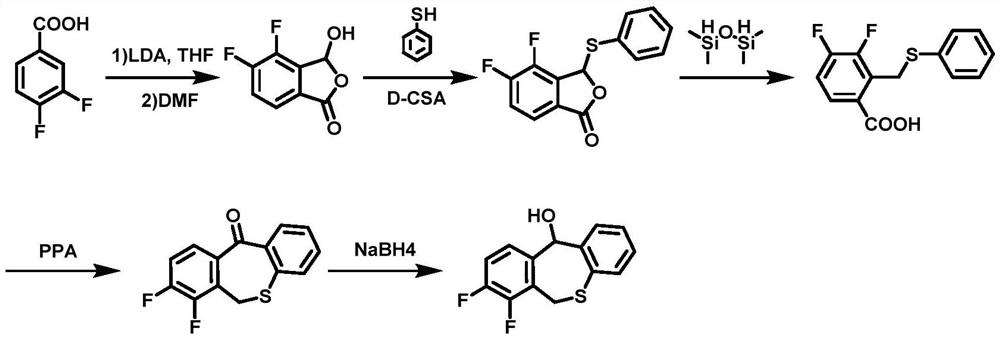
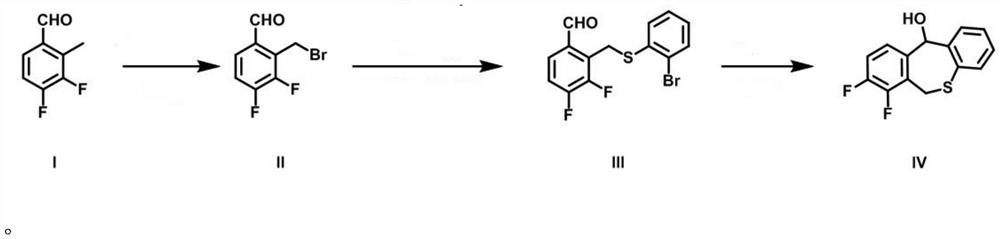
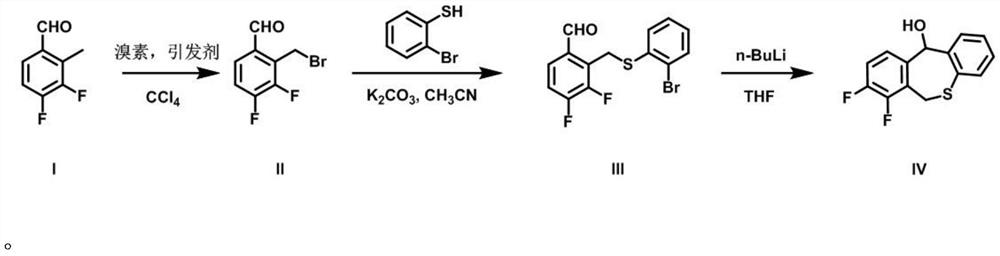
A kind of preparation method of baloxavir key intermediate and intermediate thereof
An intermediate and key technology, applied in the field of medicine, can solve the problems of limiting the expansion of reaction substrates and high safety risks, and achieve the effects of facilitating the expansion of substrates, high yields, and short synthetic routes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Add 3,4-difluoro-2-methylbenzaldehyde (10g, 64mmol), N-bromosuccinimide (NBS) (14.68g, 76.9mmol), azobisiso Butyronitrile (AIBN) (1.05g, 6.4mmol), 100mL carbon tetrachloride, argon protection, stirred at 60°C for 10h, and monitored the reaction by TLC. After the reaction was completed, add 100 mL of water, extract with dichloromethane (50 mL×3), combine the dichloromethane phases, wash with saturated brine (10 mL×3), dry over anhydrous sodium sulfate, remove the organic phase by distillation under reduced pressure, and perform silica gel column chromatography Afterwards, 14.2 g of intermediate II was obtained, with a yield of 94.3%.
[0041] 1 H NMR (300MHz, Chloroform-d) δ10.36(s, 1H), 7.63(d, J=8.2Hz, 1H), 7.43(d, J=8.2Hz, 1H), 4.54(s, 2H)
[0042] Add 2-bromothiophenol (9.65g, 51.06mmol), sodium hydride (5.11g, 127.64mmol), 100mL N,N-dimethylformamide into a 250mL two-neck flask, add intermediate Ⅱ3 under argon protection , 4-difluoro-2-bromomethylbenzaldehyde (10...
Embodiment 2
[0047] In a 250mL round bottom flask, add 3,4-difluoro-2-methylbenzaldehyde (10g, 64mmol), liquid bromine (12.28g, 76.9mmol), benzoyl peroxide (BPO) (1.55g, 6.4mmol ), 100mL N,N-dimethylformamide, under argon protection, stirred at 60°C for 12h, and monitored the reaction by TLC. After the reaction was completed, 100 mL of water was added, extracted with ethyl acetate (50 mL×3), the ethyl acetate phases were combined, washed with water (50 mL×3), washed with saturated brine (10 mL×3), dried over anhydrous sodium sulfate, and evaporated under reduced pressure to remove organic After column chromatography, 13.6 g of intermediate II was obtained, with a yield of 90.4%.
[0048] 1 H NMR (300MHz, Chloroform-d) δ: 10.36(s,1H), 7.63(d,J=8.2,1H), 7.43(d,J=8.2,1H), 4.54(s,2H)
[0049] Add intermediate II 3,4-difluoro-2-bromomethylbenzaldehyde (10g, 42.55mmol), anhydrous potassium carbonate (17.64g, 127.64mmol), 100mL anhydrous acetonitrile, and argon protection in a 250mL two-necked ...
Embodiment 3
[0054] The preparation method of embodiment 3 is the same as that of embodiment 1, except that bromine is montmorillonite in step (1), solvent A is dimethyl sulfoxide, and formula (I) 3,4-difluoro-2-methano The molar ratio of base benzaldehyde and bromine feeding is 1:2, and the molar ratio of feeding with initiator is 1:0.3. The temperature of the heating and stirring reaction was 80° C., and the time was 8 hours.
[0055] In the step (2), the alkali is anhydrous sodium bicarbonate, and the solvent B is acetone; the mol ratio of the intermediate II described in the step (2) and 2-bromothiophenol is 1:1.5, and the molar ratio of the alkali is 1:1.5. The ratio is 1:5. The reaction temperature of heating and stirring overnight was 65°C.
[0056] In step (3), the base is phenyllithium, and the solvent C is anhydrous 1,4-dioxane; the molar ratio of intermediate III to the base is 1:1.2. The reaction temperature at low temperature is -40°C, and the time is 0.5h.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com