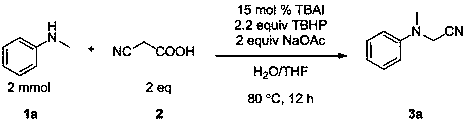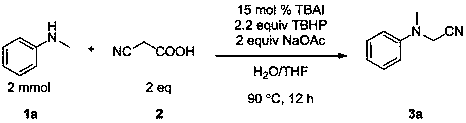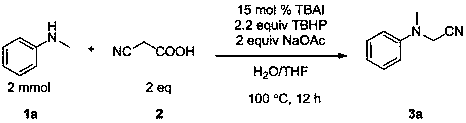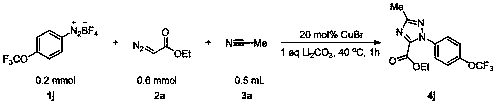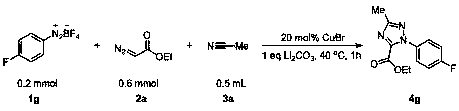Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
40results about How to "Reaction economy" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Method for preparing amino-acid ester
InactiveCN103113247AReact SafeReact greenCarbamic acid derivatives preparationOrganic compound preparationSodium acetatePtru catalyst
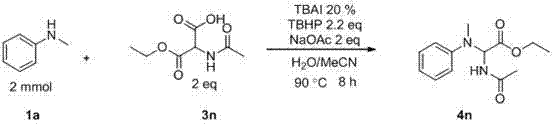
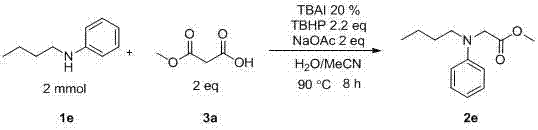
The invention discloses a method for preparing amino-acid ester. In the presence of an oxidant, the method adopts an amine compound and malonic ester as reactants, an iodide as a catalyst and sodium acetate as alkali; a product amino-acid ester is prepared through nucleophilic substitution in a polar solvent; the general formula of the chemical structure of malonic ester is shown in the decstiption; and the iodide is one of I2, TBAI, NIS and IBr. According to the method disclosed by the invention, the reaction activity of the catalyst is high, the reaction conditions are moderate, the application range of substrate is wide, the aftertreatment is convenient, the yield of the target product is high, the preparation process is simple and green and environment-friendly, and the raw materials are widely available.
Owner:SUZHOU UNIV
Hydrogen peroxide-hydrochloric acid oxidation and desulfurization method
InactiveCN103184068AEasy to buyLow priceTreatment with plural serial refining stagesDispersityReaction temperature
The invention discloses a hydrogen peroxide-hydrochloric acid oxidation and desulfurization method. The hydrogen peroxide-hydrochloric acid oxidation desulfurization method comprises the following steps of: oxidizing sulfide in fuel oil into sulphone or sulfoxide with strong polarity in an acid medium by using hydrogen peroxide, and extracting the sulphone or sulfoxide by using a polar solvent, thus removing sulfide in the fuel oil; controlling the potential of an oxidizing agent and the reaction speed by controlling the concentration of the hydrogen peroxide and hydrochloric acid and reaction temperature; and reinforcing the dispersity of the fuel oil in water through an organic matter chaotropic agent, accelerating the reaction speed, and extracting the sulphone or sulfoxide from the fuel oil by using the polar solvent. The hydrogen peroxide-hydrochloric acid oxidation and desulfurization method has advantages of low cost, high oxidation and desulfurization efficiency and very good popularization and application prospect in petrochemical industries.
Owner:HUAIYIN TEACHERS COLLEGE
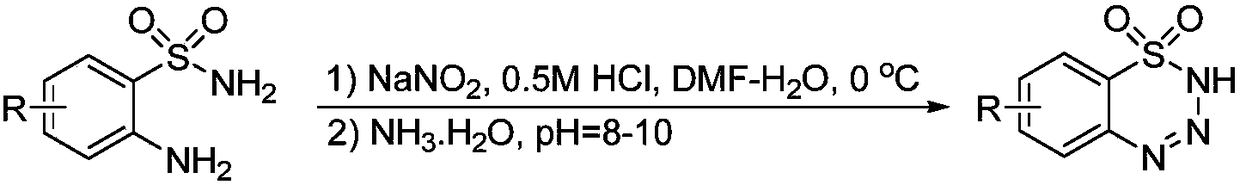
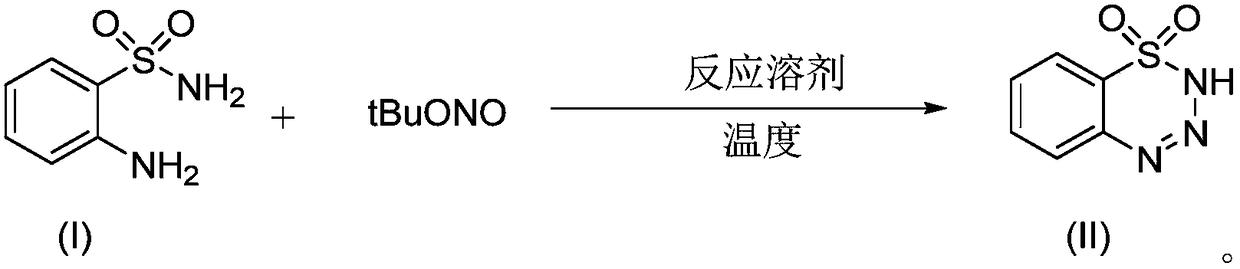
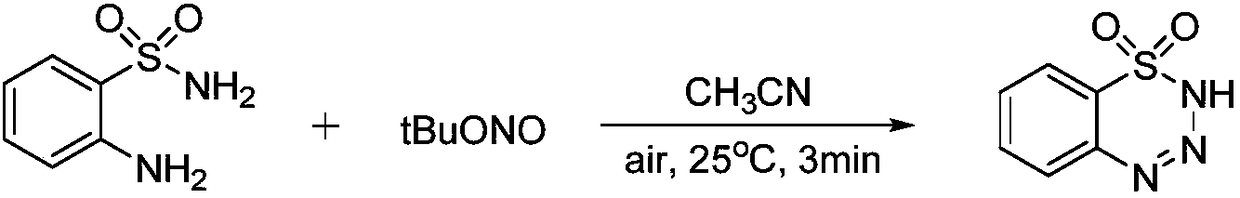
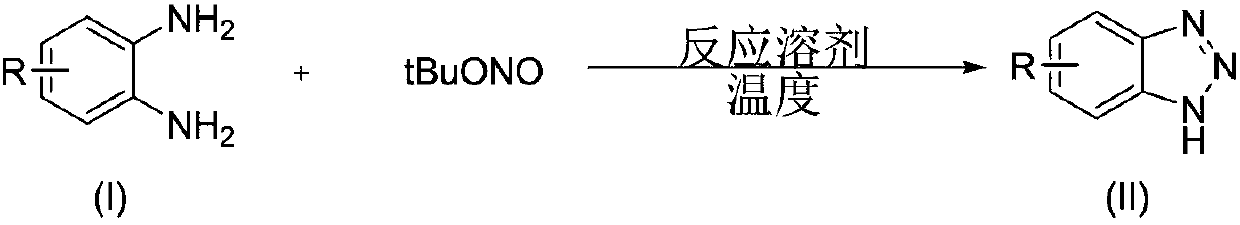
Preparation method of 1,2,3,4-benzoxatriazine-1,1(2H)-dioxide
The invention discloses a preparation method of 1,2,3,4-benzoxatriazine-1,1(2H)-dioxide. The preparation method is characterized in that 2-aminobenzenesul fonamide and tert-butyl nitrite are taken asreaction materials and have a diazo-reaction in a reaction solvent to obtain 1,2,3,4-benzoxatriazine-1,1(2H)-dioxide, and the reaction temperature is 0 DEG C to 50 DEG C. The preparation method has the beneficial effects that the reaction is efficient, the yield is high, the operation is convenient, and the postprocessing is simple; an oxidizing reagent or a catalyst are not required to be added;the reaction condition is mild, the reaction is carried out at a room temperature, the 1,2,3,4-benzoxatriazine-1,1(2H)-dioxide is easy to prepare, and the reaction is green and economical.
Owner:WENZHOU UNIVERSITY
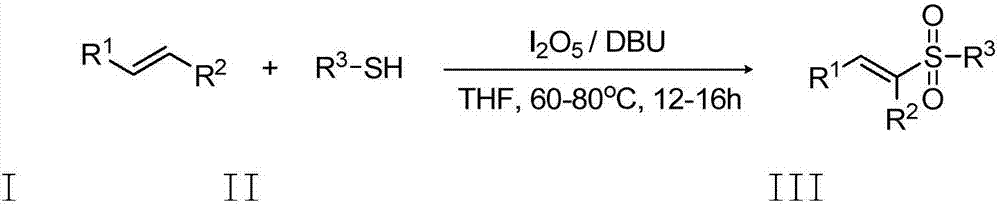
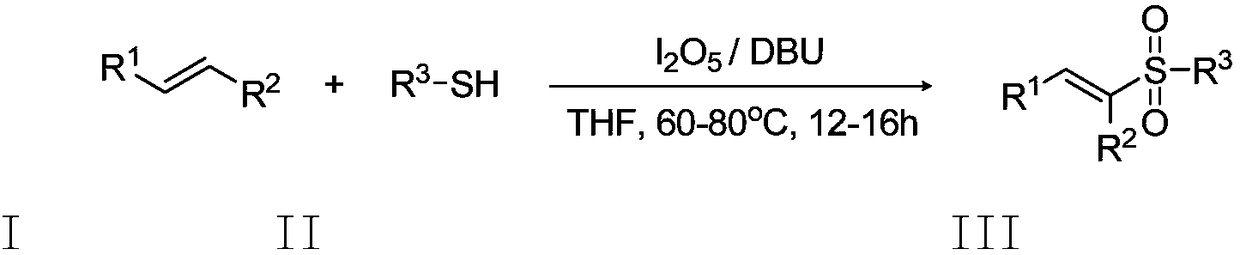
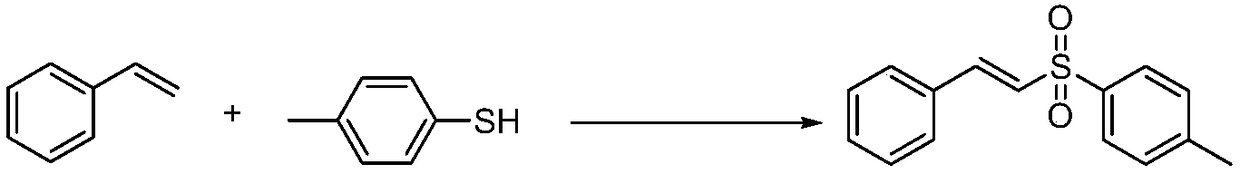
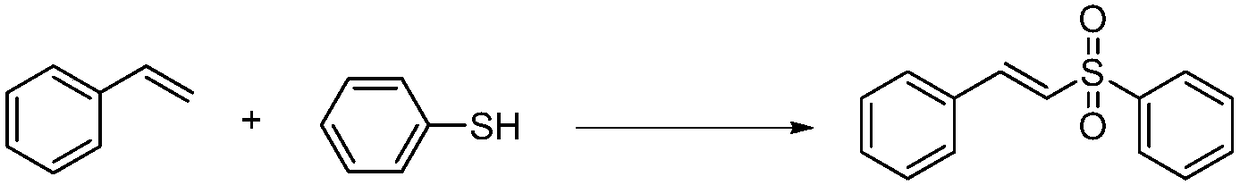
Preparation method of E-vinyl sulfones compound
InactiveCN107417582ARaw materials are cheap and easy to getAtom economy is highOrganic chemistryOrganic compound preparationOrganic baseOxygen
The invention discloses a preparation method of an E-vinyl sulfones compound. The preparation method comprises the following steps: dissolving raw materials of olefin and thiophenol (thioalcohol) into a tetrahydrofuran solvent; then adding lodine pentaoxide and organic base DBU (1,8-diazabicyclo undec-7-ene); directly reacting for 12 to 16 hours under 60 to 80 DEG C; separating and purifying a coarse product after stopping reacting, thus obtaining the E-vinyl sulfones compound. The preparation method has the advantages that raw materials are simple and can be easily obtained, the price is cheap, the reaction requires no metal catalyst, metal pollution is avoided, the operation is simple and is free of harsh conditions of water free, oxygen free and the like, the adaptive range of a substrate is wide, the reaction stereoselectivity is high, the process conditions are stable, and a prepared product is easy to purify.
Owner:QUFU NORMAL UNIV
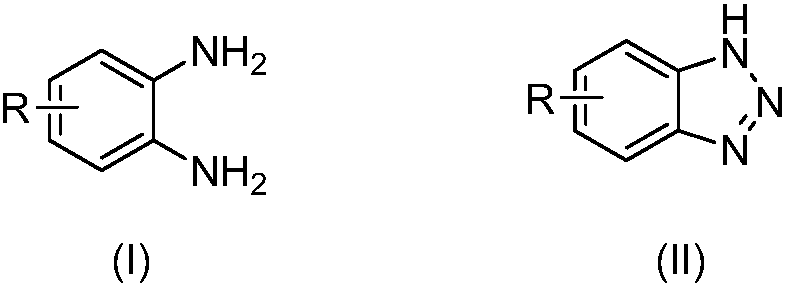
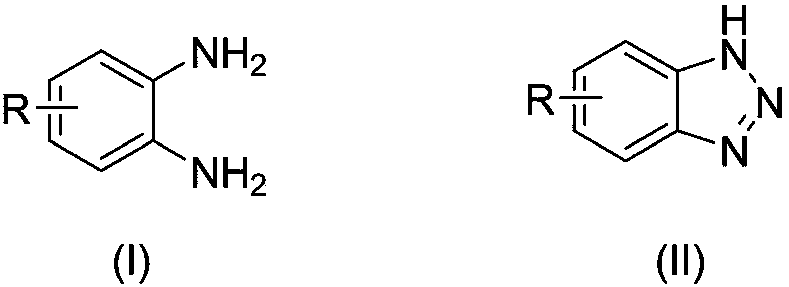
Benzotriazole compounds and a preparing method thereof
The invention relates to benzotriazole compounds and a preparing method thereof. o-phenylenediamine or an o-phenylenediamine compound n-butyl nitrite are adopted as reaction raw materials and are subjected to an intramolecular diazo-reaction at room temperature in a reaction solvent to obtain the corresponding benzotriazole compound. According to the method, the range of substrates is wide, reaction conditions are mild, after-treatment is simple, and a product yield and quality are high. A novel synthesis route and method for the benzotriazole compounds are developed. The benzotriazole compounds have good application potentials and research value.
Owner:WENZHOU UNIVERSITY
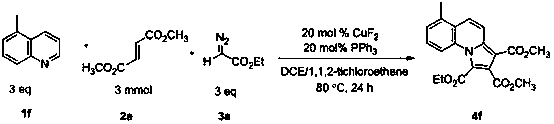
Indolizine derivative and preparation method thereof
The invention discloses an indolizine derivative and a preparation method thereof. A cheap copper compound is taken as a catalyst, a pyridine derivative, an olefinic derivative and a heavy nitrogen derivative are taken as reactants, and the catalyst and the reactants are subjected to cyclization reaction in a mixed solvent to obtain a product indolizine. The catalyst prepared with the method disclosed by the invention is cheap, can be easily obtained, is high in reaction activity, is moderate in reaction conditions, only needs to be carried out in the air, has a wide primer applicable range and is convenient in aftertreatment, and a final product can be obtained through simple column chromatography after reaction is finished. A target product has the advantages of high yield, simple preparation process, environment protection and wide raw material resource. The indolizine derivative conforms to the requirement and the direction of modern green chemistry development and has a potential industrial application value.
Owner:SUZHOU UNIV
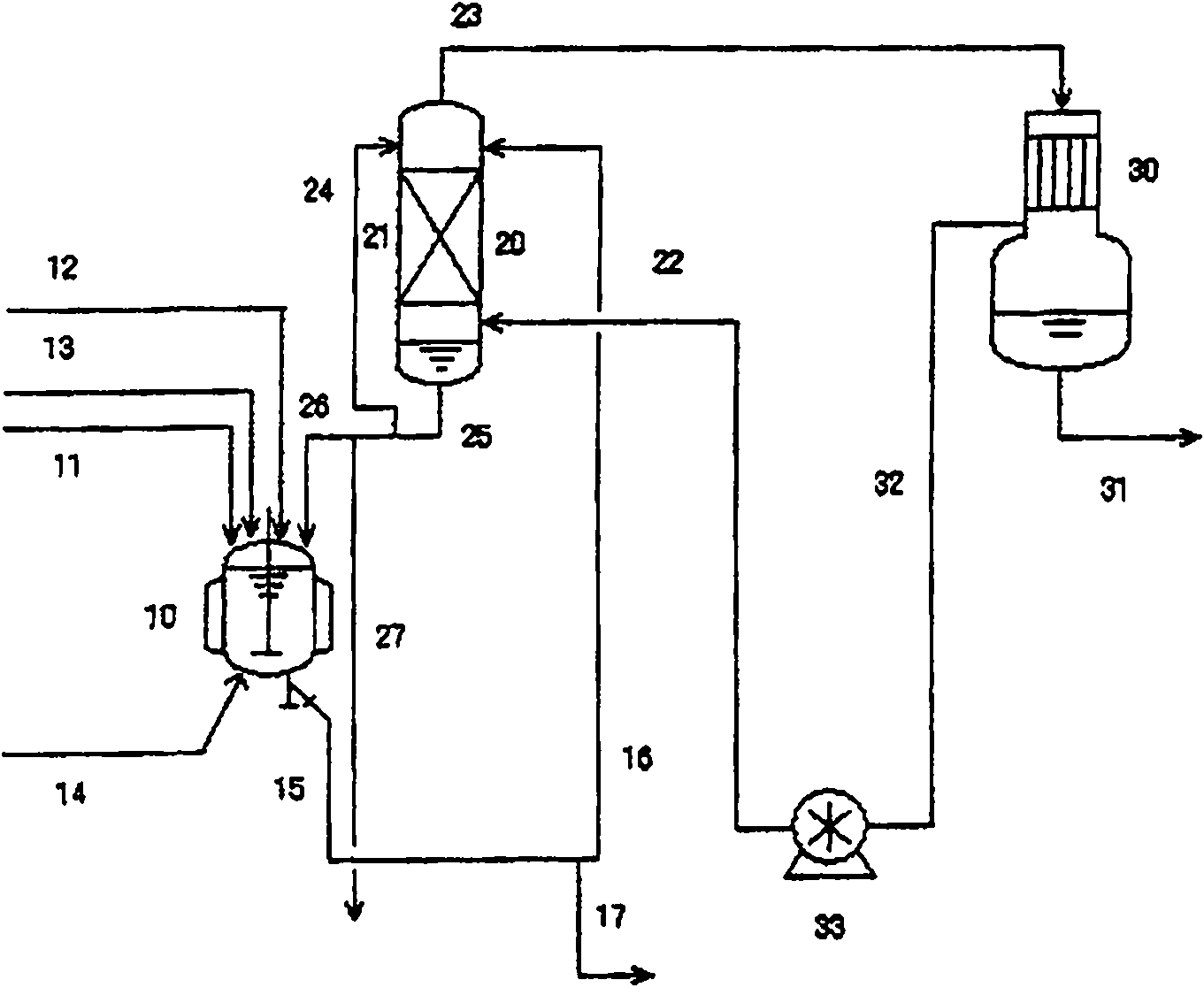
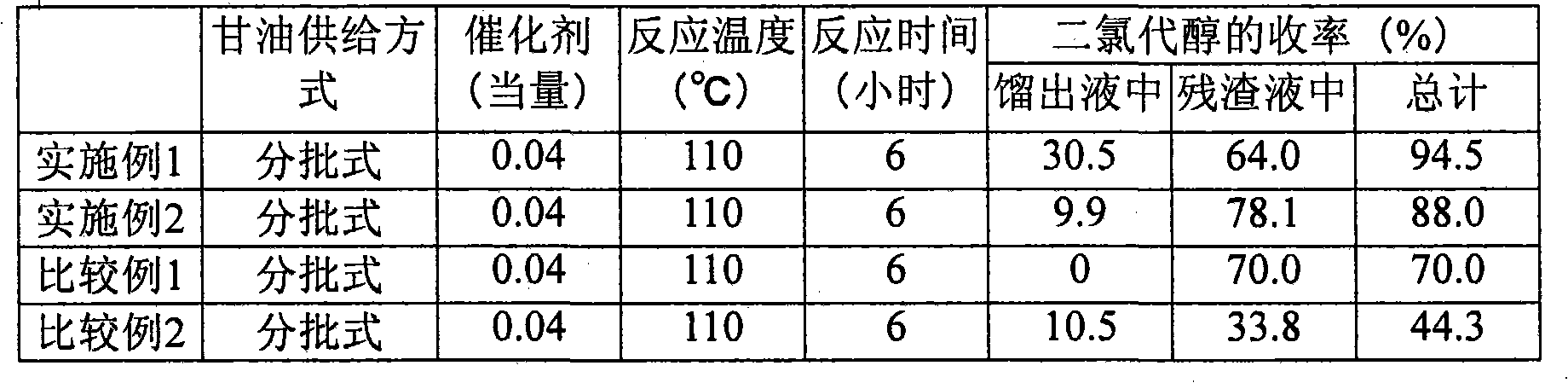
Process for producing chlorohydrin
InactiveCN101808968AReact efficiently and economicallyReaction economyPreparation by halogen introductionHydroxy compound separation/purificationGlycerolAliphatic hydrocarbon
A process for producing chlorohydrins through reaction of a polyhydroxylated aliphatic hydrocarbon, such as glycerol, and / or an ester of polyhydroxylated aliphatic hydrocarbon with a chlorinating agent, which process realizes high reaction efficiency and economic advantage. The process for producing chlorohydrins comprises the steps of (A) reacting a polyhydroxylated aliphatic hydrocarbon and / or an ester of polyhydroxylated aliphatic hydrocarbon with a chlorinating agent and (B) bringing the thus obtained reaction mixture into countercurrent contact with a diffuser gas so as to cause at leasta potion of reaction product to be entrained by the diffuser gas and drawn out from the reaction mixture. Preferably, the process further comprises the step of (C) condensing at least a portion of the reaction product entrained by the diffuser gas.
Owner:OSAKA SODA CO LTD
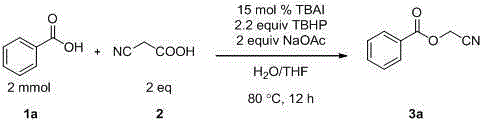
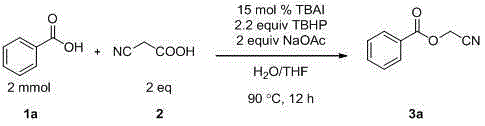
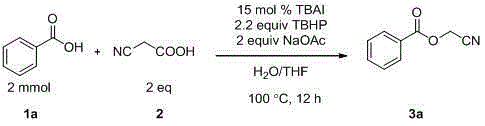
Preparation method of cyanomethyl ester
InactiveCN105294495AIncrease profitNo generationCarboxylic acid nitrile preparationOrganic compound preparationSodium acetatePtru catalyst
The invention discloses a preparation method of cyanomethyl ester. In the presence of an oxidizing agent, a carboxylic acid compound and cyanoacetic acid are used as reactants, iodide is used as a catalyst, and sodium acetate is used as alkali, so as to prepare the cyanomethyl ester in a mixed solvent through nucleophilic substitution. According to the method, the reactivity of the catalyst is high, reaction conditions are mild, the application range of substrates is wide, the postprocessing is convenient, the yield of target products is high, a preparation process is simple, green and environmentally friendly, and the sources of used raw material are wide.
Owner:SUZHOU UNIV
Simple preparation method of isoxazoline
The invention discloses a simple preparation method of isoxazoline. The simple preparation method is characterized in that a series of isoxazoline compounds are efficiently synthesized by using aldehyde, p-toluenesulfonhydrazide, olefin and tert-butyl nitrite as substrates, copper chloride as a catalyst and tetramethylethylenediamine (TMEDA) as alkali through the one-pot two-step method. The method has the advantages that the catalyst is cheap, reaction is economical, good substrate universality is achieved, the raw materials are easy to obtain, later-stage functionalization is more convenient, reaction conditions are mild, and gram-level scale reaction is good; and especially, the compounds with medium yield can be obtained without the catalyst, post-treatment is simple and convenient, the application of the compoumds in drug molecule synthesis and large-scale industrialization is facilitated, and the requirements and trends of modern green chemistry and medicinal chemistry are met.
Owner:SUZHOU UNIV
Preparation method of tert-butyl acrylate
InactiveCN111099996AHigh yieldReduce the temperatureMolecular sieve catalystsOrganic compound preparationMolecular sieveTert-butyl acrylate
The invention discloses a preparation method of tert-butyl acrylate, which comprises the following steps: acrylic acid, a catalyst, a polymerization inhibitor and isobutene are added into a reactor, and esterification reaction is carried out at 20-50 DEG C under the pressure of 1-3 MPa at the isobutene volume space velocity of 0.5-5.0 / h to synthesize tert-butyl acrylate, wherein the feeding molarratio of the acrylic acid to the polymerization inhibitor to the isobutene is 1: (0.05-0.2): (0.2-0.8), the catalyst is selected from at least one of strongly acidic cation exchange resin, p-toluenesulfonic acid and an acid group modified SBA-15 mesoporous molecular sieve, the polymerization inhibitor is a mixture of tert-butyl catechol and tert-butyl alcohol, and the mass ratio of tert-butyl catechol to tert-butyl alcohol is 1: (1-3). According to the preparation method of tert-butyl acrylate, through the combination of the special catalyst and the polymerization inhibitor, the yield of esterification reaction is greatly improved, the reaction temperature is reduced, and the reaction is milder, greener and more economical.
Owner:TAICANG YUNTONG BIOCHEM ENG
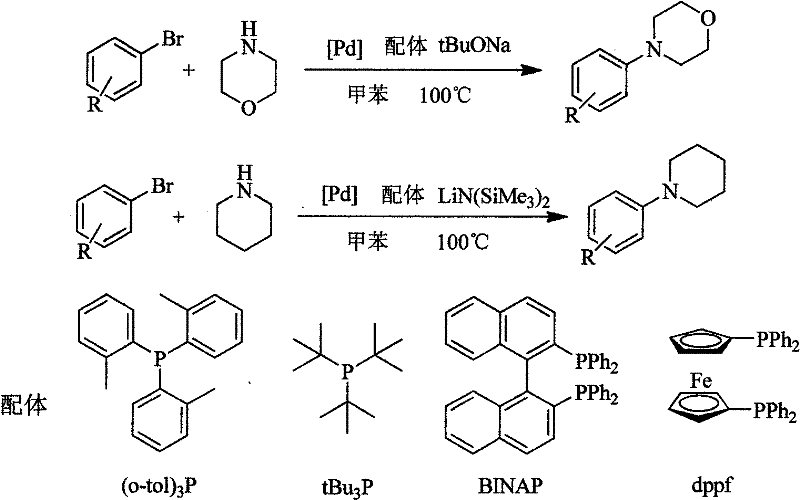
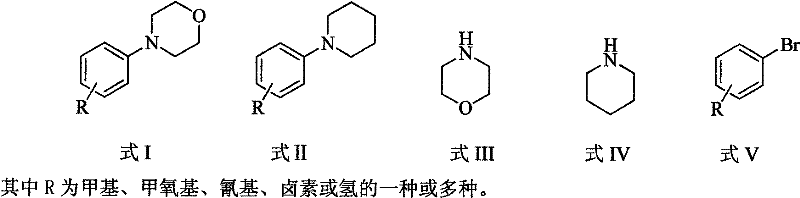
Method for easily preparing aryl morpholine and aryl piperidine
The invention relates to a method for easily preparing aryl morpholine (formula I) and aryl piperidine (formula II). The preparation method mainly comprises the following steps of: reacting morpholine (formula III) or piperidine (formula IV) with aryl bromide (formula V) in an organic solvent in the presence of a catalytic amount of palladium compound with a simple structure, a phosphorus (phosphonium) ligand with a simple structure and an alkali at a certain temperature for 3-10 hours; and performing column chromatography isolation to obtain aryl morpholine (formula I) or aryl piperidine (formula II). Compared with the conventional method for preparing aryl morpholine and aryl piperidine, the method has the advantages of easiness, readily-available catalysts and ligands, low preparation cost, high yield and the like.
Owner:EAST CHINA UNIV OF SCI & TECH
Nitrogen-doped graphene quantum dot-iron ion composite nano-enzyme and preparation and application thereof
ActiveCN113289654AReact greenDoes not involve acid or alkaline mediaMaterial analysis by observing effect on chemical indicatorColor/spectral properties measurementsDoped graphenePeroxidase
The invention discloses a nitrogen-doped graphene quantum dot-iron ion composite nano-enzyme and a preparation method and application thereof, and belongs to the technical field of nano-enzymes. The preparation method of the nitrogen-doped graphene quantum dot-iron ion composite nano-enzyme comprises the following steps: synthesizing nitrogen-doped graphene quantum dots by using 1-aminopyrene as a carbon source and a nitrogen source and H2O as a reaction medium through a one-step hydrothermal method, and compounding the nitrogen-doped graphene quantum dots with Fe < 3 + > to obtain the nitrogen-doped graphene quantum dot-iron ion composite nano-enzyme. The nano-composite has the activity of pseudo-peroxidase. The nitrogen-doped graphene quantum dot-iron ion composite nano enzyme can be used as pseudo-peroxidase to distinguish hydroquinone and isomers thereof, and selective colorimetric detection of hydroquinone is realized. The preparation method is simple, and the synthesized nano-enzyme is high in activity.
Owner:ZHEJIANG SCI-TECH UNIV
Method for preparing isoxazoline
ActiveCN112028848AReaction economyBroad substrate versatilityOrganic chemistryPtru catalystOrganic solvent
The invention relates to a method for preparing isoxazoline, which comprises the steps of reacting olefin, a diazo compound and tert-butyl nitrite in an organic solvent at the temperature of 25-50 DEGC under the action of a Lewis acid catalyst to obtain isoxazoline after complete reaction. Lewis acid is used as a catalyst for synthesizing isoxazoline, reaction conditions are mild and can be carried out under the condition that the temperature is as low as the room temperature, use of transition metal is avoided, and the product yield is high.
Owner:SUZHOU UNIV
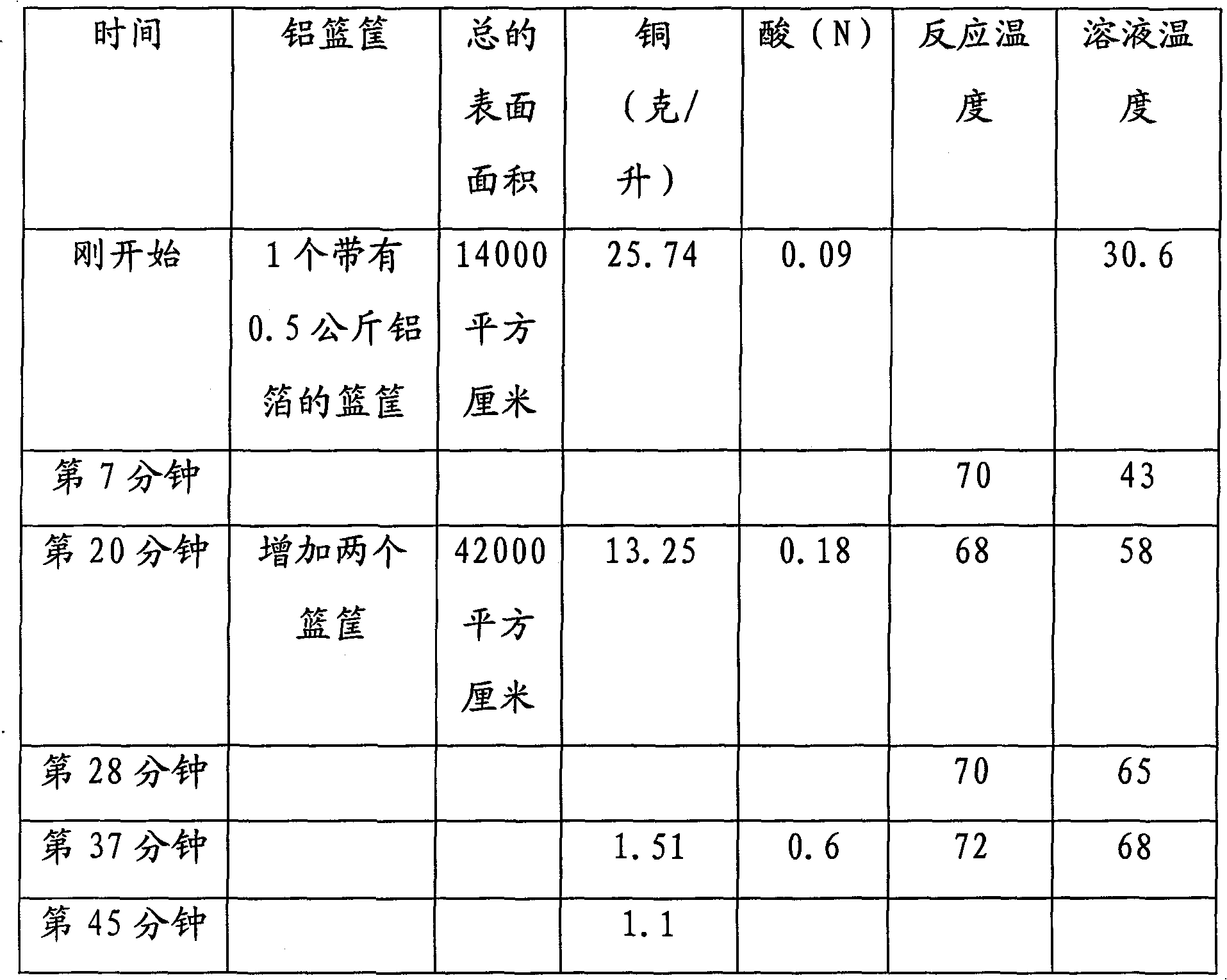
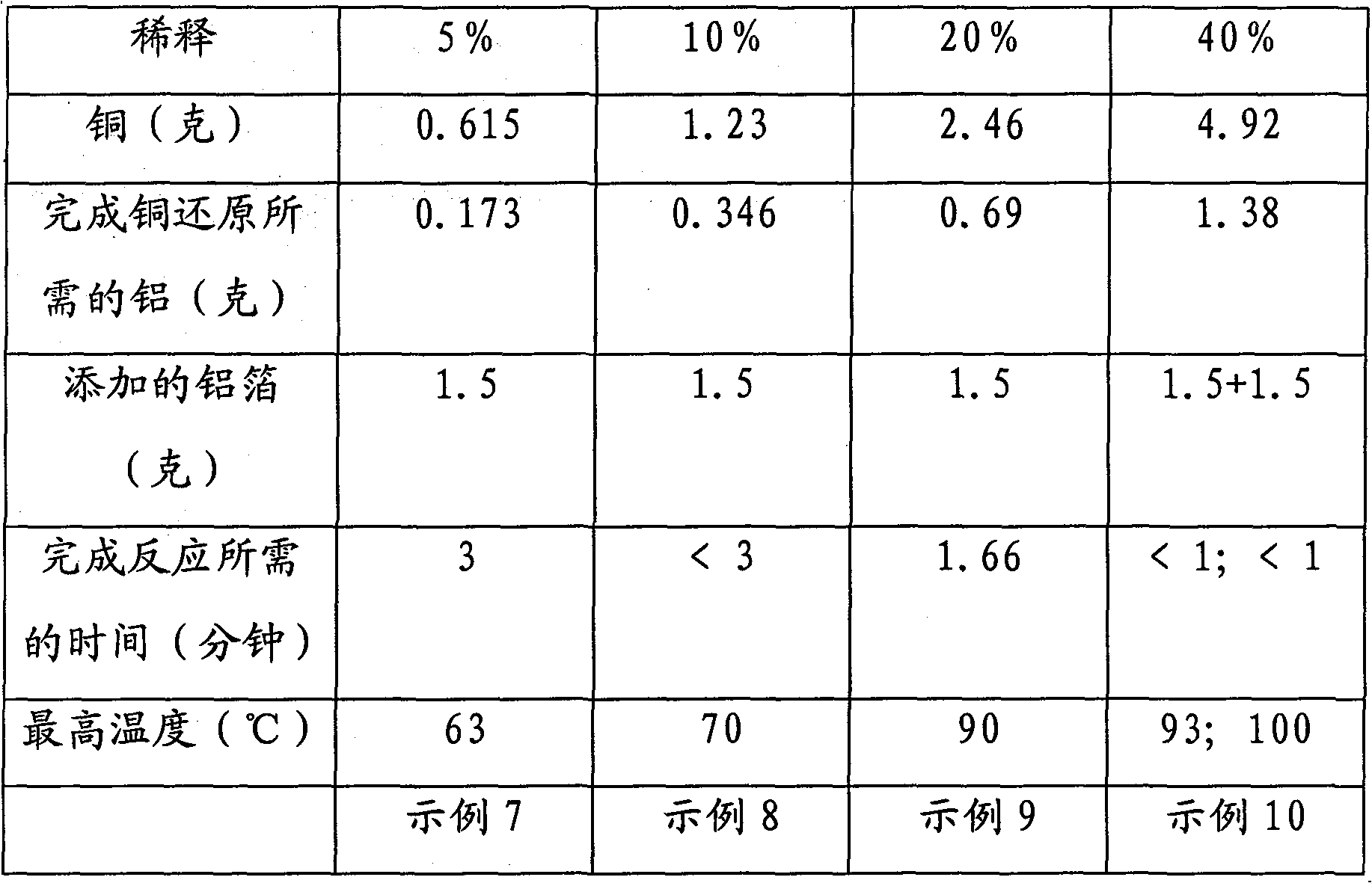
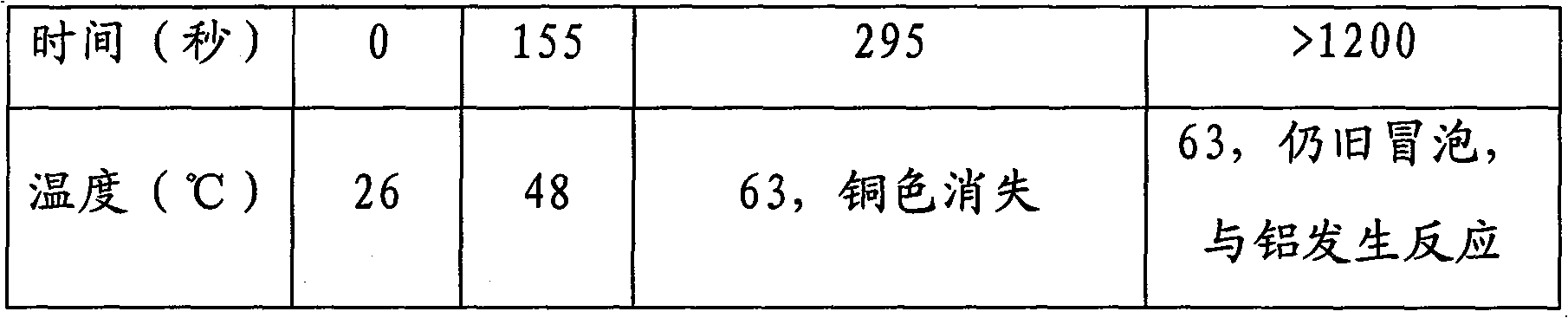
Technique for processing and recovering disuse acidic copper etchant by metal aluminum
InactiveCN101209874BRapid responseThorough responseWaste water treatment from metallurgical processWater/sewage treatment by reductionSolid reactionChemical products
The invention discloses a technology for treating and recycling waste acid copper etchant with metal aluminum. The technology first controls variables such as temperature, copper concentration of waste acid copper etchant, etc. and then adopts one or more treating spouts filled with the metal aluminum to treat the copper etchant, and then copper is separated out as metal. The technology can be used in batch or continuous mode, the treating spouts applied are connected in series or in parallel or in series-parallel and heating or cooling is carried out to control the required temperature. The inside of the treating spouts can be optionally provided with an embedded separation mechanism so as to keep solid reaction product. The treated acid etchant contains dissolved aluminum salt and can be used as chemical product or raw material for waste treatment.
Owner:DAREN TECH
Method for preparing 1,4-dicarbonyl derivative
ActiveCN103467225BReduce usageEasy to purifyCarbamic acid derivatives preparationCarboxylic acid nitrile preparationPtru catalystOxidative coupling of methane
Owner:安徽(淮北)新型煤化工合成材料基地管理委员会科技服务中心
Tactico derivatives and their preparation methods
ActiveCN108373453BWide applicabilityMany reaction stepsPhysical/chemical process catalystsOrganic chemistryArylPtru catalyst
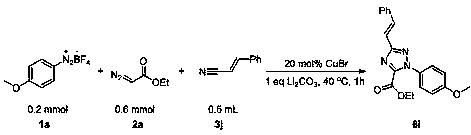
The invention discloses a triazole derivative and a preparation method thereof. According to the preparation method, aryl diazonium fluoroborate, a diazoester derivative and organic nitrile are takenas reaction substrates, transition metal is taken as a catalyst, inorganic alkali is taken as an additive, and the triazole derivative is prepared by virtue of cyclization reaction. The preparation method has the characteristics that the reaction is relatively economical, the substrates are relatively wide in universality, the later-period functionalization is relatively easily realized, reactionconditions are mild, the reaction can be carried out in air, the dose of a catalyst is low, the post-treatment is simple and convenient, and the purification and industrial utilization of a product are promoted; and meanwhile, the raw materials including reactants and catalysts adopted in the preparation method are cheap and easily available, the reaction constitution is reasonable, a ligand is not required, reaction steps are few, the relatively high yield can be realized only through one-step reaction, and the preparation method is accordant with the requirements and directions of current environment-friendly chemistry and pharmaceutical chemistry and is suitable for screening high-activity triazole derivative drugs.
Owner:ZHANGJIAGANG INST OF IND TECH SOOCHOW UNIV +1
A kind of method for preparing oxalate
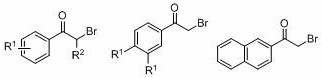
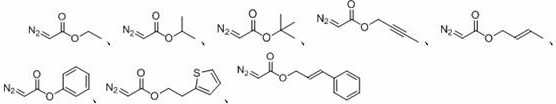
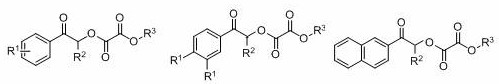
ActiveCN108863777BToxicReaction economyOrganic compound preparationCarboxylic acid esters preparationPtru catalystOrganic dye
The invention discloses a method for preparing oxalate: using diazo compounds and α-Br ketones as reaction substrates, and using O 2 As oxygen source and oxidizing agent, visible light is used as energy source, organic dye is used as photocatalyst, and oxalate is obtained through free radical process in organic solvent. The method used in the present invention has the following characteristics: the reaction is more environmentally friendly and economical, the substrate has wider applicability, the later functional group is easier, the reaction condition is mild, it can be carried out in the air, the amount of photocatalyst is less, and the post-treatment is simple. At the same time, the reactants, photocatalysts and other raw materials used in the present invention are cheap and easy to obtain, the reaction composition is reasonable, no ligand is needed, the atom economy is high, the reaction steps are few, and a higher yield can be obtained with only one step of reaction, which is in line with contemporary green The requirements and direction of chemistry and sustainable development are suitable for the synthesis of asymmetrically substituted oxalates that are difficult to synthesize by traditional methods.
Owner:SUZHOU UNIV
A kind of preparation method of e-alkenyl sulfone compound
InactiveCN107417582BAvoid pollutionImprove responseOrganic chemistryOrganic compound preparationOrganic baseOxygen
Owner:QUFU NORMAL UNIV
Application of diphenylphosphine methyl-substituted calix [4] arene
ActiveCN101927183BReduce dosageReaction economyOrganic-compounds/hydrides/coordination-complexes catalystsSulfonic acid amide preparationPtru catalystEconomic benefits
The invention discloses an application of diphenylphosphine methyl-substituted calix [4] arene serving as a catalyst for aza-Baylis-Hillman reaction and an application method by which an aza-Baylis-Hillman affixture with high yield can be prepared. The method is characterized in that the reaction condition is wild, the catalyst dosage is less, the catalyst recovery and reuse are easy and the like, thereby having greater implementation value and social and economic benefits.
Owner:ZHEJIANG UNIV OF TECH
A kind of method for preparing 1,4-dihydrooxazine
ActiveCN109705052BToxicReaction economyOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsPtru catalystAcyl group
Owner:SUZHOU UNIV
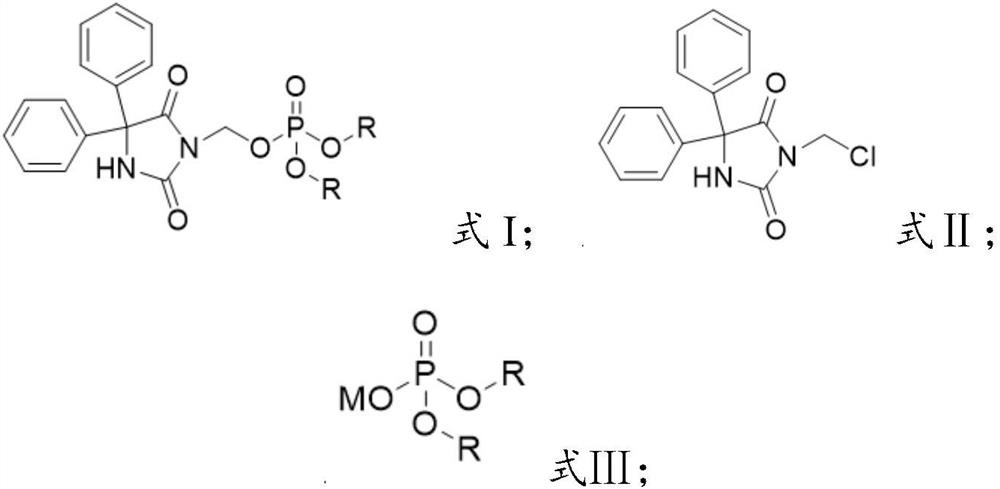
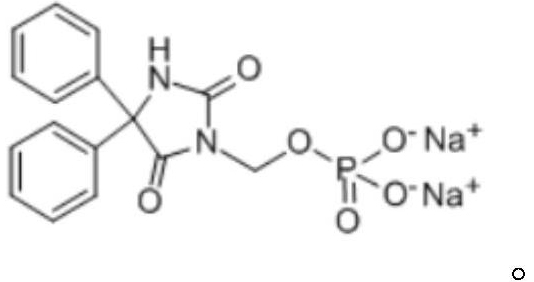
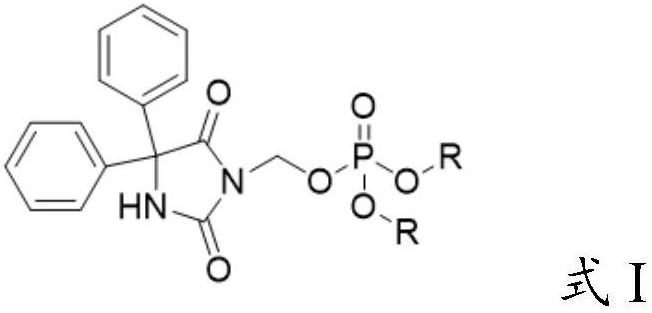
Preparation method of fosphenytoin sodium intermediate
PendingCN114685561AReaction economyAvoid Heavy Metal ResiduesGroup 5/15 element organic compoundsOrganic chemistry methodsChloride potassiumInorganic salts
The invention relates to the technical field of drug synthesis, in particular to a preparation method of a fosphenytoin sodium intermediate, which comprises the following steps: A) under the action of a catalyst, reacting a compound as shown in a formula II with a compound as shown in a formula III in a solvent a; and B) mixing a product obtained after the reaction in the step A) with a solvent b, cooling, and crystallizing to obtain the compound shown in the formula I. In the preparation process of the compound as shown in the formula I, expensive dibenzyl phosphate silver salt does not need to be used, the reaction is more economical, and residues of heavy metals such as silver are avoided; meanwhile, inorganic salts such as potassium chloride and sodium chloride can be effectively removed without a desalting step, so that the operation steps are simplified, and the production efficiency is improved. Moreover, the preparation method provided by the invention can obtain higher yield and purity, is simple and convenient to operate, high in production efficiency, safe and suitable for industrial mass production, and has a good market application prospect.
Owner:SICHUAN CREDIT PHARMA
A method for preparing fully substituted amidines
ActiveCN108383760BReaction economyBroad substrate versatilitySilicon organic compoundsOrganic-compounds/hydrides/coordination-complexes catalystsPtru catalystHigh activity
The invention discloses a method for preparing total substitution amidine. Sulfonamide derivatives, nitrile derivatives and diazo derivatives are used as reaction substrates, transition metal is usedas a catalyst, and four-component cascade reaction is carried out in organic solvents to prepare the total substitution amidine. The method has the advantages that the reaction is economical, the substrates are high in universality, functionalization in late periods can be facilitated, raw material waste can be prevented, reaction conditions are mild, the method can be implemented in air and is low in catalyst dosage and favorable for product purification and large-scale industrial application, and after-treatment is simple and convenient; raw materials such as reactants and the catalyst for the total substitution amidine are inexpensive and easily available, the reaction is reasonable in composition, ligands can be omitted, the method is high in atom economy, includes few reaction steps,is high in yield only by the aid of the one-step reaction, conforms to modern green chemistry and medicinal chemistry requirements and directions, is suitable for screening high-activity amidines medicines and is suitable for large-scale industrial production, and gram-scale reaction can be effectively carried out.
Owner:ZHANGJIAGANG INST OF IND TECH SOOCHOW UNIV +1
Preparation method of aporphine alkaloids and intermediates thereof
The invention discloses a preparation method of aporphine alkaloid intermediates, wherein the preparation method of the aporphine alkaloid intermediates comprises the steps: under the actions of a palladium catalyst and an additive, carrying out alkylation reaction on N-aryl-quinoline-2-formamide and ethyl bromoacetate to obtain the aporphine alkaloid intermediates. The invention further disclosesvarious aporphine alkaloids with various functional groups and derivatives of the apophenanthrene alkaloids, which can be synthesized by taking the intermediate preparation method as a key step through the steps of reduction, cyclization and the like. Compared with the prior art, the route has the advantages that initial raw materials are easy to obtain, the method is economical and efficient, meanwhile, the substrate applicability is high, various functional groups on the aromatic ring do not influence ring construction, and synthesis of various aporphine alkaloids can be achieved.
Owner:WENZHOU MEDICAL UNIV
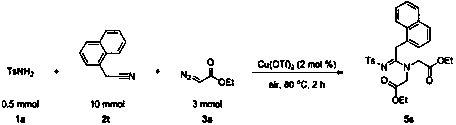
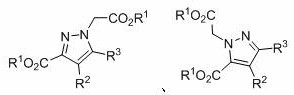
Multi-substituted pyrazole and its preparation method
The invention discloses a method for preparing polysubstituted pyrazoles, and develops a new catalytic system for aryl diazonium salts, using alkynoate and diazo ester derivatives as reaction substrates, using toluene as solvent, through cyclization / N ‑H insertion reaction to prepare polysubstituted pyrazoles. The method used in the present invention has the following characteristics: economical reaction, wider substrate universality, mild reaction conditions, can be carried out in the air, less catalyst consumption, convenient post-treatment, and is beneficial to product purification and industrial application. At the same time, the raw materials such as reactants and catalysts used in the present invention are cheap and easy to obtain, the reaction composition is reasonable, no ligand is needed, the reaction steps are few, and only one step reaction can achieve excellent yield, which meets the requirements and requirements of contemporary green chemistry and medicinal chemistry. direction.
Owner:SUZHOU UNIV
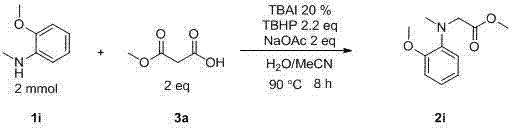
Method for preparing amino-acid ester
InactiveCN103113247BReact SafeReact greenCarbamic acid derivatives preparationOrganic compound preparationSodium acetatePtru catalyst
The invention discloses a method for preparing amino-acid ester. In the presence of an oxidant, the method adopts an amine compound and malonic ester as reactants, an iodide as a catalyst and sodium acetate as alkali; a product amino-acid ester is prepared through nucleophilic substitution in a polar solvent; the general formula of the chemical structure of malonic ester is shown in the decstiption; and the iodide is one of I2, TBAI, NIS and IBr. According to the method disclosed by the invention, the reaction activity of the catalyst is high, the reaction conditions are moderate, the application range of substrate is wide, the aftertreatment is convenient, the yield of the target product is high, the preparation process is simple and green and environment-friendly, and the raw materials are widely available.
Owner:SUZHOU UNIV
A kind of preparation method of α-cyanoamine
ActiveCN105237435BIncrease profitLow toxicityCarboxylic acid nitrile preparationOrganic compound preparationSodium acetateAcetic acid
The invention discloses a method for preparing α-cyanoamine. In the presence of an oxidizing agent, an amine compound and cyanoacetic acid are used as reactants, iodide is used as a catalyst, and sodium acetate is used as a base. The product α-cyanoamine is prepared by nuclear substitution reaction. The method of the invention has high catalyst reactivity, mild reaction conditions, wide substrate application range, convenient post-processing, high yield of target product, simple preparation process, green and environmental protection, and wide sources of raw materials.
Owner:SUZHOU UNIV
Method for synthesizing acrylic alkoxy ethyl ester or methacrylate alkoxy ethyl ester
ActiveCN101475475BLess corrosiveNo pollution in the processOrganic compound preparationCarboxylic acid esters preparationMethacrylatePhenothiazine
Owner:NANJING FORESTRY UNIV +2
Sialic acid Neu5Ac derivative as well as preparation method and application thereof
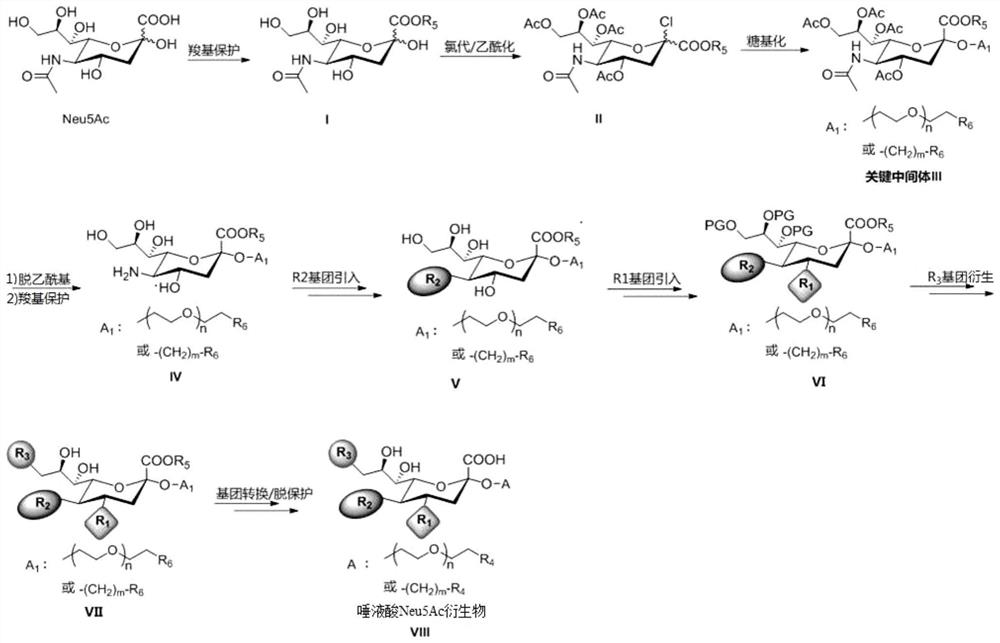
PendingCN114437157AEnable therapeutic applicationsRealize detectionSugar derivativesBiological material analysisSide chainSialic acid
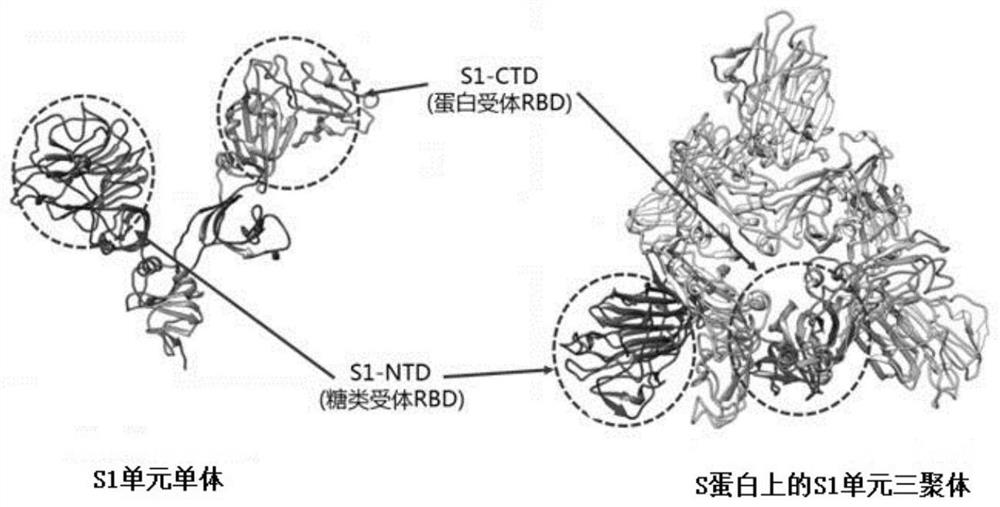
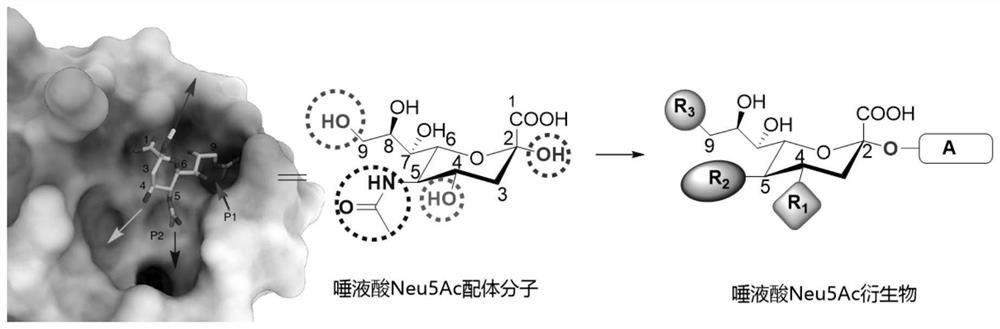
The invention discloses a sialic acid Neu5Ac derivative as well as a preparation method and application thereof, the structure of the derivative is shown as follows: R1 is selected from hydroxyl, methoxyl or substituted methoxyl; r2 is selected from an acetamido group, a substituted acetamido group, a benzamido group, a substituted benzamido group, an alkoxycarbonylamido group, a triazolyl group or a substituted triazolyl group; r3 is selected from a hydroxyl group, a methoxy group, a substituted methoxy group, an acetamido group, a substituted acetamido group, a sulfonamide group, a substituted sulfonamide group or a phosphamide group; a is or-(CH2) m-R4, and R4 is selected from amino, sulfydryl or azido; n is selected from 0, 1, 2, 3, 4, 5, 6 and 7; m is selected from 2, 3, 4, 5, 6, 7, 8, 9 and 10. The derivative provided by the invention not only can be combined with the S protein on the surface of the new coronavirus in a high-affinity manner, but also is endowed with wider application including detection of the new coronavirus, treatment of new coronapneumonia and the like through a derivative functional side chain.
Owner:SHENZHEN INST OF ADVANCED TECH
A kind of method for preparing isoxazole compound
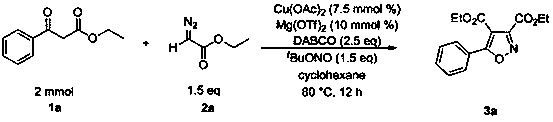
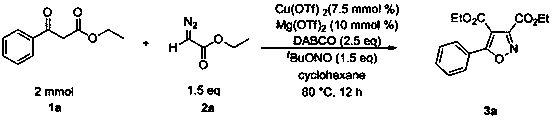
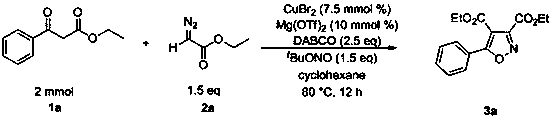
ActiveCN107445912BReact SafeReact greenOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsEthylenediaminePtru catalyst
The invention discloses a method for preparing isoxazole compounds, using β-keto acid ester, α-diazoic acid ester and tert-butyl nitrite as reaction substrates, copper compounds as catalysts, Lewis acid as additives, three Ethylenediamine (DABCO) is a base, and the fully substituted oxazole product is prepared by a three-component reaction; the method used in the present invention has the following characteristics: the reactivity of the catalyst is high, the reaction conditions are mild, and the substrate has a wide range of applications. The post-treatment is convenient, the yield of the target product is high, the preparation process is simple, and the sources of raw materials used are extensive.
Owner:SUZHOU UNIV
1,2,4-triazole and its preparation method
ActiveCN108276350BMany reaction stepsReaction economyGroup 4/14 element organic compoundsHigh activityGreen chemistry
The invention discloses 1,2,4-triazole and a preparation method thereof. The 1,2,4-triazole is prepared by taking aryl diazonium fluoborate, a diazo ester derivative and organic nitrile as reaction substrates, taking transition metal as a catalyst and taking inorganic alkali as an additive through cyclization reaction. The method has the following characteristics: the reaction is more economic, the substrate universality is higher, late functionalization is realized easily, the reaction condition is mile, the reaction can be carried out in air, the use amount of the catalyst is small, aftertreatment is simple and convenient, and purification and industrialized application of products are facilitated. Meanwhile, the raw materials such as the reactants and the catalyst are cheap and easily available, the reaction composition is reasonable, ligand is not needed, the reaction steps are few, high yield can be obtained only by one-step reaction, the requirement and the direction of the contemporary green chemistry and medicine chemistry are met, and the preparation method is suitable for screening high-activity 1,2,4-triazole medicines.
Owner:ZHANGJIAGANG INST OF IND TECH SOOCHOW UNIV +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

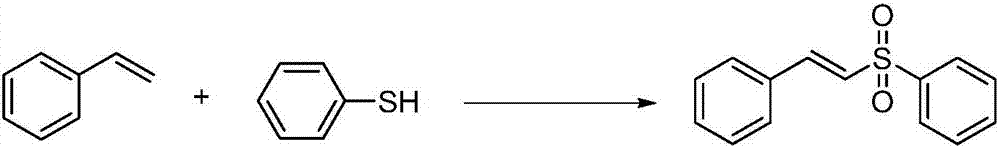
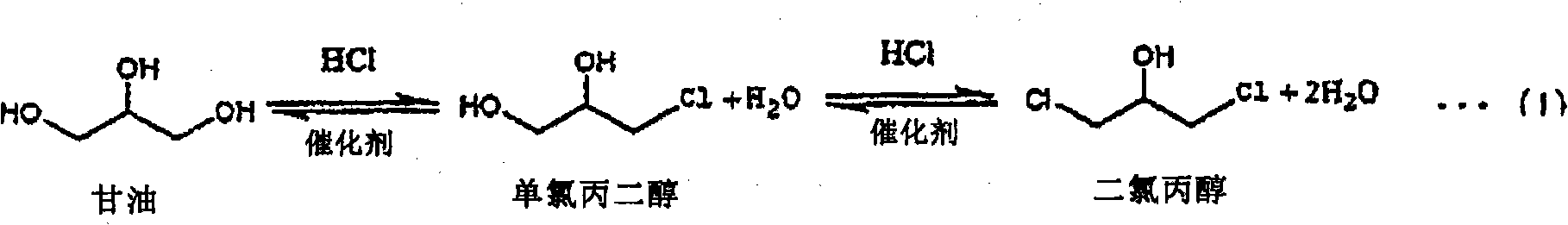
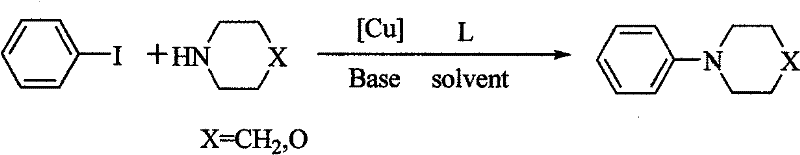

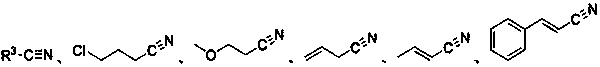
![Application of diphenylphosphine methyl-substituted calix [4] arene Application of diphenylphosphine methyl-substituted calix [4] arene](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/1d65b32c-8ba9-422a-8674-7a4a034566c5/DEST_PATH_IMAGE001.PNG)
![Application of diphenylphosphine methyl-substituted calix [4] arene Application of diphenylphosphine methyl-substituted calix [4] arene](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/1d65b32c-8ba9-422a-8674-7a4a034566c5/FDA0000143676330000011.PNG)
![Application of diphenylphosphine methyl-substituted calix [4] arene Application of diphenylphosphine methyl-substituted calix [4] arene](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/1d65b32c-8ba9-422a-8674-7a4a034566c5/FDA0000143676330000012.PNG)