Compositions For Delivering Acyclovir
a technology of compositions and acyclovir, applied in the field of acyclovir formulations, can solve the problems of inability to respond to acyclovir therapy, inability to improve the bioavailability of acyclovir, and difficulty in compliance with oral acyclovir formulations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
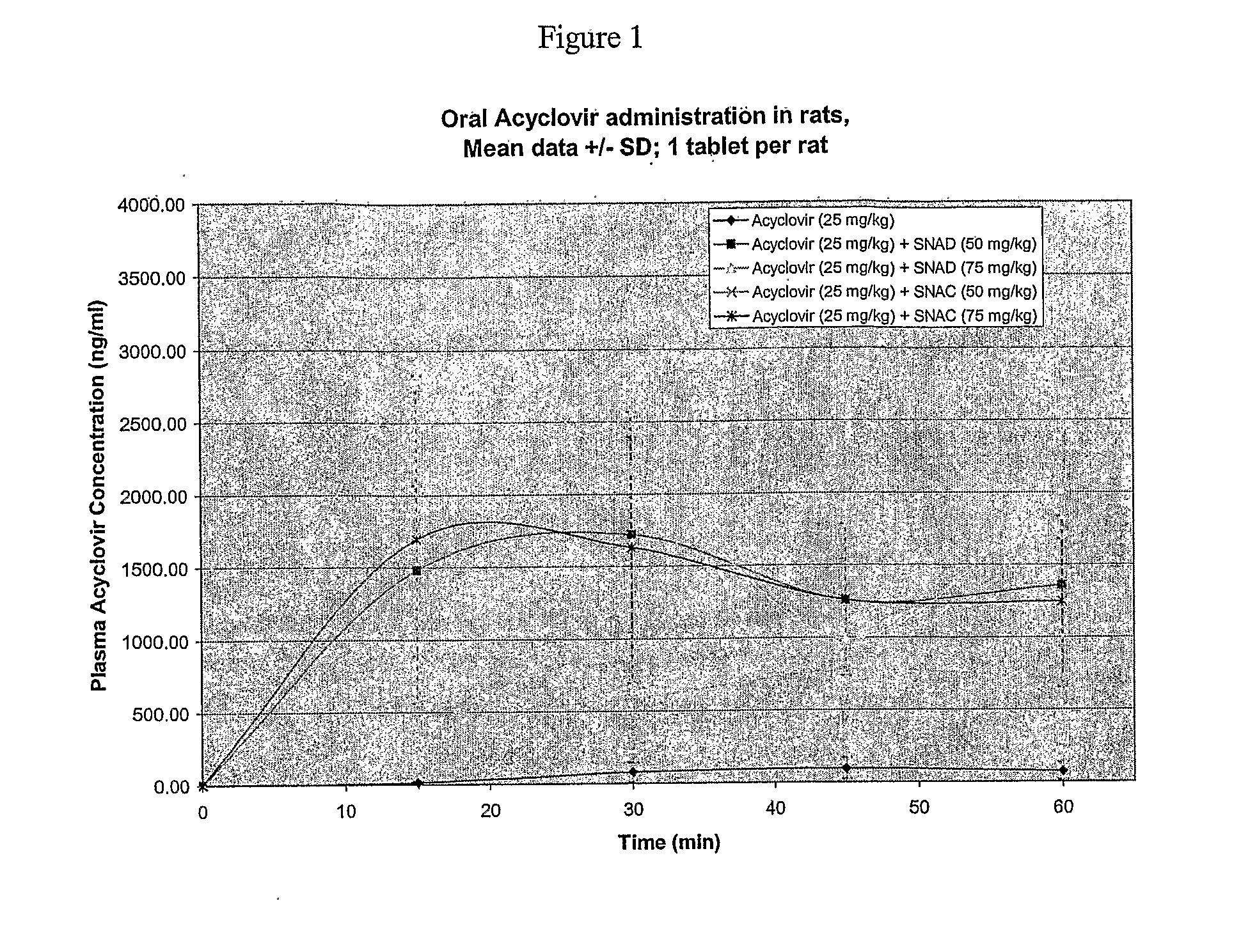
Solid Oral Delivery of Acyclovir in Rats
[0136]The dose of Acyclovir used was 25 mg / kg body weight. The dose of delivery agent was either 50 or 75 mg / kg body weight.
[0137]Approximately 6.25 mg / tablet of acyclovir was blended with either 12.5 or 18.75 mg / tablet (50 and 75 mg / kg, respectively) of delivery agent compound. Upper punch, lower punch and die of a Carver 4350 manual pellet press with a Caplet shape model sold by Natoli Engineering Company, Inc. were treated with magnesium stearate (0.1%). Approximately 6.25 mg (Acyclovir alone), 18.75 mg (Acyclovir+50 mg / kg delivery agent compound), or 25 mg (Acyclovir+75 mg / kg delivery agent compound) of mixed powder was fed into the die and a mini bead shape tablet was made at about 1000 PSI bar pressure. The resulting solid dosage forms were 2.65 mm in diameter and approximately 8.40 mm in length for the 25 mg tablets, 6.3 mm in length for the 18.75 mg tablets, and 2.1 mm in length for the 6.25 mg tablets.
[0138]Male Sprague Dawley rats (˜...
example 2
Delivery of Acyclovir in Dogs
[0141]Six different oral dosage forms (tablets) were administered to dogs: (1) unitary solid oral dosage forms comprising 80 mg of acyclovir and 240 mg of the delivery agent, the monosodium salt of N-(8-[2-hydroxybenzoyl]-amino)caprylic acid (SNAC), (2) a solid oral dosage form comprising 400 mg of acyclovir (Zovirax™, commercially available from GlaxoSmithKline), (3) a solid oral dosage form comprising 800 mg dosage of (Zovirax™, commercially available from GlaxoSmithKline), (4) a solid oral dosage form comprising 500 mg dosage of valacyclovir (Valtrex™, commercially available from GlaxoSmithKline) and (6) unitary solid oral dosage comprising 240 mg of acyclovir and 240 mg of the delivery agent SNAC. Oral administration of acylcovir alone and with a delivery agent was compared to an interveneous dosage form comprising 80 mg of acyclovir (Zovirax™ for injection, commercially available from GlaxoSmithKline).
[0142]Unitary dosages of acyclovir and delivery ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com