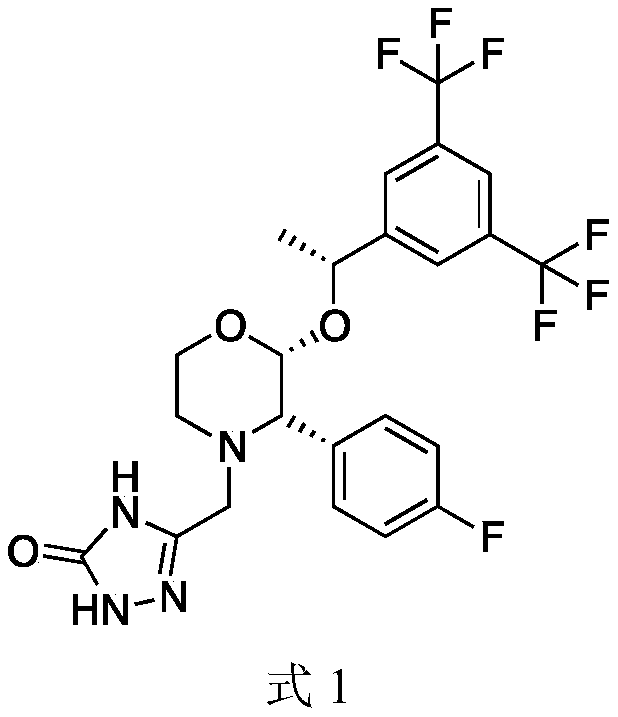
Chiral synthesis method of Aprepitant intermediate and intermediate synthesized through chiral synthesis method of Aprepitant intermediate
A technology of chiral synthesis and aprepitant, applied in asymmetric synthesis, organic chemical methods, chemical instruments and methods, etc., can solve the problems of being unsuitable for industrialized large-scale production, low product yield, and poor quality, and achieve shortening Effects of synthesis steps, high yield, and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Preparation of Chiral Ligands
[0038] At room temperature, under nitrogen protection, add Pd(PPh 3 ) 4 3.5g (3mmol) and phosphoramidite (R-monoPhos) 2.2g (6mmol) in 200mL reaction solvent anhydrous DMF, then add compound (4) 61g (0.3mol) and compound (5) 51g (0.3mol), React at 100°C, monitor the reaction by TLC, and end the reaction after 12 hours. The reaction solution was lowered to room temperature, then poured into 200ml of saturated ammonium chloride aqueous solution, extracted twice with 200ml of ethyl acetate, combined the organic phase, and the organic phase was washed with 200ml of saturated sodium chloride aqueous solution, separated, and the organic phase was reduced Concentrate under reduced pressure to obtain a crude product of compound (6), which is directly used in the next reaction.
[0039] At room temperature, under the protection of nitrogen, add the crude product of the above compound (6) to 200 mL reaction solvent anhydrous tetrahydrofuran into...
Embodiment 2
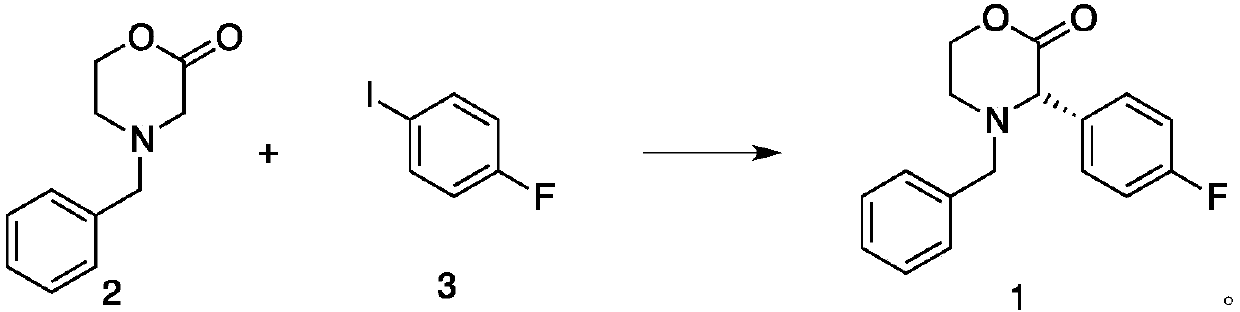
[0043] Preparation of compound (1)
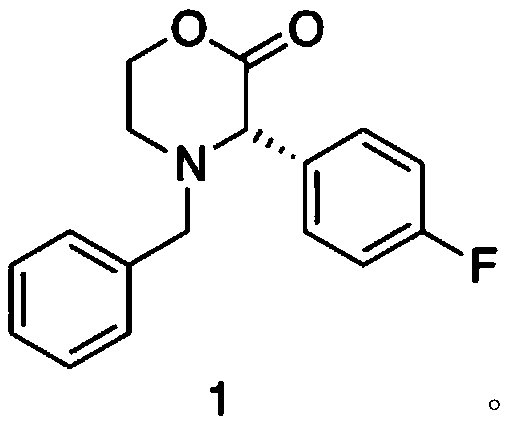
[0044] At room temperature, under the protection of nitrogen, add compound (2) 1.91kg (10.0mol) to 5L reaction solvent anhydrous toluene in 20L reactor, slowly add 5L LiHMDS toluene solution containing 2mol / L, after 1 hour After adding, after reacting at room temperature for 1 hour, add the catalyst [(t-Bu 3 P)PdBr] 277g (0.1mol), chiral ligand 46g (0.11mol), after stirring for half an hour, add compound (3) 2.44kg (11.0mol) in 2L toluene solution, TLC monitors the reaction, the reaction ends after 17 hours, slowly add 5L Saturated ammonium chloride aqueous solution was stirred for 30 minutes, separated, the aqueous phase was extracted with dichloromethane, and then the organic phases were combined, and the organic phase was concentrated (0.2mmHg) under reduced pressure to obtain the crude product of compound (1). The crude product was washed with toluene / petroleum The ether was recrystallized to obtain 2.57 kg (9.01 mol) of the refined p...
Embodiment 3
[0051] According to the synthesis method of Example 2, the difference is that the molar ratio of compound (2) to compound (3) is 1:1, the reaction temperature is 0°C, the reaction solvent is anhydrous tetrahydrofuran, and the catalyst is Pd 2 (dba) 3 , the base is LDA, the molar ratio of catalyst to compound (2) is 1:20; the molar ratio of catalyst to chiral ligand is 1:1. Compound (1) has a molar yield of 89.2%, and a purity of 98.55% by HPLC.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com