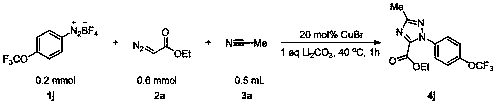1,2,4-triazole and its preparation method
A technology for triazole and diazonium salt, which is applied in the field of 1,2,4-triazole and its preparation, can solve the problems of narrow substrate range, high reaction temperature, narrow reaction substrate range and the like, and achieves economical reaction , The effect of easy availability of raw materials and wide substrate versatility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042]
[0043] The reaction flask was filled with compound 1a (0.2 mmol, 45.8 mg), CuBr (0.04 mmol, 5.8 mg), Li 2 CO 3 (14.8 mmol,), compound 3a (0.5 mL), compound 2a (0.6 mmol, 72.1 mg). Then the system was magnetically stirred and reacted at 40°C in the air for 1 hour, quenched with ethyl acetate, removed the solvent with a rotary evaporator, adsorbed on silica gel, and the product 4a was obtained by simple column chromatography with a yield of 85%. %. The main test data of the obtained product are as follows. It can be seen from the analysis that the actual synthesized product is consistent with the theoretical analysis.
[0044] 1 H NMR (400 MHz, CDCl 3 ) δ 7.33 – 7.25 (m, 4H), 4.36 (q, J = 8.0 Hz,2H), 2.51 (s, 3H), 2.43 (s, 3H), 1.34 (t, J = 8.0 Hz, 3H). 13 C NMR (101 MHz, CDCl 3 ) δ 160.79, 157.28, 144.66, 139.60, 135.29, 129.33, 125.31, 62.38,21.15, 13.89, 13.72. HRMS (ESI-TOF): Anal. Calcd. For C 13 h 15 N 3 o 2 +Na + : 284.1006,Found: 284.1015; IR ...
Embodiment 2
[0046]
[0047] The reaction flask was filled with compound 1b (0.2 mmol, 43.4 mg), CuBr (0.04 mmol, 5.8 mg), Li 2 CO 3 (14.8 mmol,), compound 3a (0.5 mL), compound 2a (0.6 mmol, 72.1 mg). Then the system was magnetically stirred and reacted at 40°C in the air for 1 hour, quenched with ethyl acetate, removed the solvent with a rotary evaporator, adsorbed on silica gel, and the product 4b was obtained by simple column chromatography with a yield of 71 %. The main test data of the obtained product are as follows. It can be seen from the analysis that the actual synthesized product is consistent with the theoretical analysis.
[0048] 1 H NMR (400 MHz, CDCl 3 ) δ 7.33 – 7.25 (m, 4H), 4.36 (q, J = 8.0 Hz,2H), 2.51 (s, 3H), 2.43 (s, 3H), 1.34 (t, J = 8.0 Hz, 3H). 13 C NMR (101 MHz, CDCl 3 ) δ 160.79, 157.28, 144.66, 139.60, 135.29, 129.33, 125.31, 62.38,21.15, 13.89, 13.72. HRMS (ESI-TOF): Anal. Calcd. For C 13 h 15 N 3 o 2 +H + : 246.1237, Found: 246.1235; IR (nea...
Embodiment 3
[0050]
[0051] The reaction flask was filled with compound 1c (0.2 mmol, 49.3 mg), CuBr (0.04 mmol, 5.8 mg), Li 2 CO 3 (14.8 mmol,), compound 3a (0.5 mL), compound 2a (0.6 mmol, 72.1 mg). Then the system was magnetically stirred and reacted at 40°C in air for 1 hour, quenched with ethyl acetate, removed the solvent with a rotary evaporator, adsorbed on silica gel, and the product 4c was obtained by simple column chromatography with a yield of 70 %. The main test data of the obtained product are as follows. It can be seen from the analysis that the actual synthesized product is consistent with the theoretical analysis.
[0052] 1 H NMR (400 MHz, CDCl 3 ) δ 7.38 – 7.30 (m, 4H), 4.36 (q, J = 8.0 Hz,2H), 2.99 (dt, J = 13.8, 6.9 Hz, 1H), 2.51 (s, 3H), 1.33 (t, J = 8.0 Hz, 3H), 1.28 (d, J = 6.9 Hz, 6H). 13 C NMR (101 MHz, CDCl 3 ) δ 160.80, 157.31, 150.40,144.63, 135.51, 126.77, 125.38, 62.38, 33.82, 23.76, 13.87, 13.72. HRMS (ESI-TOF): Anal. Calcd. For C 15 h 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com