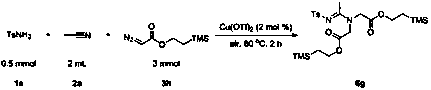
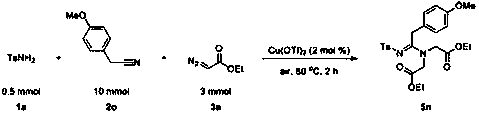
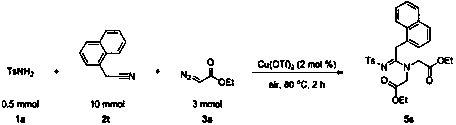
A method for preparing fully substituted amidines
A technology of full substitution and derivatives, which is applied in the preparation of sulfonic acid amides, chemical instruments and methods, catalysts for physical/chemical processes, etc. Novel, waste-free effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038]
[0039] Fill the reaction bottle with compound 1a (0.5 mmol, 85.6 mg), compound 3a (3 mmol, 316 μL), Cu(OTf) 2 (0.01 mmol, 3.6 mg) and compound 2a (2 mL). Then the system was heated at 80°C in the air for about 2 hours, quenched with saturated sodium sulfite solution, extracted with ethyl acetate (10 mL × 3), removed the solvent with a rotary evaporator, adsorbed on silica gel, passed through a simple column layer The product 4a was obtained after analysis, and the yield was 94%. The main test data of the obtained product are as follows. It can be seen from the analysis that the actual synthesized product is consistent with the theoretical analysis.
[0040] 1 H NMR (400 MHz, CDCl 3 ) δ 7.76–7.74 (m, 2H), 7.25–7.23 (m, 2H), 4.25–4.18 (m, 6H), 4.06 (q, J = 7.1 Hz, 2H), 2.50 (s, 3H), 2.39 (s, 2H), 1.28 (t, J = 7.1 Hz, 3H), 1.16 (t, J = 7.1 Hz, 3H). 13 C NMR (101 MHz, CDCl 3 ) δ 167.8,167.6, 166.6, 142.1, 140.3, 128.9, 126.1, 62.0, 61.2, 51.5, 51.2, 21.3, 1...
Embodiment 2
[0042]
[0043] Fill the reaction bottle with compound 1b (0.5 mmol, 78.6 mg), compound 3a (3 mmol, 316 μL), Cu(OTf) 2(0.01 mmol, 3.6 mg) and compound 2a (2 mL). Then the system was heated at 80°C in the air for about 2 hours, quenched with saturated sodium sulfite solution, extracted with ethyl acetate (10 mL × 3), removed the solvent with a rotary evaporator, adsorbed on silica gel, passed through a simple column layer Analysis can give product 4b, and the yield is 87%. The main test data of the obtained product are as follows. It can be seen from the analysis that the actual synthesized product is consistent with the theoretical analysis.
[0044] 1 H NMR (400 MHz, CDCl 3 ) δ 7.89– 7.86 (m, 2H), 7.53–7.43 (m, 3H), 4.2 –4.18 (m, 6H), 4.04 (q, J = 7.1 Hz, 2H), 2.52 (s, 3H), 1.27 (t, J = 7.1 Hz,3H), 1.14 (t, J = 7.1 Hz, 3H). 13 C NMR (101 MHz, CDCl 3 ) δ 167.7, 167.5, 166.8, 143.0, 131.5, 128.3, 126.0, 62.0, 61.2, 51.6, 51.3, 17.4, 13.9, 13.8. HRMS(ESI-TOF): Anal...
Embodiment 3
[0046]
[0047] Fill the reaction bottle with compound 1c (0.5 mmol, 87.6 mg), compound 3a (3 mmol, 316 μL), Cu(OTf) 2 (0.01 mmol, 3.6 mg) and compound 2a (2 mL). Then the system was heated at 80°C in the air for about 2 hours, quenched with saturated sodium sulfite solution, extracted with ethyl acetate (10 mL × 3), removed the solvent with a rotary evaporator, adsorbed on silica gel, passed through a simple column layer The product 4c can be obtained after analysis, and the yield is 83%. The main test data of the obtained product are as follows. It can be seen from the analysis that the actual synthesized product is consistent with the theoretical analysis.
[0048] 1 H NMR (400 MHz, CDCl 3 ) δ 7.91–7.86 (m, 2H), 7.16–7.10 (m, 2H), 4.26–4.17 (m, 6H), 4.06 (q, J = 7.1 Hz, 2H), 2.53 (s, 3H), 1.29 (t, J = 7.1 Hz,3H), 1.16 (t, J = 7.1 Hz, 3H). 13 C NMR (101 MHz, CDCl 3 ) δ 167.7, 167.5, 166.8, 164.3 ( J = 251 Hz), 139.3 ( J = 3 Hz), 128.7 ( J = 9 Hz), 115.4 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com