Acyclovir ethosome and preparation method thereof
A technology of ethosomes and systems, which is applied in the direction of liposome delivery, antiviral agents, active ingredients of heterocyclic compounds, etc., can solve the lack of research on the stability of ethosomes, affect the stability of ethosome preparations, and encapsulation The efficiency is not high, to achieve the effect of improving bioavailability, improving stability, and good affinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
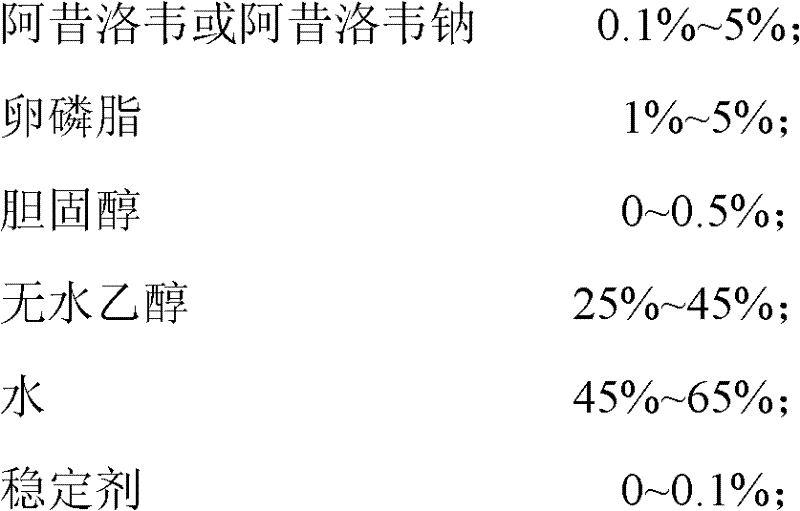
[0019] prescription for
[0020]
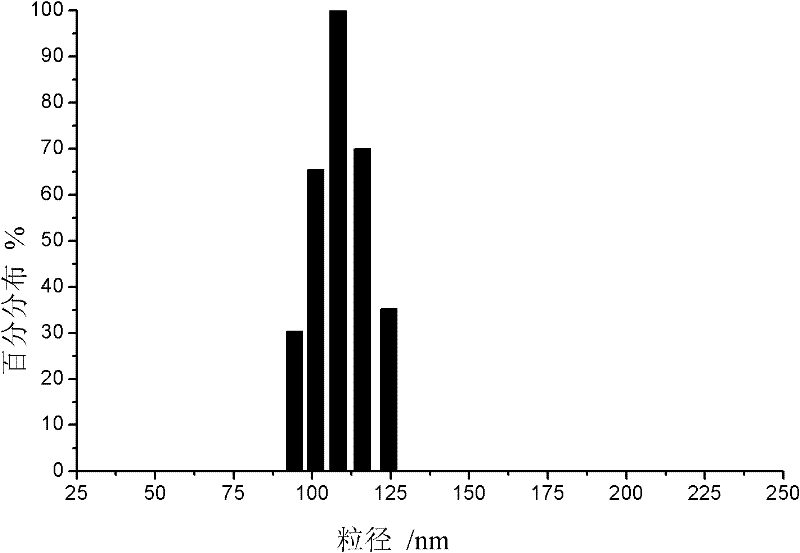
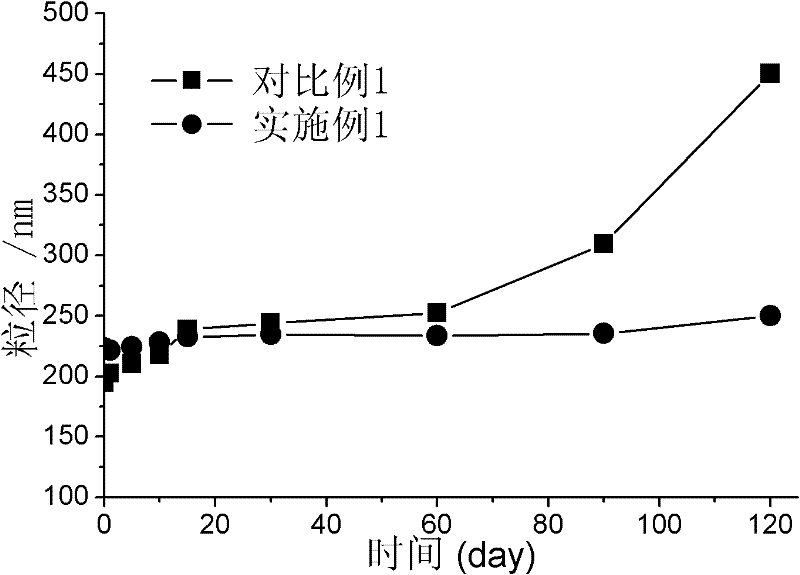
[0021] Preparation method: Dissolve the prescribed amount of acyclovir, phospholipids and cholesterol in absolute ethanol, dissolve PEG6000 in water for injection, mix and stir at room temperature, then slowly add water for injection under airtight conditions, and continue stirring for 30 minutes. After standing still for 1 hour, ultrasonication was performed for 8 minutes, and acyclovir ethosomes were obtained by grading with a 0.45 μm microporous filter membrane. The ethosome particle size was 223.5 nm and the scattering intensity was 69.6 Kcps as measured by a laser scattering instrument. It was 0.249; the encapsulation efficiency was 71.2%.
Embodiment 2
[0023] prescription for
[0024]
[0025] Preparation method: Dissolve the phospholipids, cholesterol and acyclovir in the prescription amount in absolute ethanol, slowly add 1% 6-O-quaternary ammonium salt chitosan aqueous solution under room temperature and stirring under airtight conditions, and then slowly Add water for injection, continue to stir for 30 minutes, after standing for 1 hour, ultrasonic for 10 minutes, use a 0.45 μm microporous filter membrane to granulate to obtain acyclovir ethosome, and the ethosome particle size measured on the laser scattering instrument is 165.9 nm, the scattering intensity is 62.9Kcps, the distribution is 0.205; the encapsulation efficiency is 65.3%.
Embodiment 3
[0027] prescription for
[0028]
[0029] Preparation method: Weigh the prescribed amount of phospholipids, cholesterol, acyclovir sodium and acyclovir and dissolve them in absolute ethanol, slowly add water for injection under room temperature and stirring under airtight conditions, continue to stir for 30 minutes, and let stand After 1 hour, ultrasound for 10 minutes, and a 0.45 μm microporous filter membrane was used to granulate the aciclovir ethosome. The ethosome particle size was 80.2 nm, the scattering intensity was 47.8 Kcps, and the distribution was 0.268 ; The encapsulation efficiency was 58.3%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com