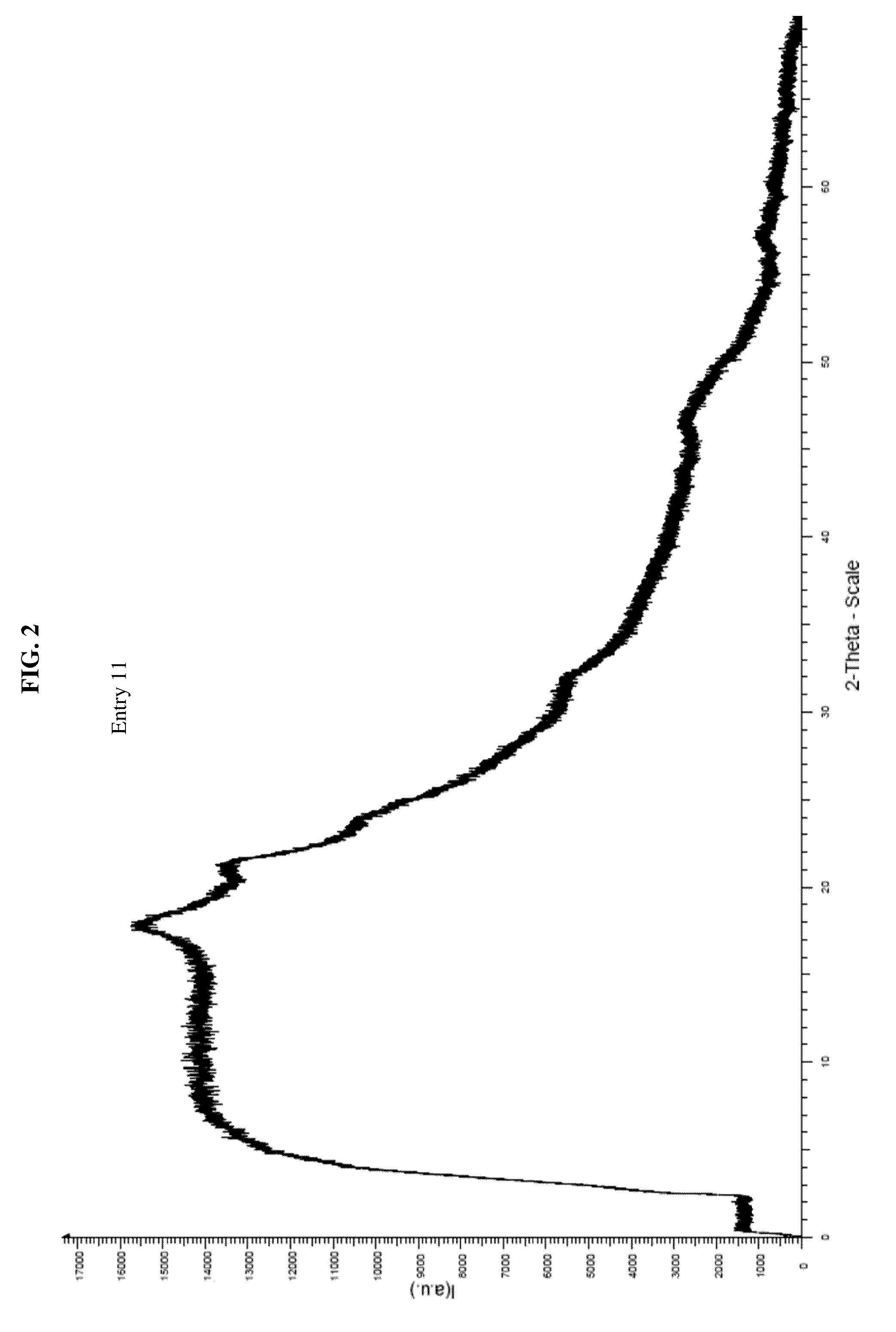
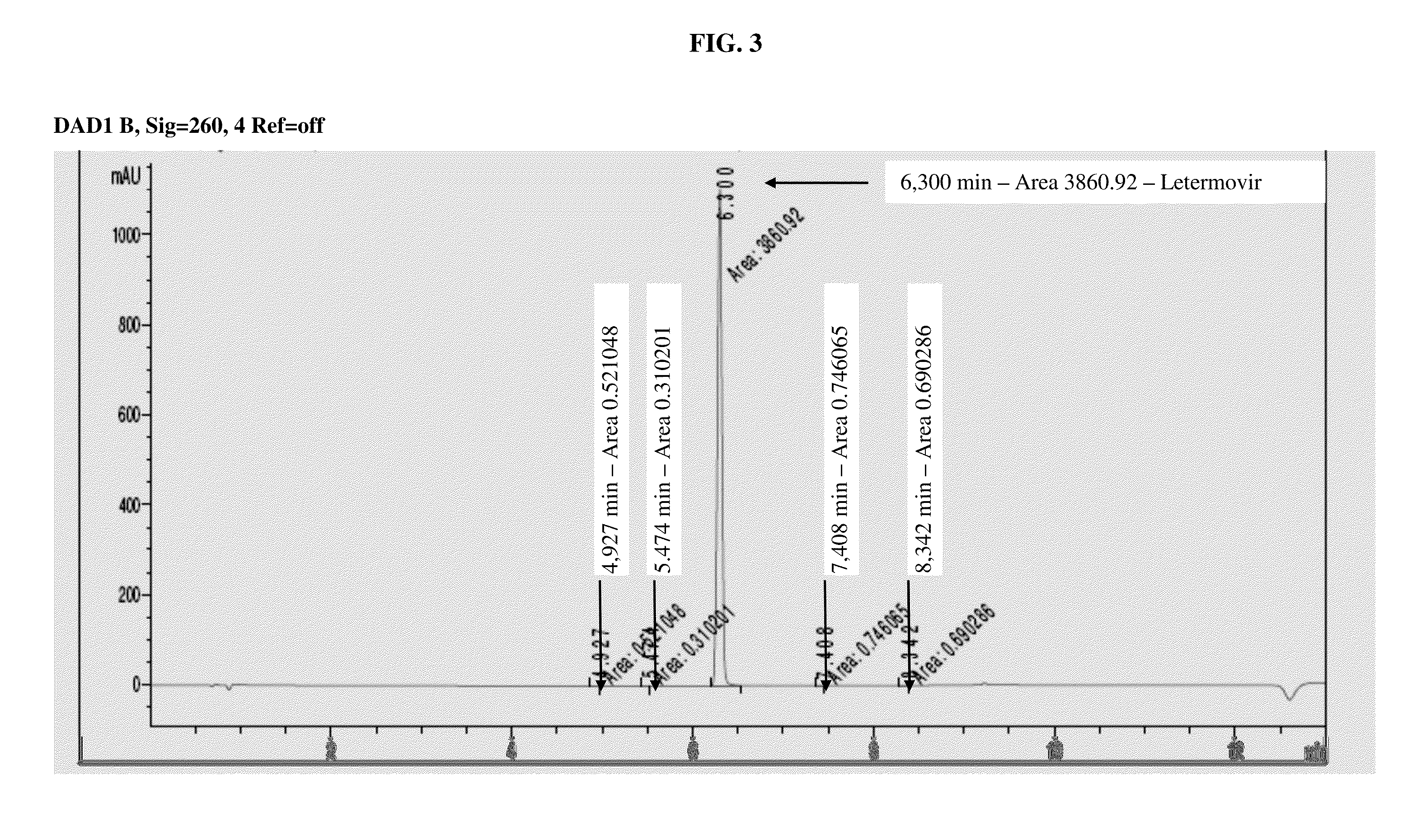
Amorphous letermovir and solid pharmaceutical formulations thereof for oral administration
ActiveUS20160145216A1Uniform thickness and weightDissolve fastOrganic active ingredientsOrganic chemistryDiseaseImmediate release
The present invention provides for amorphous Letermovir and orally administrable solid pharmaceutical formulations thereof (immediate release formulation). Said amorphous Letermovir is suitable for immediate release formulations when isolated out of an organic solution by either roller-drying said organic solution in a volatile organic solvent, in particular acetone, at a temperature of 30° C. to 60° C., and subsequently drying the amorphous Letermovir obtained, or isolating said amorphous Letermovir by precipitation from water miscible solvents selected from acetone or acetonitrile into excess water as anti-solvent, and subsequently filtrating or centrifuging the amorphous Letermovir obtained.The immediate release formulations of amorphous Letermovir are intended for use in methods of prophylaxis or methods of treatment of diseases associated with the group of Herpesviridae, preferably associated with cytomegalovirus (CMV), even more preferably associated with human cytomegalovirus (HCMV).
Owner:AIC246 AG & CO KG
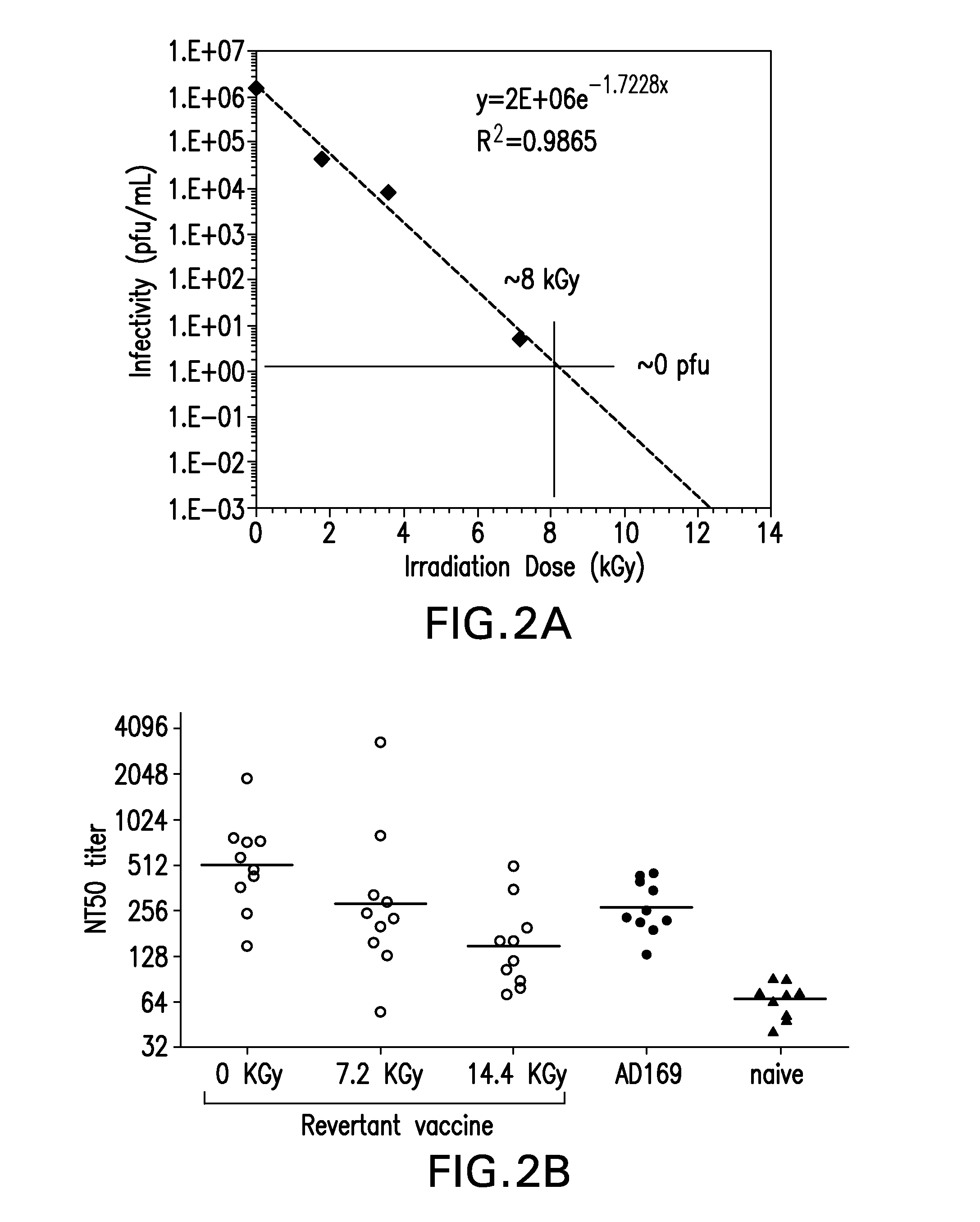
Conditional replicating cytomegalovirus as a vaccine for cmv
ActiveUS20140220062A1Decrease likelihood of infectionReduce replicationSugar derivativesViral antigen ingredientsCytomegalovirus diseaseViral replication
The present invention relates to methods of inducing an immune response to cytomegalovirus (CMV) using a genetically modified CMV that is conditionally replication defective. The methods of the invention can be used to treat and / or prevent primary CMV infection, infection due to reactivation of a latent CMV and a super-infection of a different strain of CMV that had been previously encountered. The present invention also relates to a replication defective CMV which has been recombinantly altered to allow for external control of viral replication. Compositions comprising the replication defective CMV are also encompassed by the present invention.
Owner:MERCK SHARP & DOHME LLC
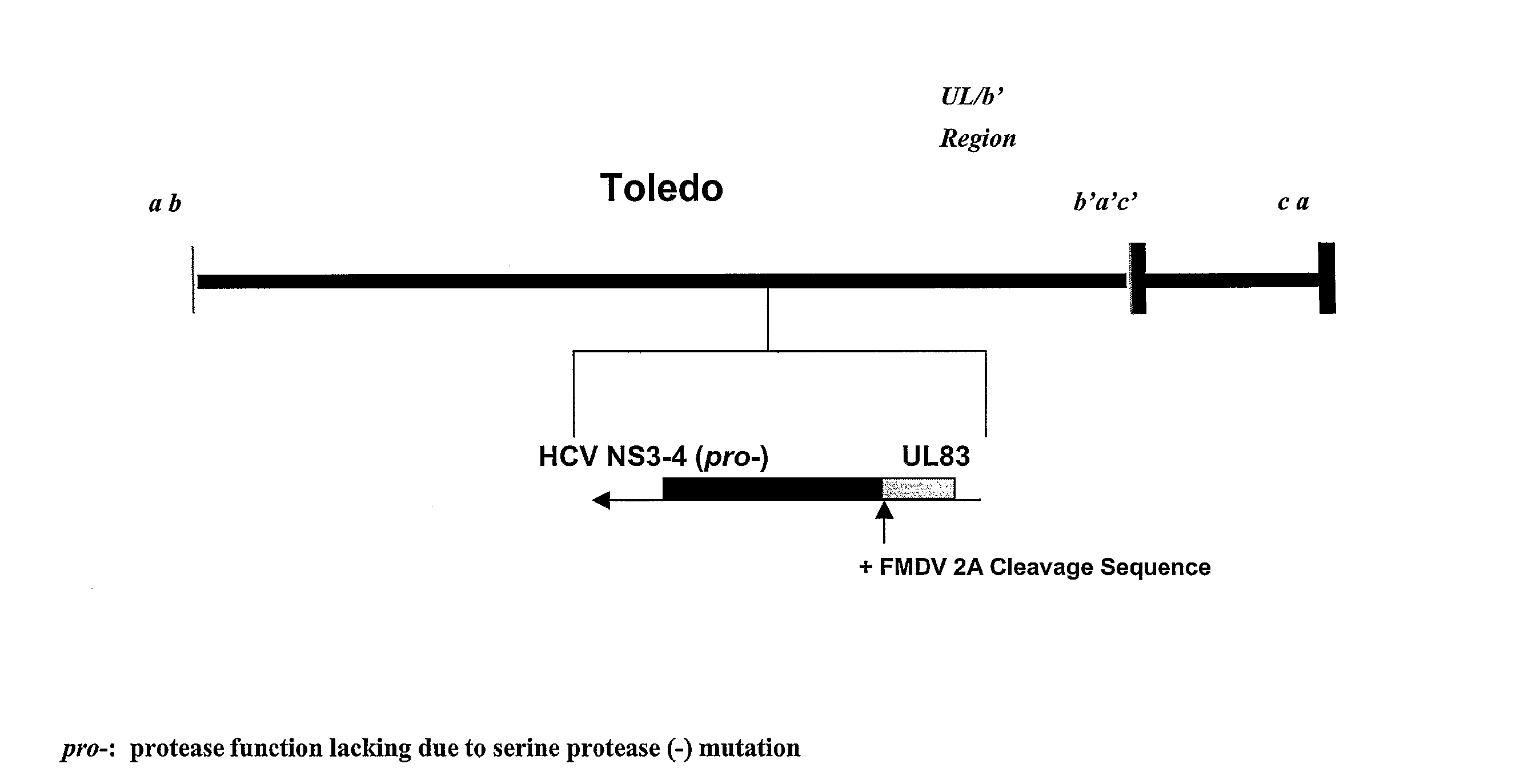
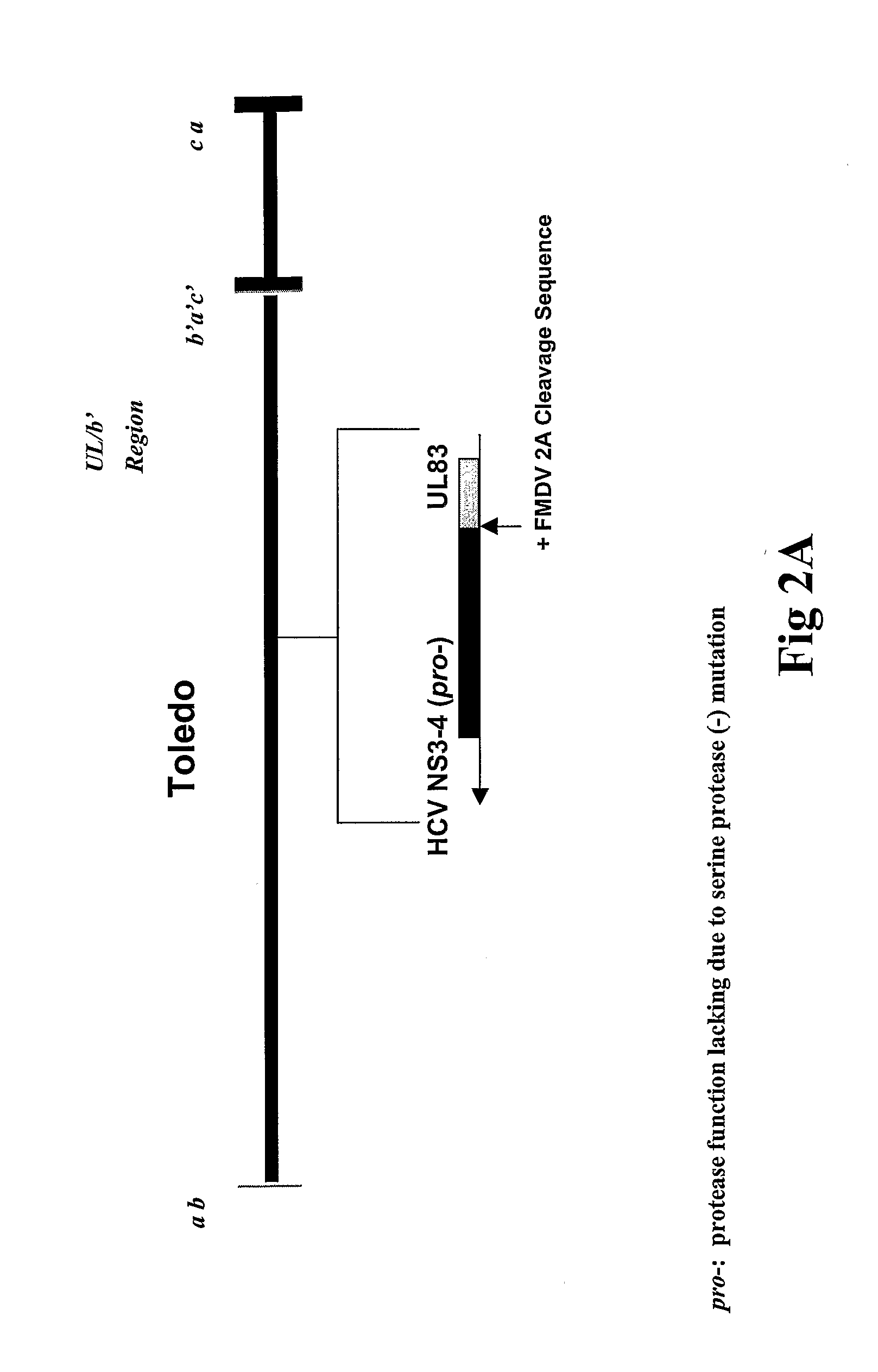
Attenuation of cytomegalovirus virulence
InactiveUS20070292455A1Viral antigen ingredientsMicrobiological testing/measurementUltrasound attenuationVirulent characteristics
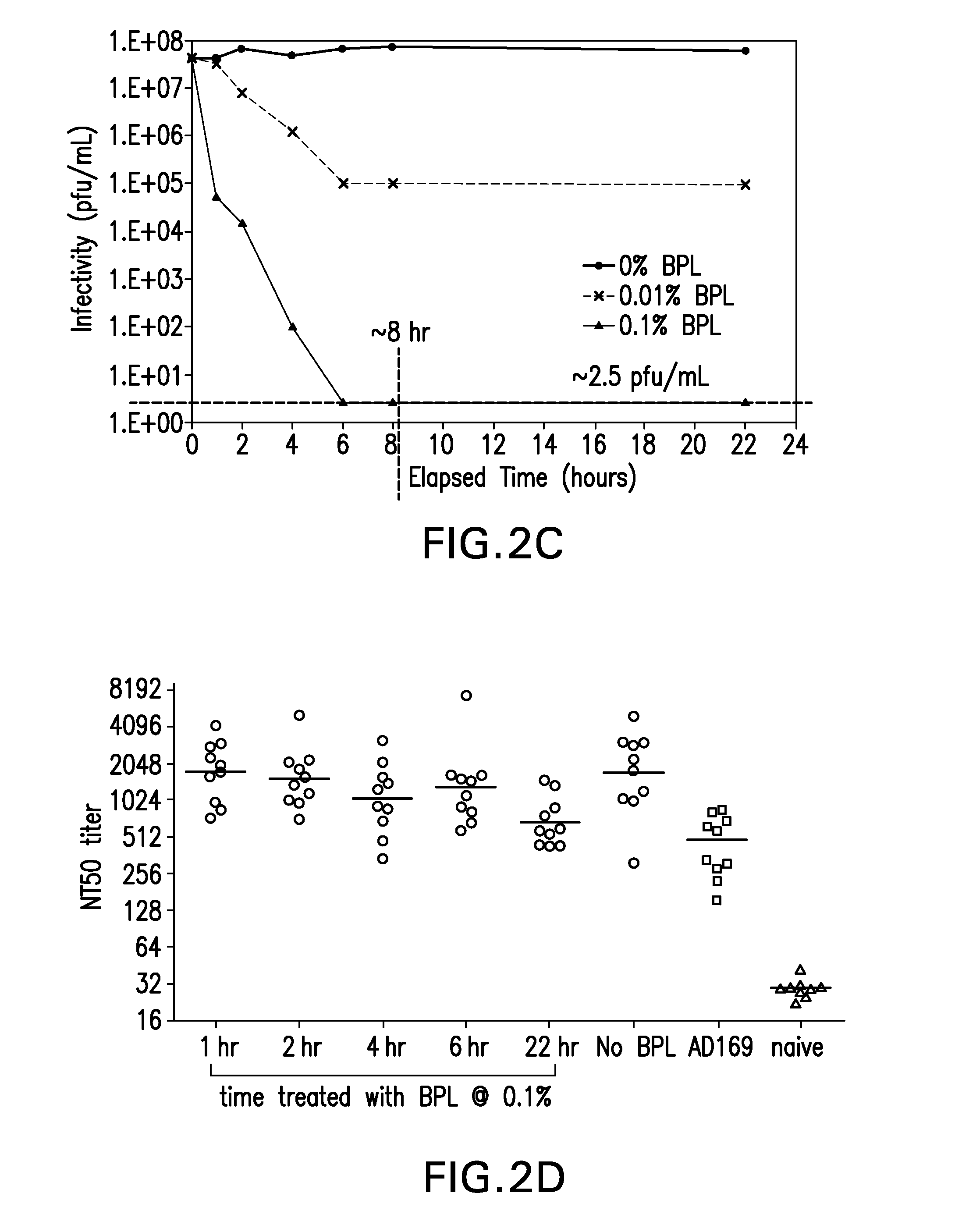
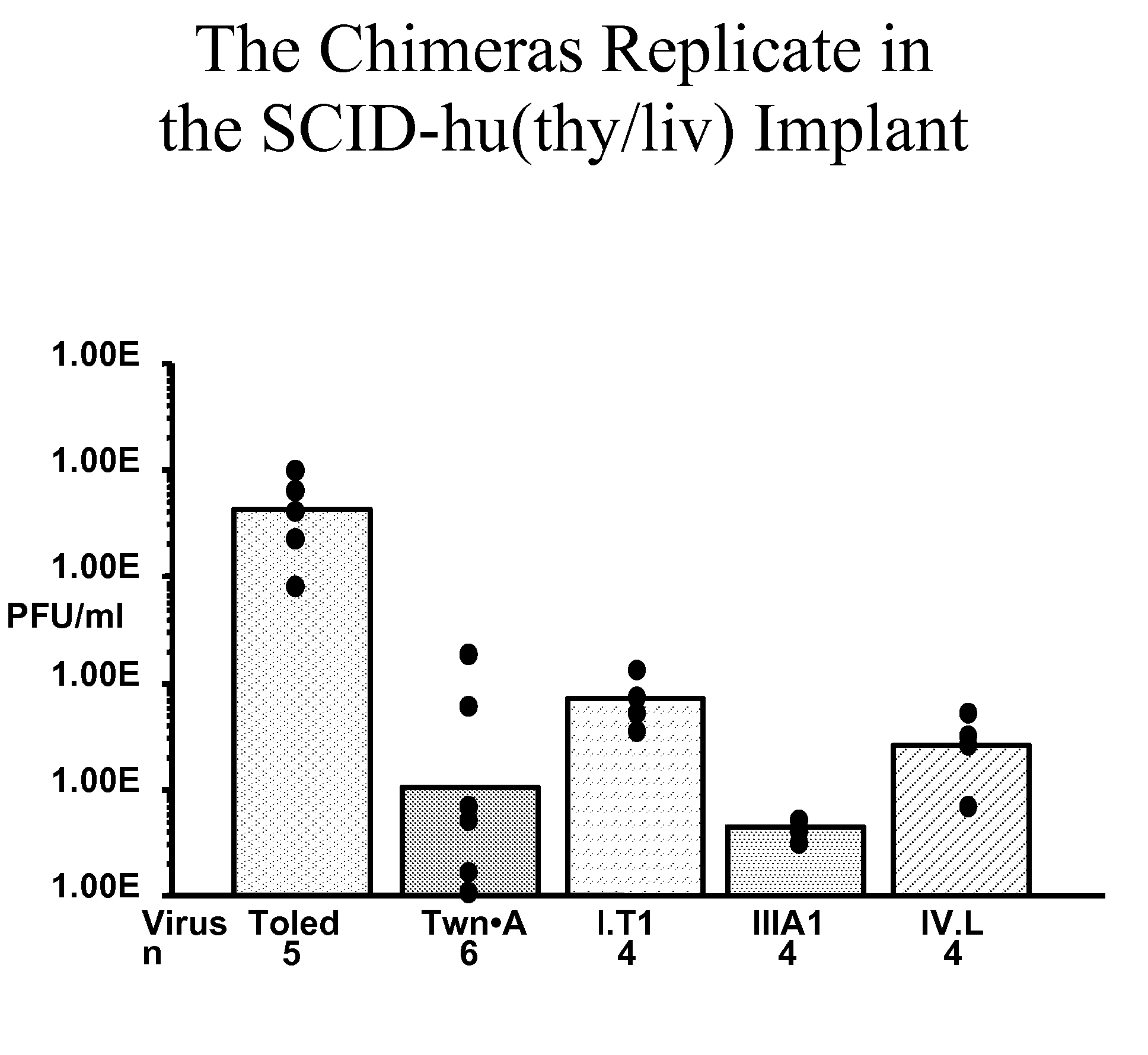
A method is provided for attenuating a cytomegalovirus comprising functionally disrupting an open reading frame of a Toledo genome region or its homolog and making chimeric CMV virus genomes.
Owner:MEDIMMUNE LLC
Recombinant herpesvirus of turkey (HVT) and preparation method thereof
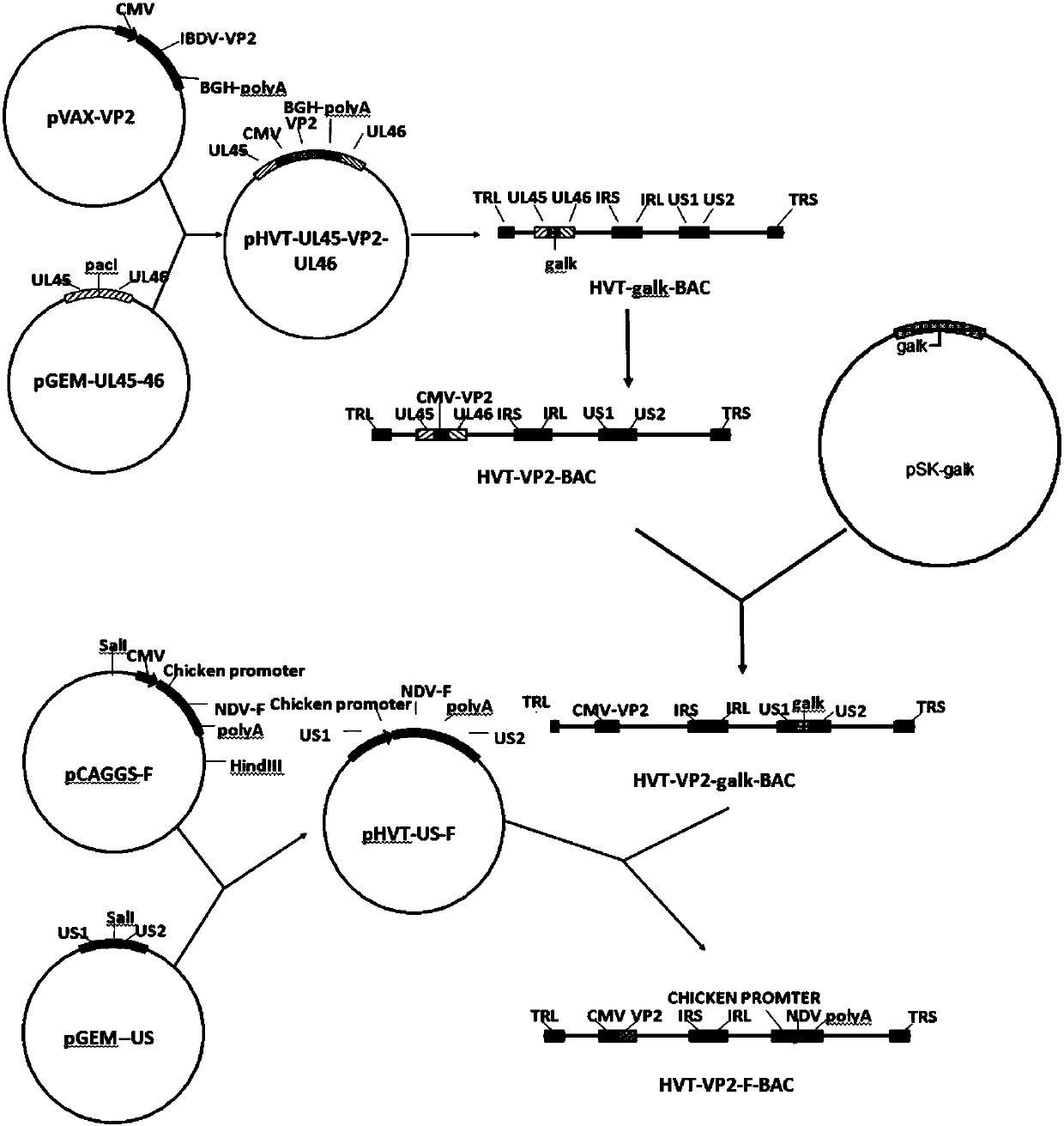
InactiveCN107815469AReduce manufacturing costSsRNA viruses negative-senseVirus peptidesAntigenBacterial artificial chromosome
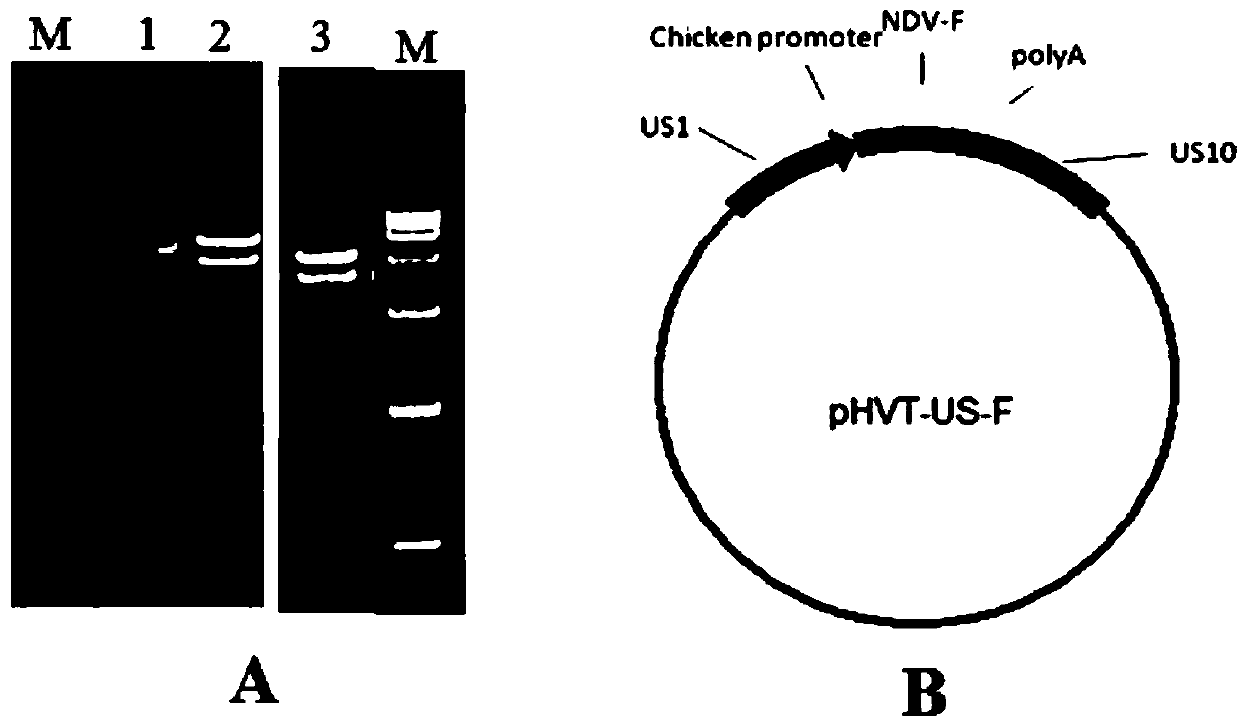
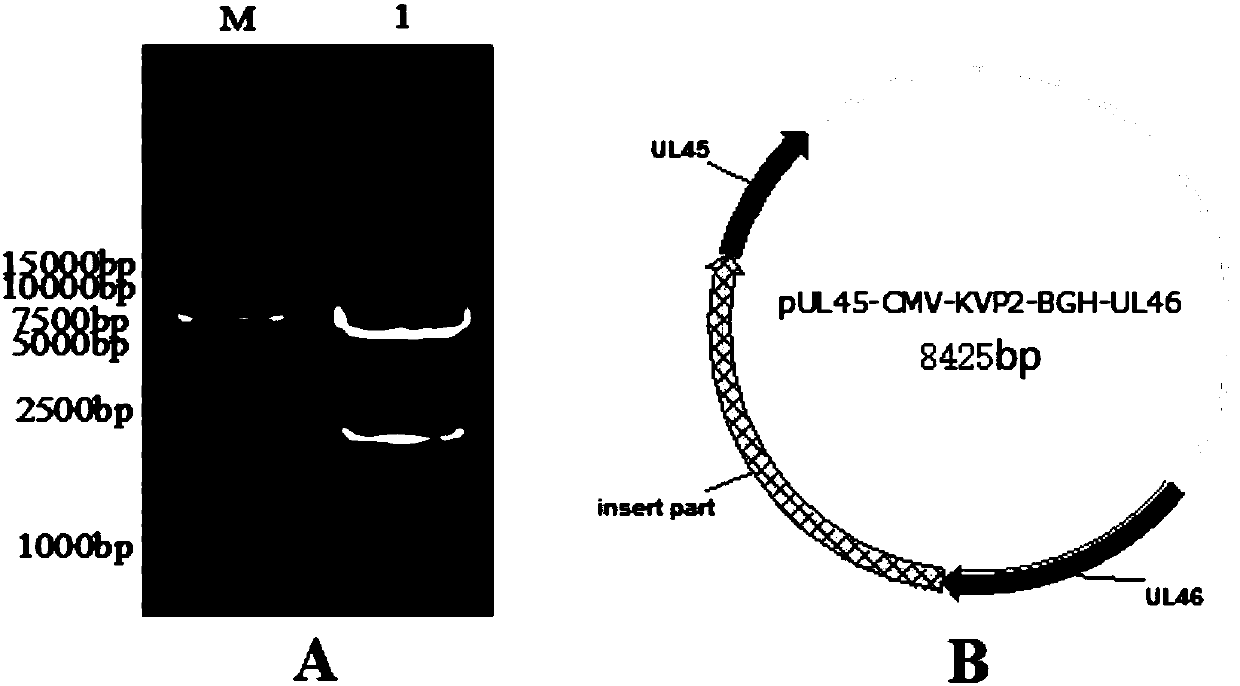
The invention belongs to the technical field of biology and in particular discloses a recombinant herpesvirus of turkey (HVT). The recombinant HVT is capable of simultaneously expressing NDV and IBDVprotective antigen genes, and is a bacterial artificial chromosome (BAC) based on the HVT. The construction method comprises the following steps: inserting a VP2 gene expression cassette of an infectious bursal disease virus LX strain under regulation of a cytomegalovirus promoter CMV into a UL45-46 non-essential region of the HVT genome by utilizing a gene recombination technology; and insertingan F gene expression cassette of a newcastle diseases virus PX02 / 3 strain under regulation of a chicken beta-actin promoter pec into another non-essential region US1-10 of the HVT genome. The recombinant herpesvirus of turkey is a recombinant triplet poultry vaccine candidate strain capable of controlling Marek's disease of chickens and effectively resisting Newcastle disease and infectious bursaldisease.
Owner:BEIJING ACADEMY OF AGRICULTURE & FORESTRY SCIENCES
Application of polycyclic polyketides in preparation of anti-herpesvirus drugs
The invention discloses application of polycyclic polyketides in the preparation of anti-herpesvirus drugs. According to the invention, the polyketides are found to inhibit diseases caused by herpes simplex virus (HSV) type I, HSV-2, varicella zoster virus (VZV) and cytomegalo virus (CMV) infection. Compared with drugs such as acyclovir on the market, the compound in the invention exhibits considerable activity, but the mechanism of action is different, and the compound overcomes the drug resistance of the drugs currently on the market. Thus, these compounds have good application prospects inthe treatment of diseases caused by HSV-1, HSV-2, VZV and CMV infection.
Owner:JINAN UNIVERSITY
Mammalian cells expressing cytomegalovirus antigens
This invention relates to cytomegalovirus (CMV) proteins suitable for vaccine uses. Provided herein are mammalian host cells, in particular CHO cells, in which the sequence(s) encoding CMV proteins gH, gL, pUL128, pUL130, pUL131 (or a complex-forming fragment thereof) are stably integrated into the genome.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Use of endogenous viral vaccine in chimeric antigen receptor T cell therapy
ActiveUS11116834B2Polypeptide with localisation/targeting motifImmunoglobulin superfamilyViral VaccineTGE VACCINE
Provided herein are, inter alia, methods and compositions including T cells expressing (i) a recombinant CAR protein which includes a peptide binding site and is capable of specifically binding cancer-specific antigens and (ii) a T cell receptor specific for a viral antigen (e.g., a CMV pp65 protein). The engineered T cells provided herein may be used in combination with a viral vaccine (e.g. cytomegalovirus (CMV) Triplex Vaccine) to treat a variety of cancers. The methods described herein also permit in viva expansion of CMV-specific CAR T cells, instead of or in addition to ex vivo expansion, avoiding excessive T cell exhaustion that results in some cases from ex vivo manufacturing.
Owner:CITY OF HOPE
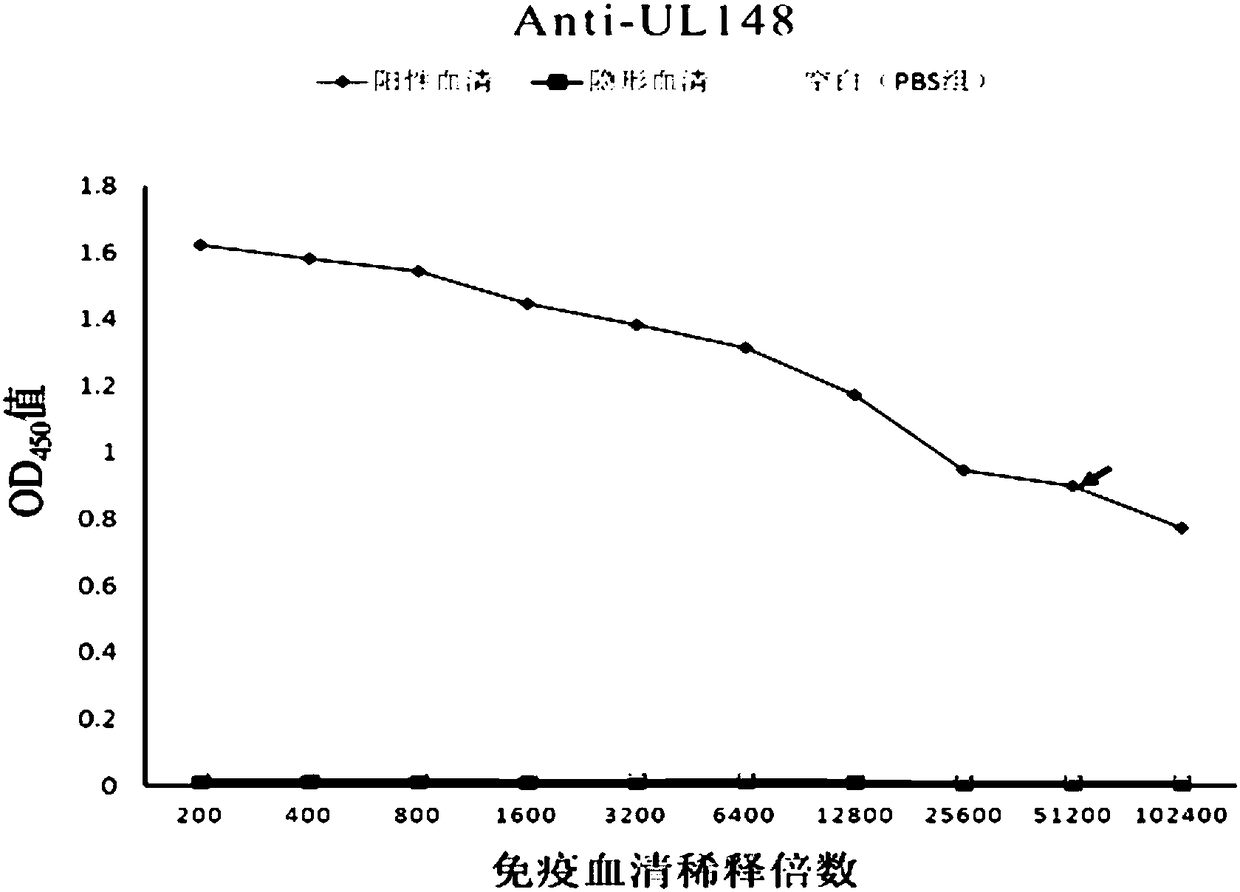
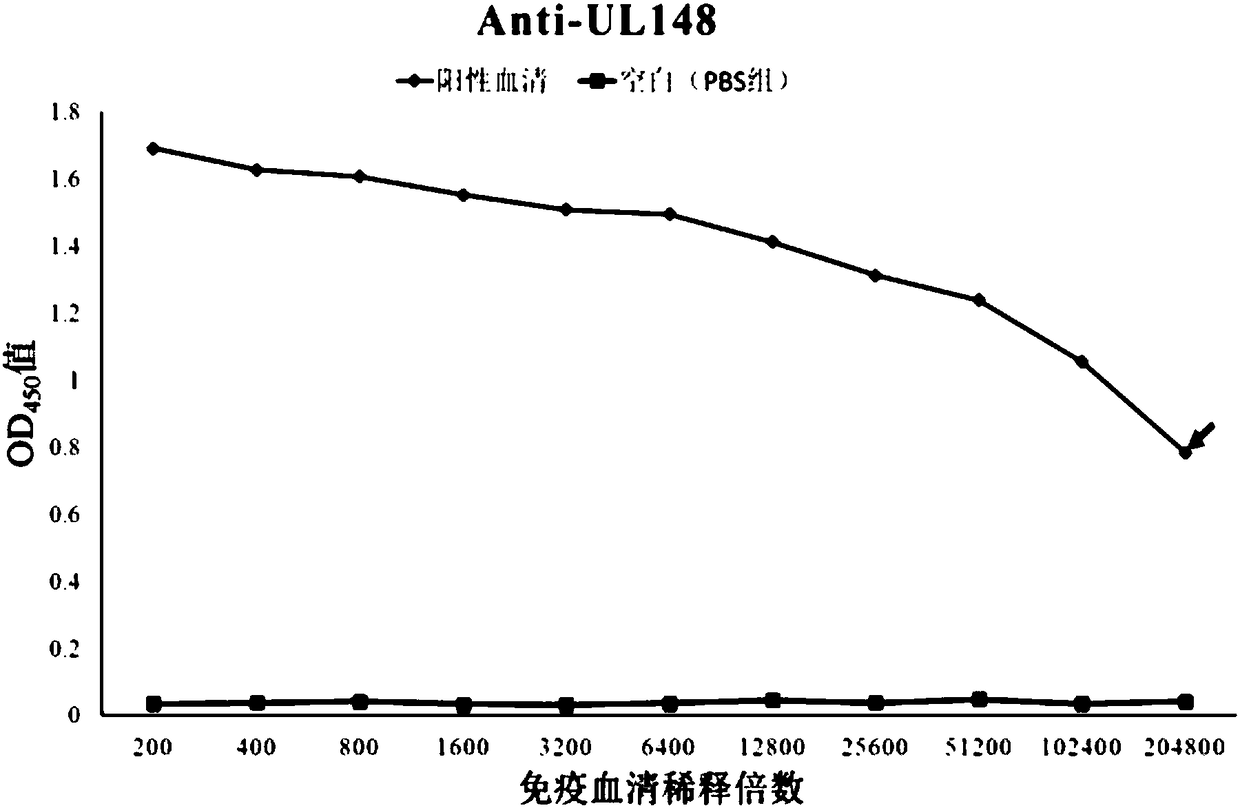
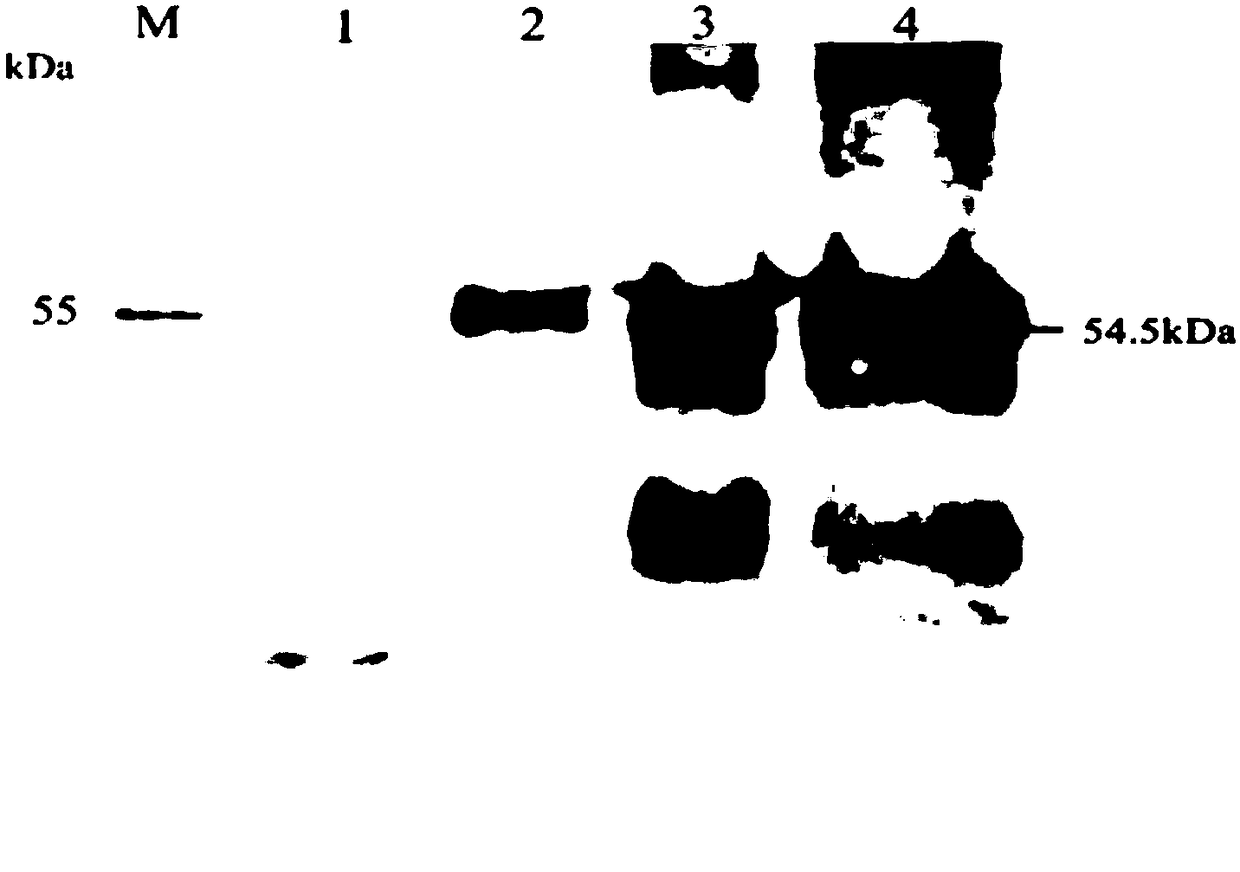
Human cytomegalovirus (HCMV) anti-UL148 polyclonal antibody and preparation method and application thereof
The invention discloses a human cytomegalovirus (HCMV) anti-UL148 polyclonal antibody and a preparation method and application thereof. The HCMV anti-UL148 polyclonal antibody is obtained by taking UL148 recombinant fusion protein as an antigen to perform immunization on a rabbit to obtain serum and then performing purification treatment on the serum. The prepared polyclonal antibody has high purity and strong specificity; the UL148 recombinant protein is used for detecting the specificity of the polyclonal antibody; the Western blot result shows that for the HFF (Human Foreskin Fibroblast) infected with the HCMV, expression of UL148 genes is detected through the Western blot; and the polyclonal antibody can specifically recognize UL148 recombinant protein of which the size is about 54.5kDa. The polyclonal antibody disclosed by the invention can be used for development of HCMV diagnostic kits and HCMV related protein detection kits.
Owner:JINAN UNIVERSITY
Cytomegalovirus GB antigen
The invention relates to a cytomegalovirus (CMV) gB polypeptide comprising at least a portion of a gB protein extracellular domain comprising a fusion loop 1 (FL1) domain and a fusion loop 2 (FL2) domain, wherein at least one of the FL1 and FL2 domains comprises at least one amino acid deletion or substitution.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Gene therapy using genetically modified viral vectors
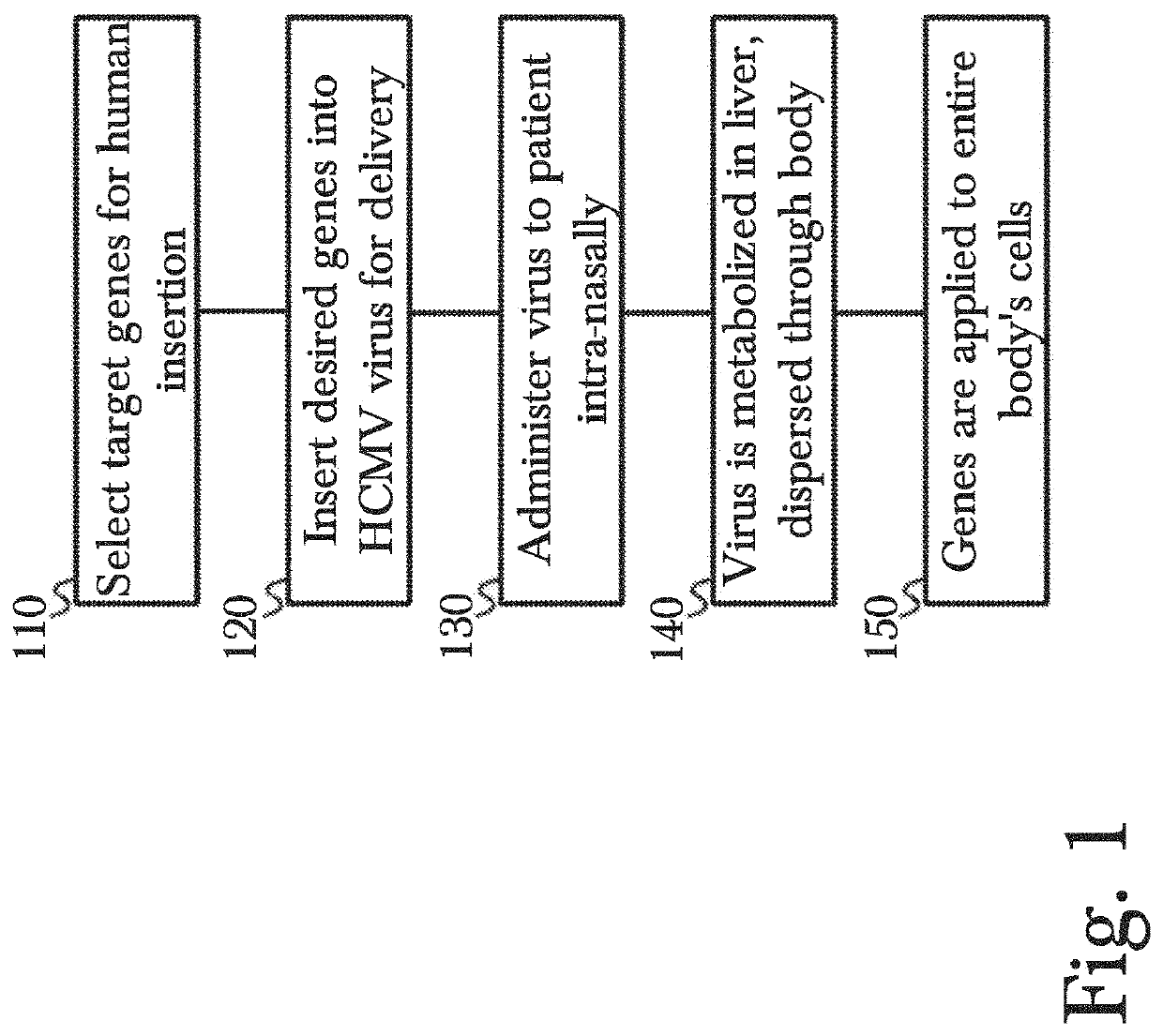
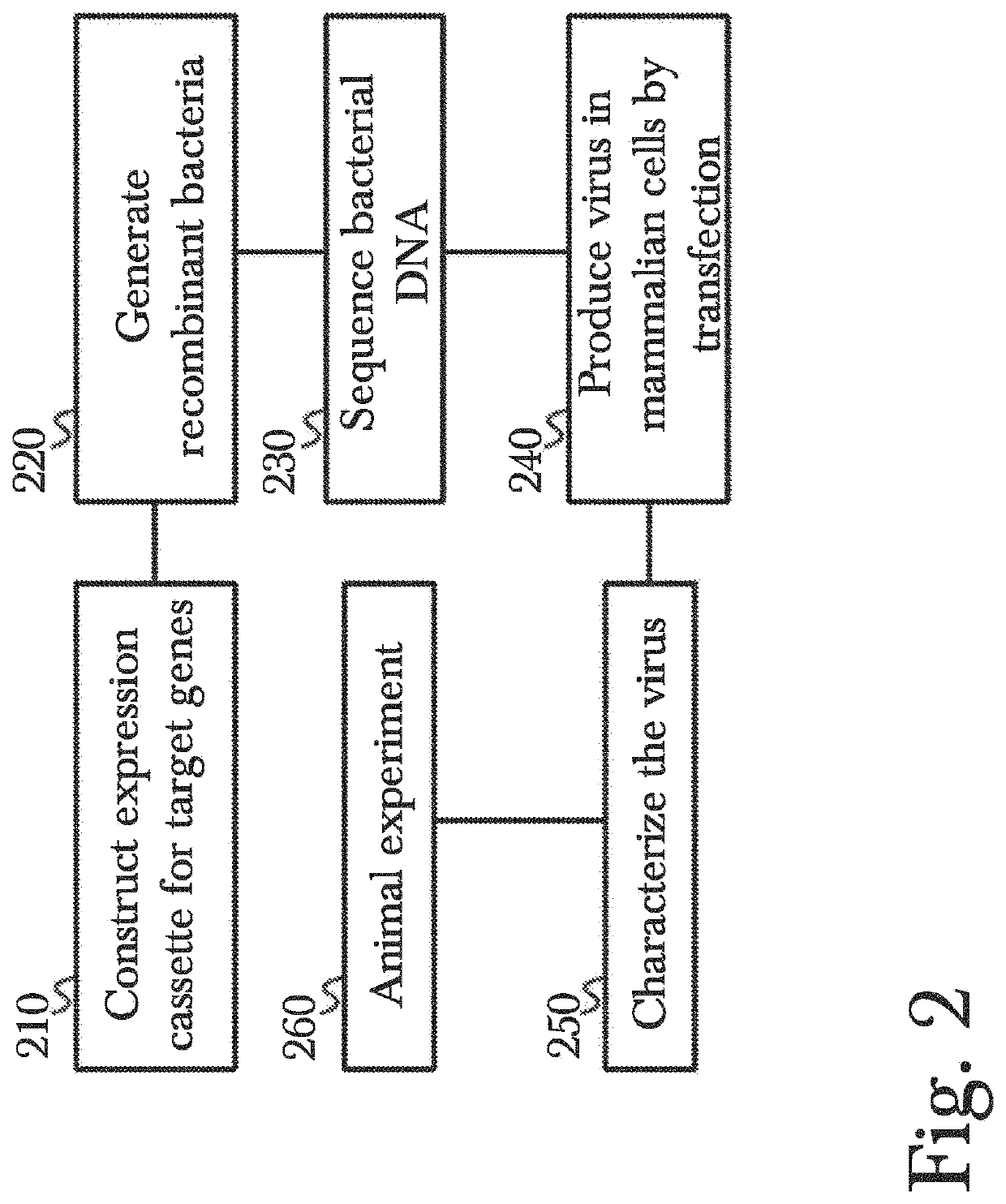
PendingUS20220177921A1Easy to insertAffordable and effective gene therapyGenetic therapy composition manufacturePeptidesReverse transcriptaseViral vector
Disclosed are methods for gene therapy by administration of genetically modified viral vectors. Gene therapy vectors can include a cytomegalovirus vector encoding one or more therapeutic donor genes such as human telomerase reverse transcriptase (hTERT). These vectors can be used in exemplary gene therapy methods for maintaining or improving one or more aspects of a recipient's physiological wellness and / or longevity. The recombinant viral vector can be administered or received intranasally or as an injectable therapeutic
Owner:BIOVIVA USA
Method of detecting and quantifying cytomegalovirus
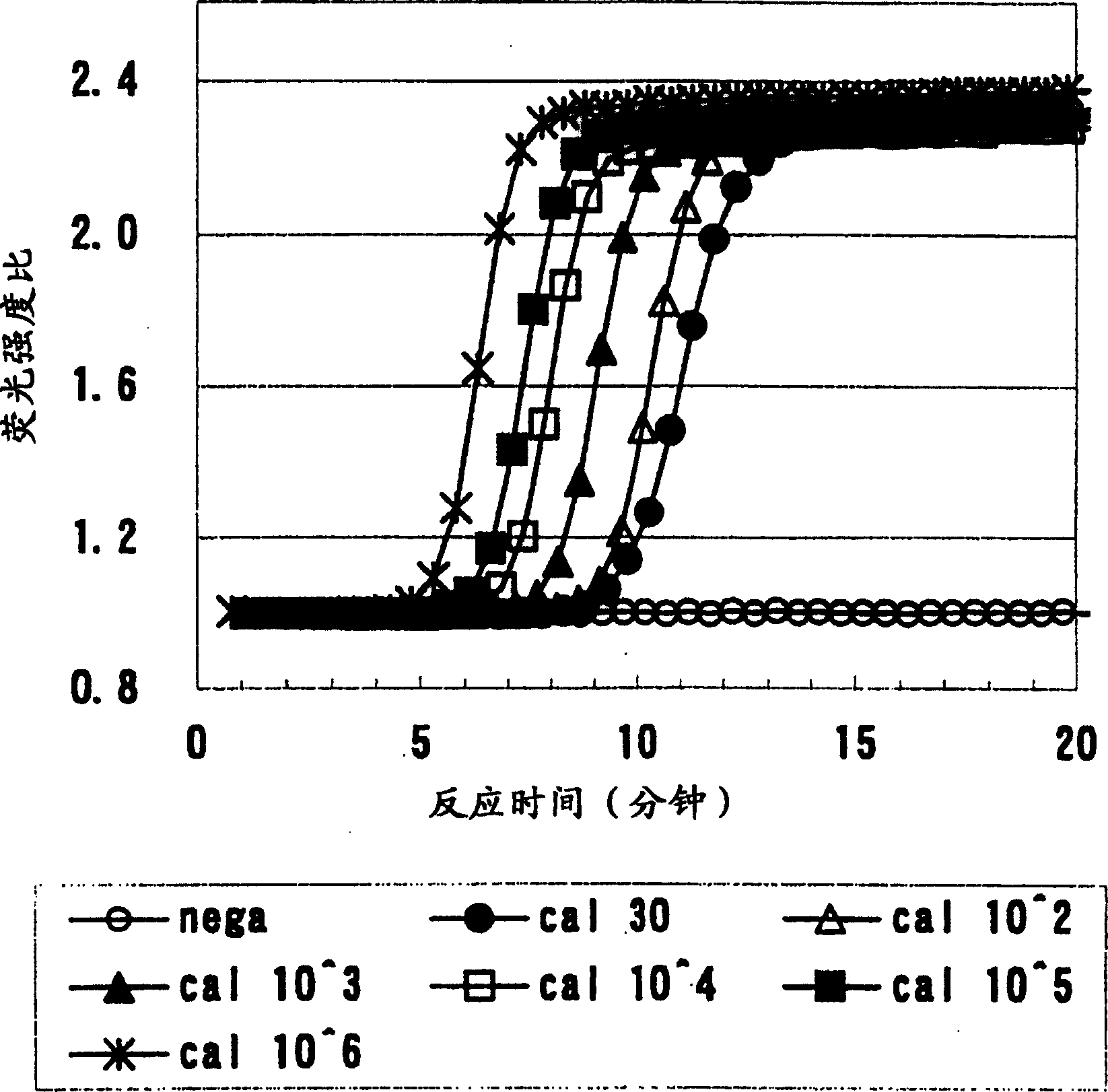
InactiveCN1888074AHigh sensitivityMicrobiological testing/measurementFermentationFluorescenceOligonucleotide Primer
Provided are detection and assay of CMV that can rapidly amplify and detect the beta 2.7 gene originating from CMV at a certain temperature through a one-step operation. The mRNA of beta 2.7 gene originating from CMV is amplified by utilizing the combination of specifically amplifiable oligonucleotide primers and the RNA amplification step comprising these combinations of oligonucleotide primers is measured and detected by using an oligonucleotide probe that is marked with an intercalated fluorescent dye, to settle above topic.
Owner:TOSOH CORP
Cytomegalovirus-based immunogenic preparations
This disclosure relates to methods of using recombinant replication-deficient cytomegalovirus (CMV) to generate a long-term, repeatedly stimulated T cell-based immune response in a subject, for instance against a heterologous antigen expressed by the cytomegalovirus. It further relates to methods of using a recombinant replication deficient CMV as an anti-cancer immunogenic preparation.
Owner:OREGON HEALTH & SCI UNIV
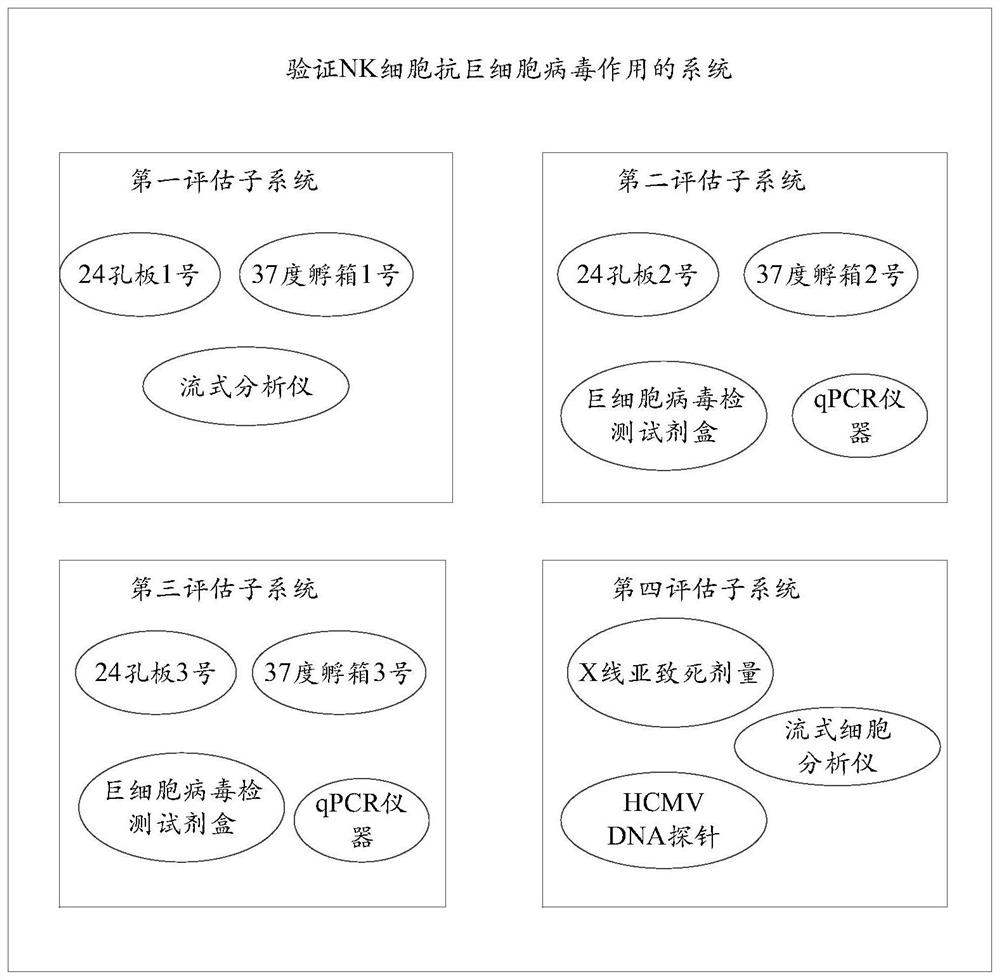
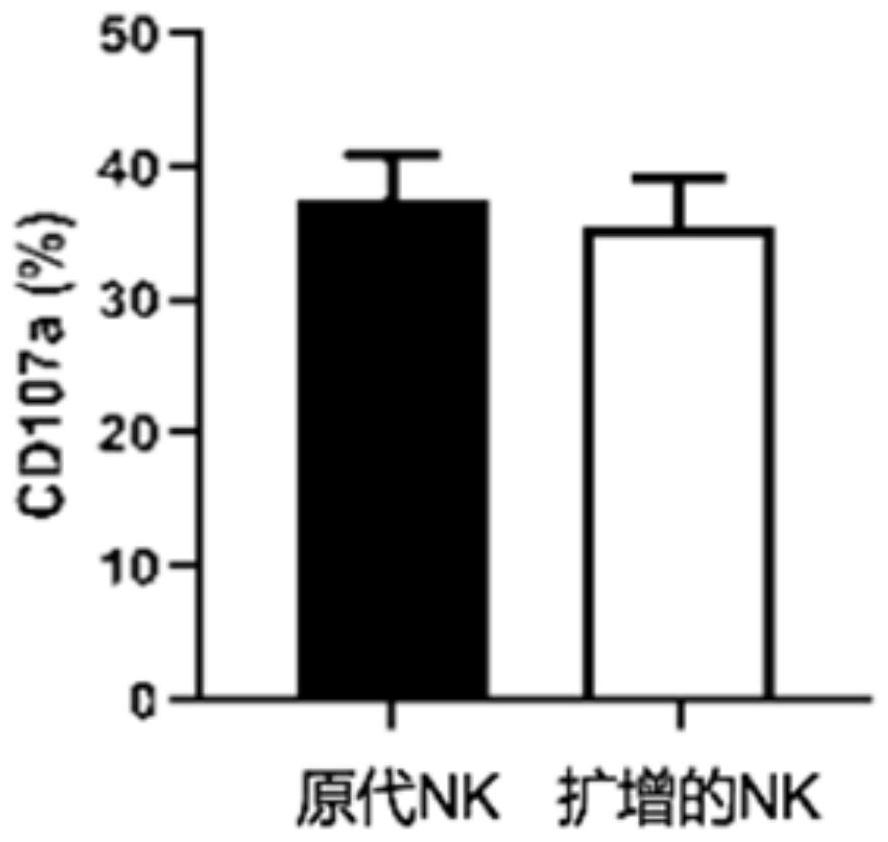
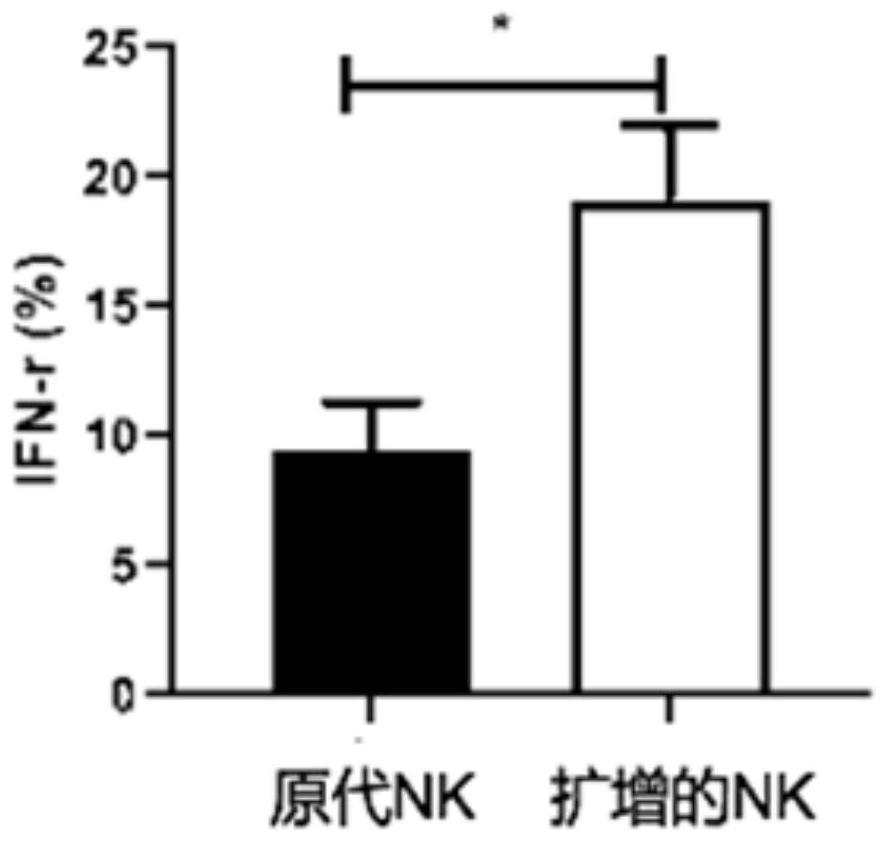
System for evaluating anti-cytomegalovirus effect of NK cells
ActiveCN113109570AGrowth inhibitionMicrobiological testing/measurementBiological testingNatural Killer Cell Inhibitory ReceptorsRe infection
The invention provides a system for evaluating the anti-cytomegalovirus effect of NK cells, and the system comprises a first evaluation subsystem which is used for evaluating the anti-cytomegalovirus function of the NK cells; a second evaluation subsystem, used for evaluating the function of the NK cells in inhibiting cytomegalovirus amplification; a third evaluation subsystem, used for evaluating the re-infection ability of the cytomegalovirus acted by the NK cells; and a fourth evaluation subsystem, used for evaluating the removal function of the NK cells on cytomegalovirus. Through the system provided by the invention, the anti-cytomegalovirus effect of the natural killer cells (NK cells) is scientifically and normatively evaluated, and a scientific and systematic reference basis is provided for the application of the natural killer cells in the anti-cytomegalovirus effect, so that the system provided by the invention has a wide application prospect.
Owner:PEOPLES HOSPITAL PEKING UNIV
Substituted heterocyclic compounds and methods of use
The present invention relates to pyrimidinones and pyridones and derivatives thereof, and pharmaceutically acceptable salts thereof. Also included is a method of treatment of inflammation, rheumatoid arthritis, Pagets disease, osteoporosis, multiple myeloma, uveititis, acute or chronic myelogenous leukemia, pancreatic β cell destruction, osteoarthritis, rheumatoid spondylitis, gouty arthritis, inflammatory bowel disease, adult respiratory distress syndrome (ARDS), psoriasis, Crohn's disease, allergic rhinitis, ulcerative colitis, anaphylaxis, contact dermatitis, asthma, muscle degeneration, cachexia, Reiter's syndrome, type I diabetes, type II diabetes, bone resorption diseases, graft vs. host reaction, Alzheimer's disease, stroke, myocardial infarction, ischemia reperfusion injury, atherosclerosis, brain trauma, multiple sclerosis, cerebral malaria, sepsis, septic shock, toxic shock syndrome, fever, myalgias due to HIV-1, HIV-2, HIV-3, cytomegalovirus (CMV), influenza, adenovirus, the herpes viruses or herpes zoster infection in a mammal comprising administering an effective amount a compound as described above.
Owner:AMGEN INC
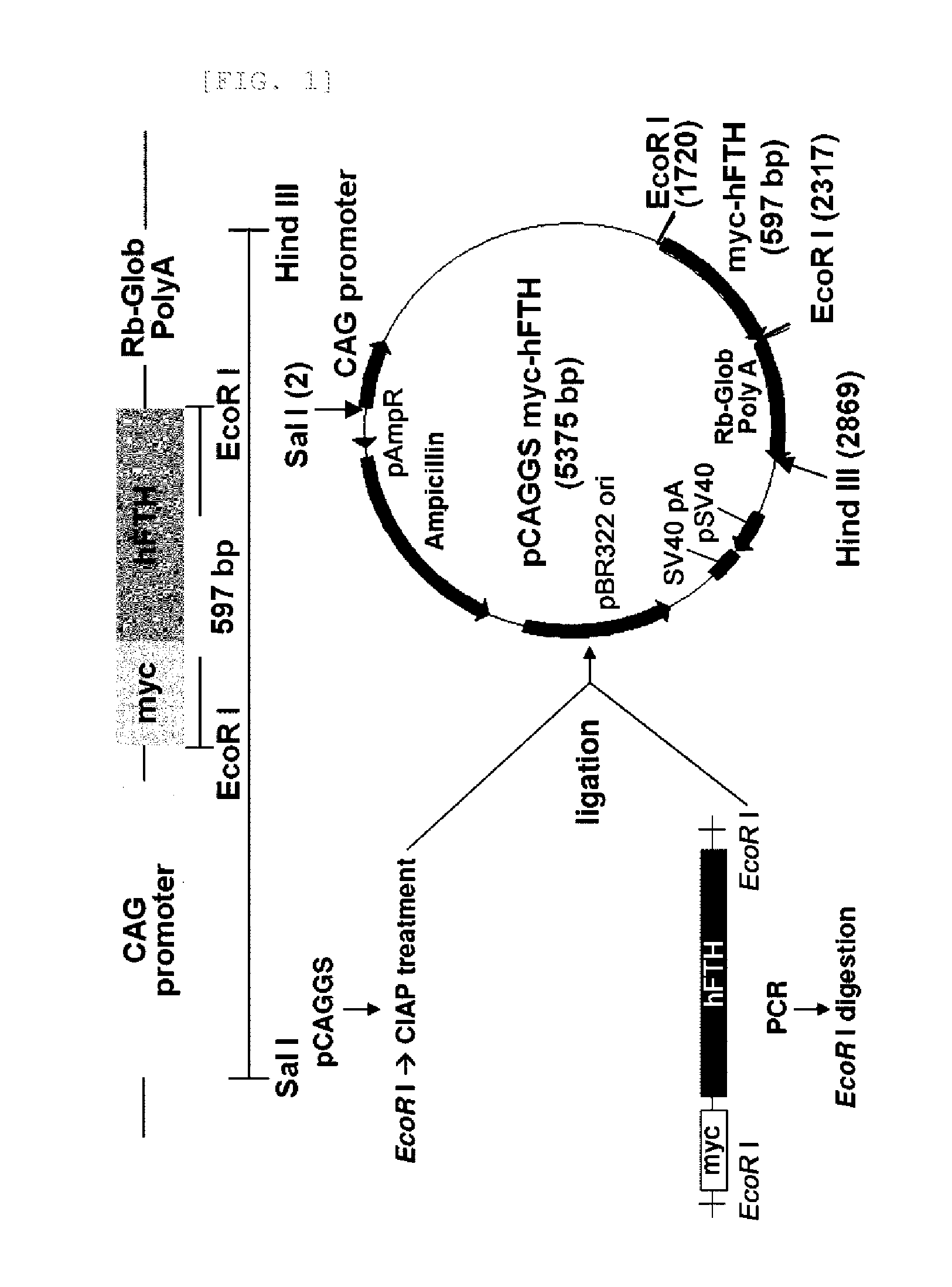
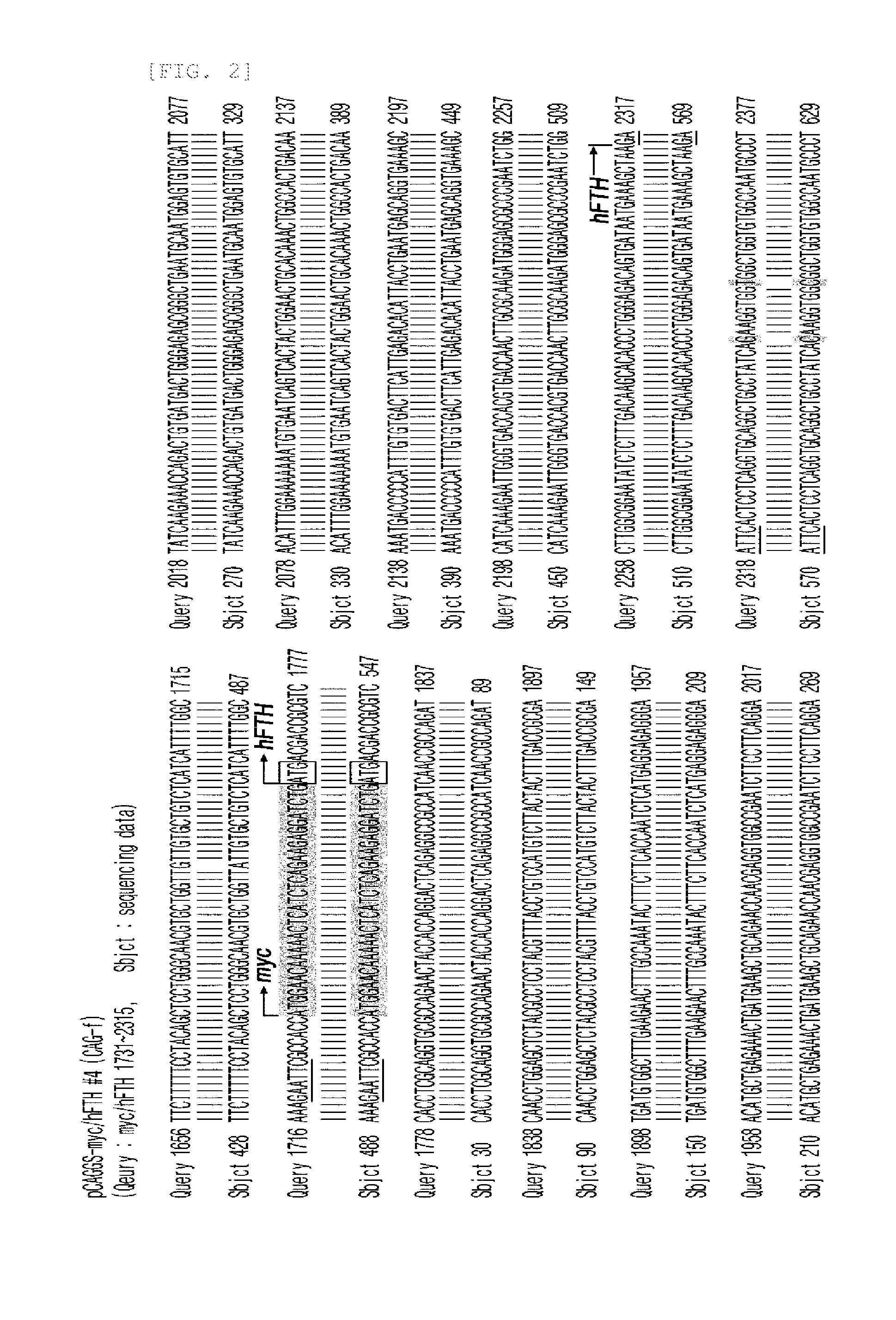
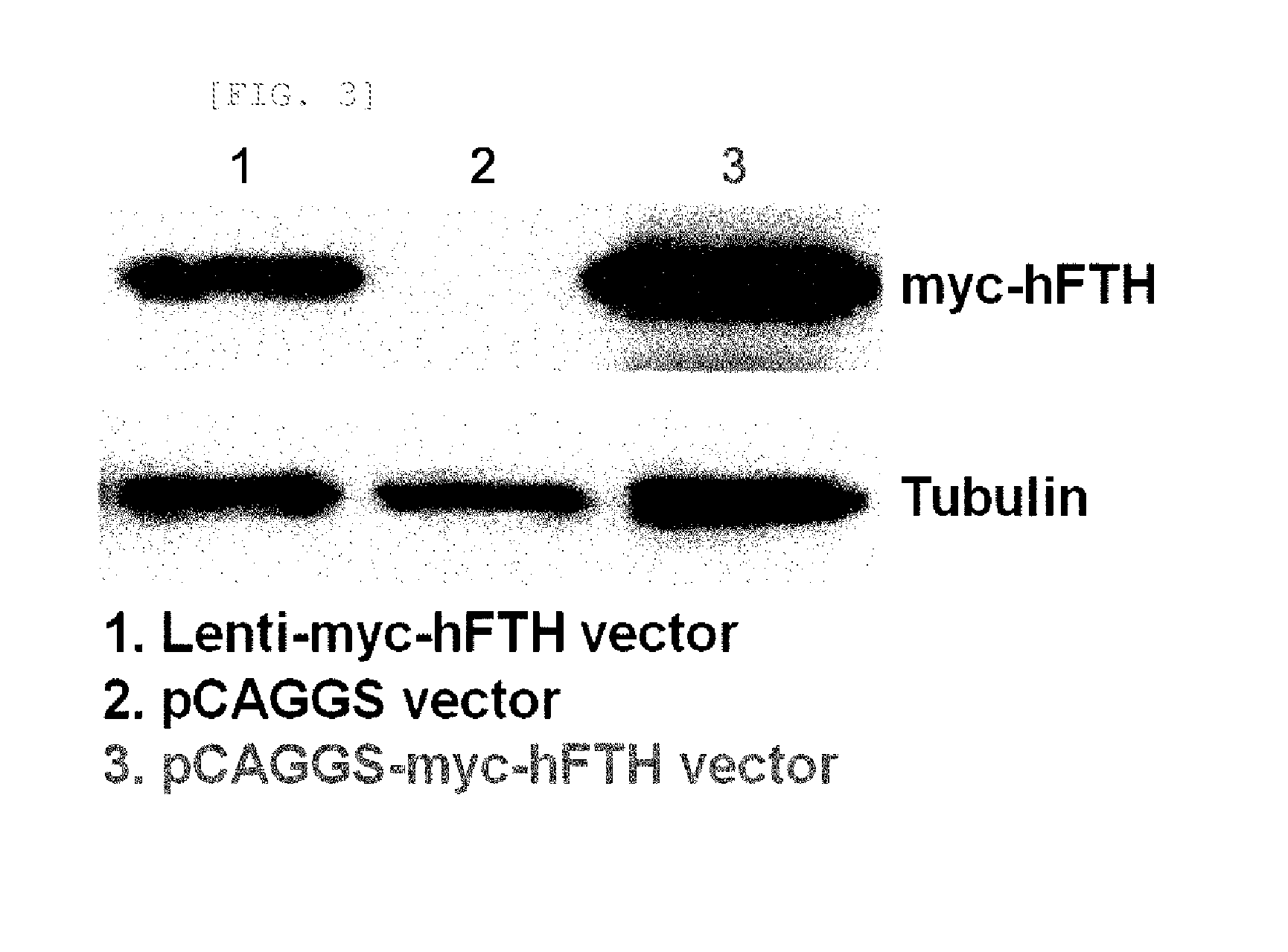
Transgenic mouse for expressing human ferritin in tissue non-specific manner and use thereof
The present invention relates to a recombinant vector and a transgenic mouse for expressing human ferritin in a tissue non-specific manner, and more particularly, to a vector prepared by operably linking a human ferritin gene to a promoter including a cytomegalovirus (CMV) early enhancer element and a β-actin promoter, and a transgenic mouse expressing human ferritin in a tissue non-specific manner, which is transformed with the vector. Further, the present invention relates to a method for preparing a transgenic mouse, and a method for monitoring cell or tissue therapy using the transgenic mouse.
Owner:SEOUL NAT UNIV R&DB FOUND
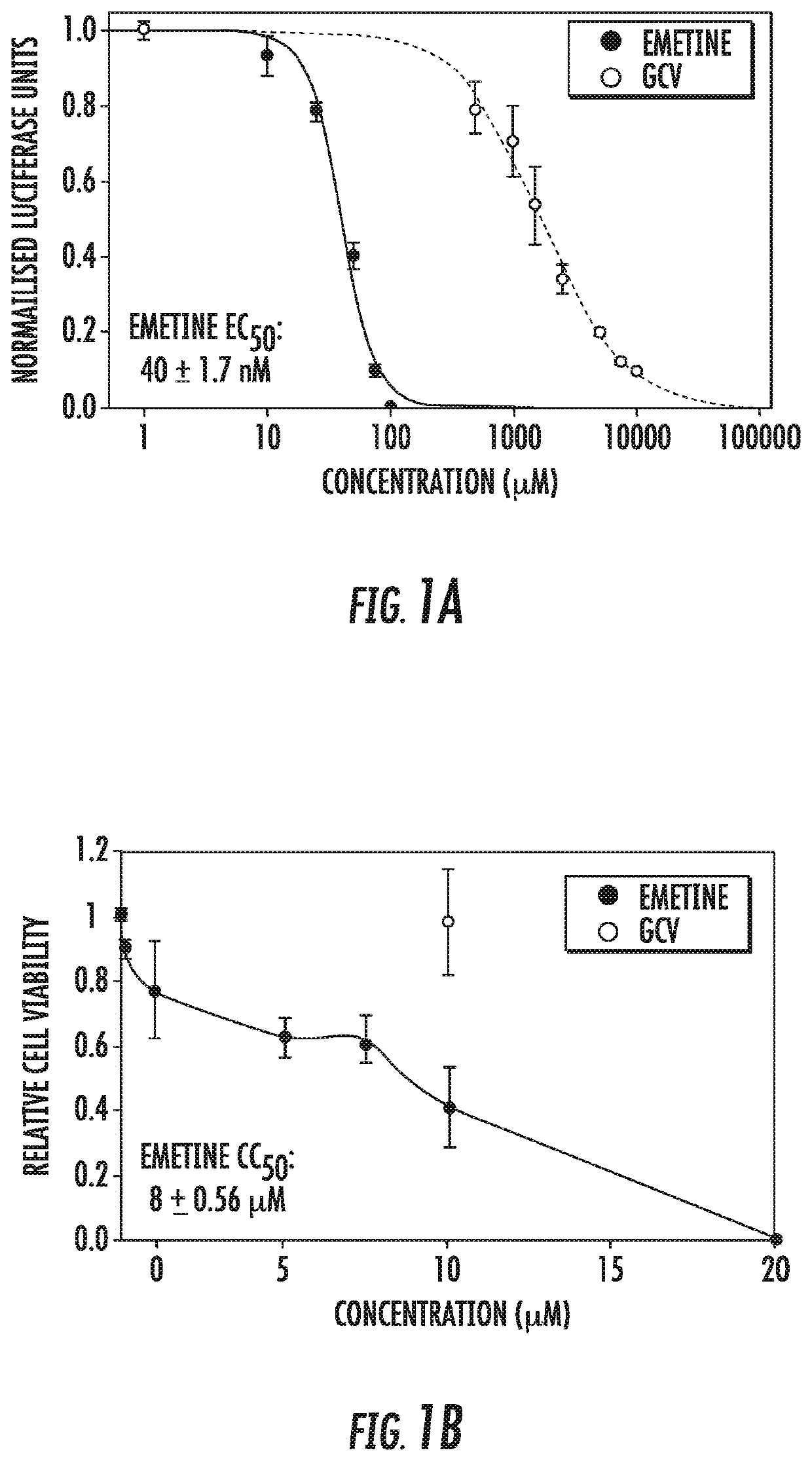
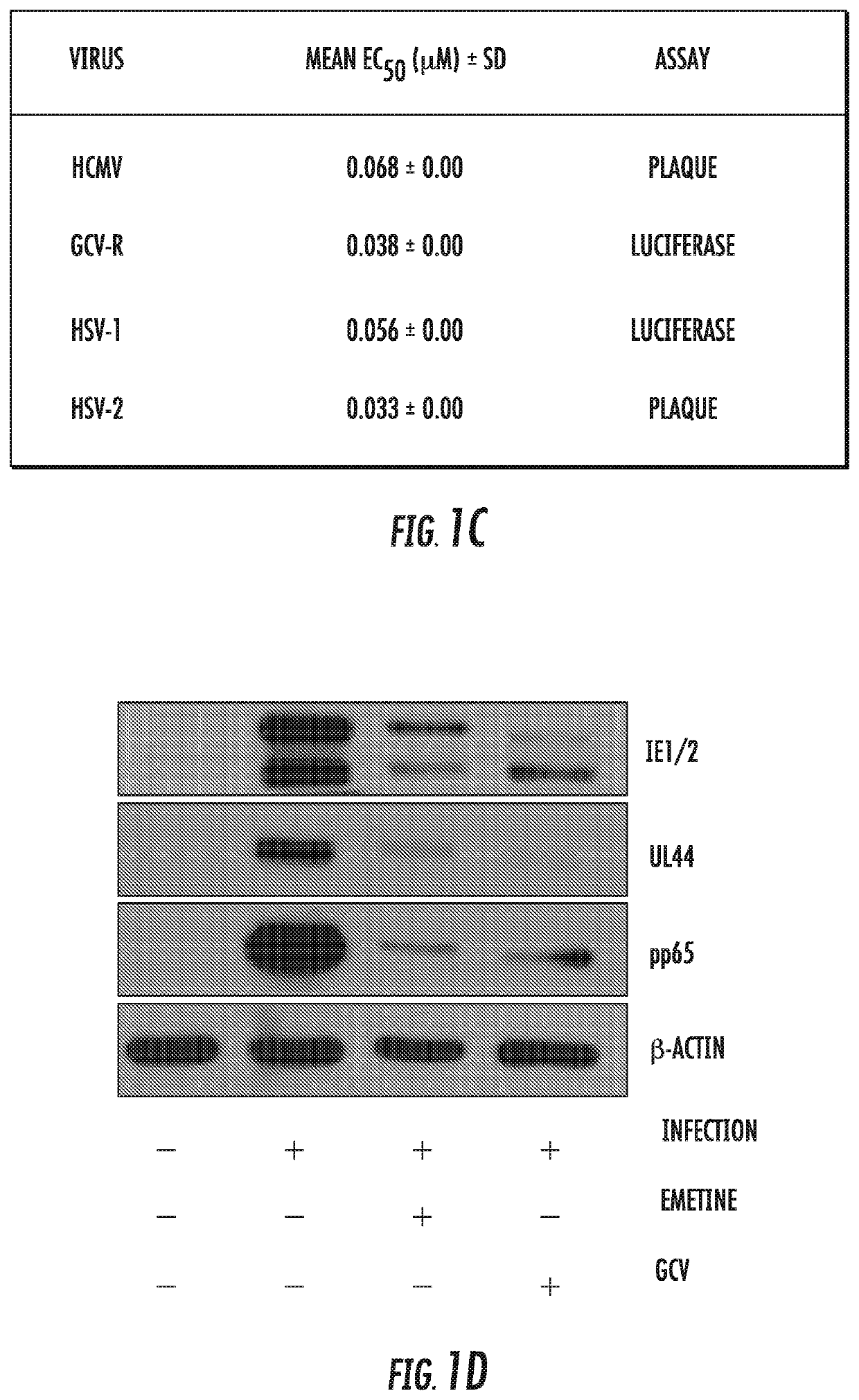
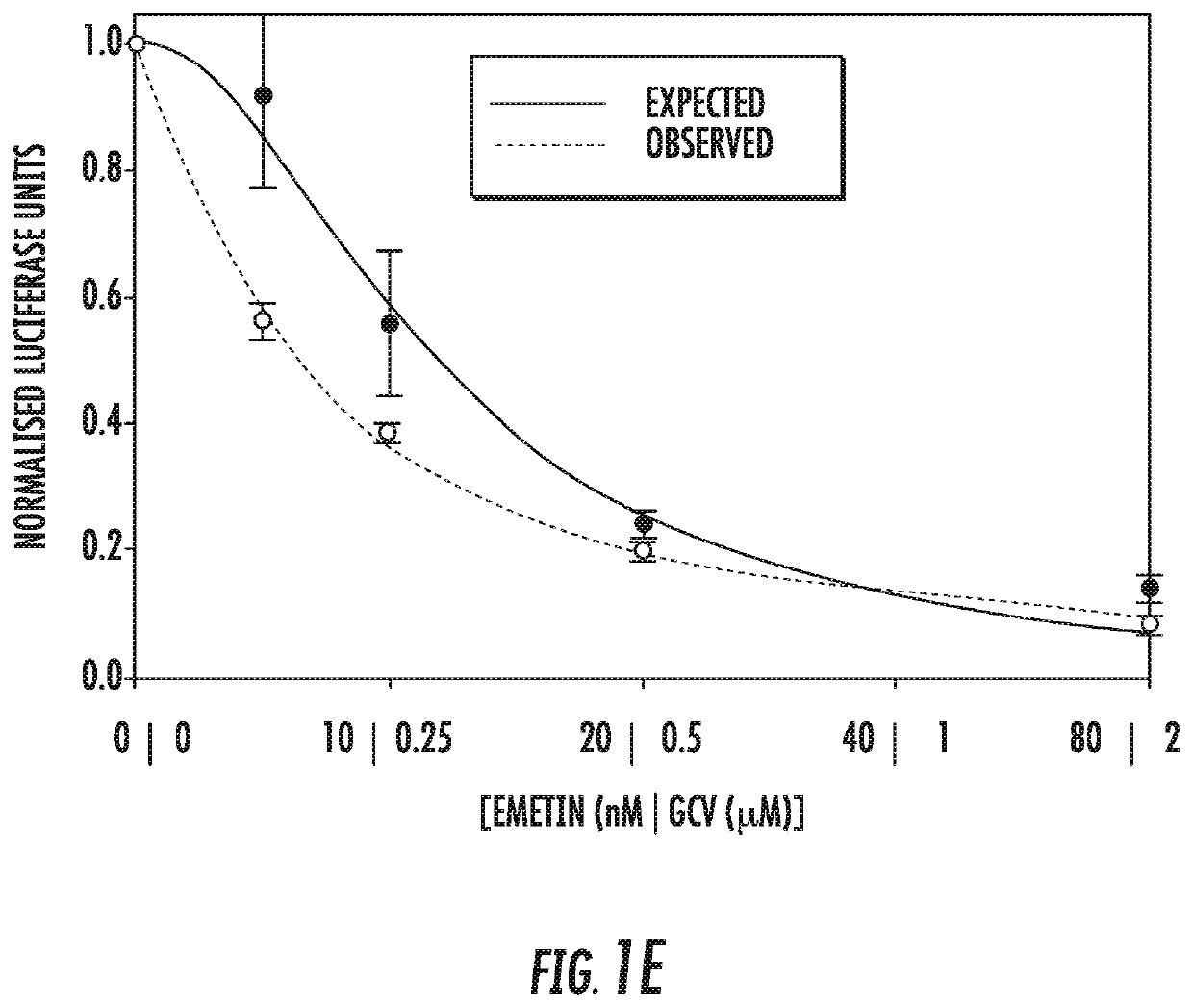
Use of low dose emetine for inhibition of human cytomegalovirus (HCMV)
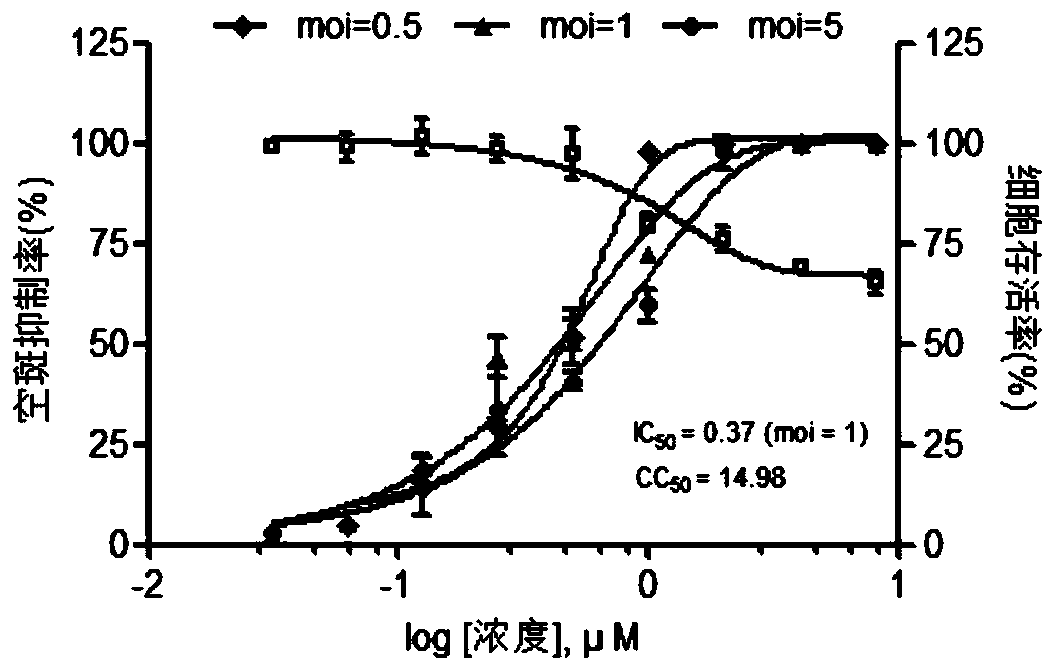
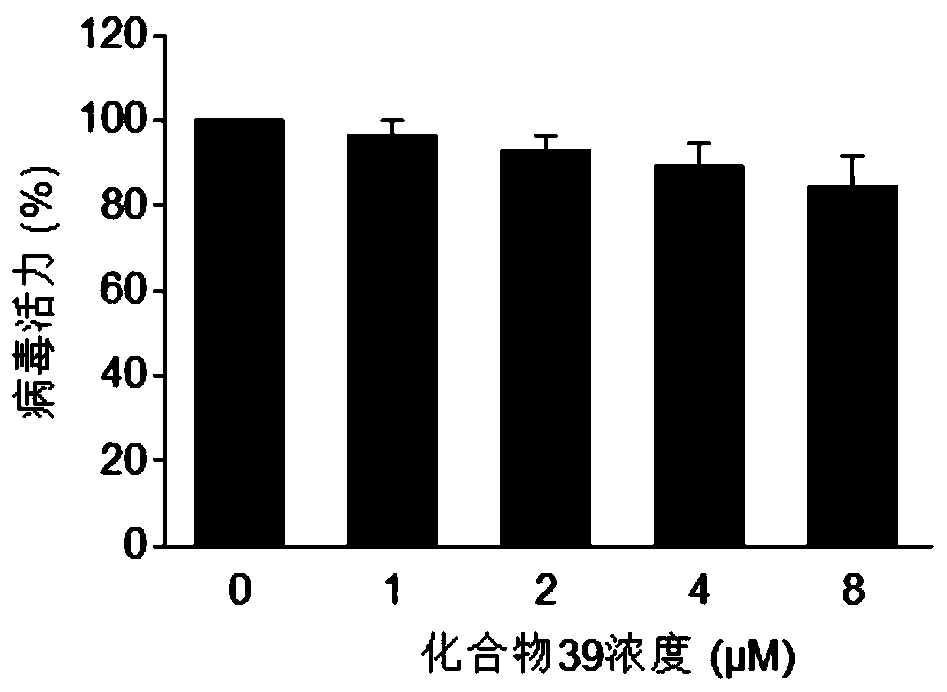
ActiveUS11253511B2Improve efficacyInhibition of replicationDipeptide ingredientsPharmaceutical delivery mechanismAdjuvantPharmaceutical drug
The present invention relates to the field of virology. More specifically, the present invention provides methods and compositions useful for prevention and treatment of human cytomegalovirus (CMV). In one embodiment, a pharmaceutical composition comprises (a) emetine or a derivative thereof; (b) a human cytomegalovirus (HCMV) drug; and (c) a pharmaceutically acceptable carrier. In certain embodiments, the pharmaceutical composition further comprises an adjuvant. In a specific embodiment, the HCMV drug is ganciclovir. In such embodiments, emetine is present at about 1 / 10 to about 1 / 100 the normal dosage for amebiasis.
Owner:UNITED STATES OF AMERICA +1
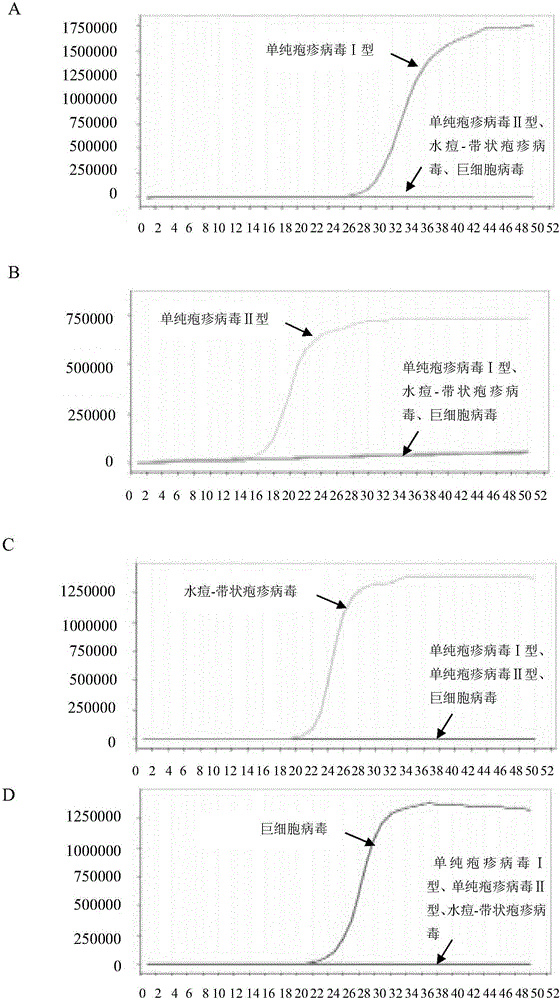
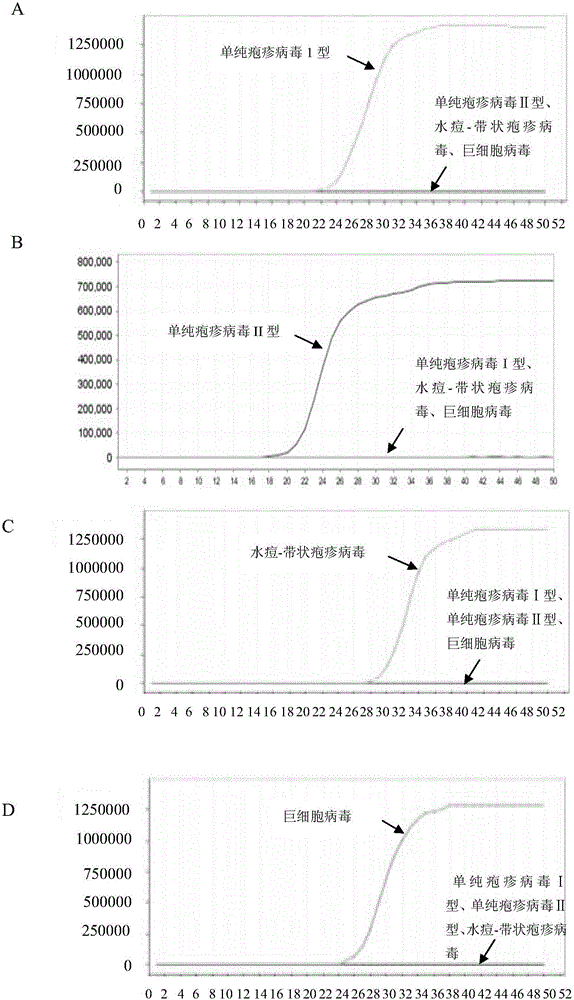
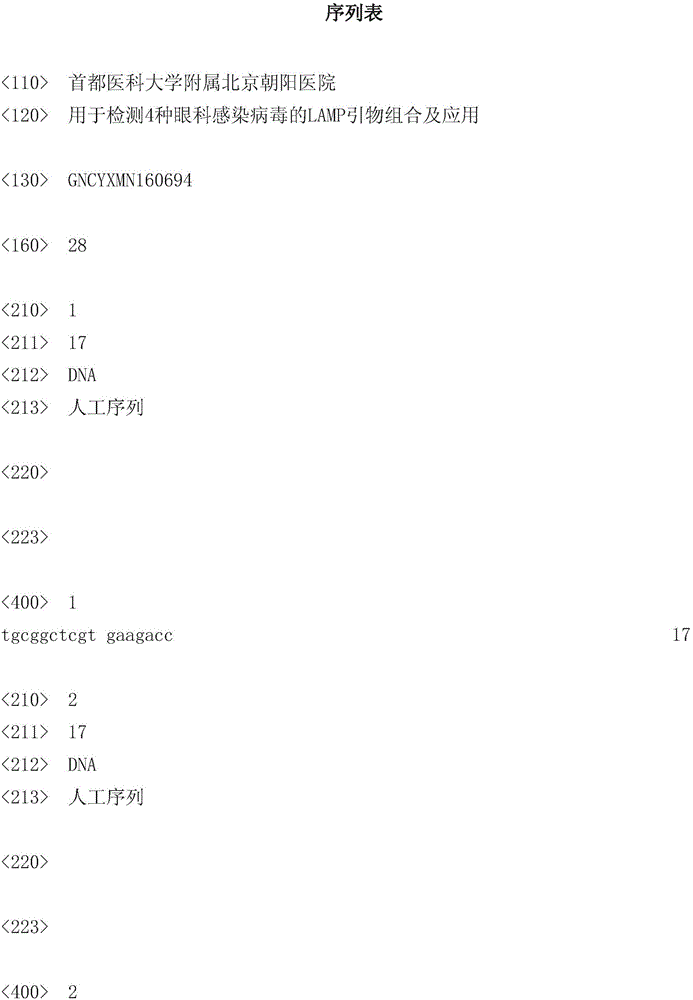
LAMP (loop-mediated isothermal amplification) primer combination for detecting four types of eye infected viruses and application
ActiveCN106048088AMicrobiological testing/measurementMicroorganism based processesLoop-mediated isothermal amplificationSingle strand dna
The invention discloses an LAMP (loop-mediated isothermal amplification) primer combination for detecting four types of eye infected viruses and an application. The primer combination comprises 24 single-stranded DNA molecules shown in sequences from 1 to 24. The invention also discloses the application of the primer combination, and further provides a method for identifying herpes simplex virus type I, herpes simplex virus type II, varicella-zoster viruses or cytomegalo viruses, a method for identifying whether the to-be-detected viruses are the herpes simplex virus type I, the herpes simplex virus type II, the varicella-zoster viruses or the cytomegalo viruses, and a method for identifying whether a to-be-detected sample is infected with the herpes simplex virus type I and / or the herpes simplex virus type II and / or the varicella-zoster viruses and / or the cytomegalo viruses. With the application of LAMP primers and the method, the herpes simplex virus type I, the herpes simplex virus type II, the varicella-zoster viruses and the cytomegalo viruses can be detected rapidly and accurately.
Owner:智德科技(无锡)有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com