Preparation method of 2, 3-naphthalimide derivative
A technology of naphthalimide and derivatives, which is applied in the field of preparation of 2,3-naphthoimide derivatives, and can solve problems such as complex synthetic routes, low yields, and limited range of substrates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] Among the present invention, the preparation method of 2,3-naphthalimide comprises the following steps:
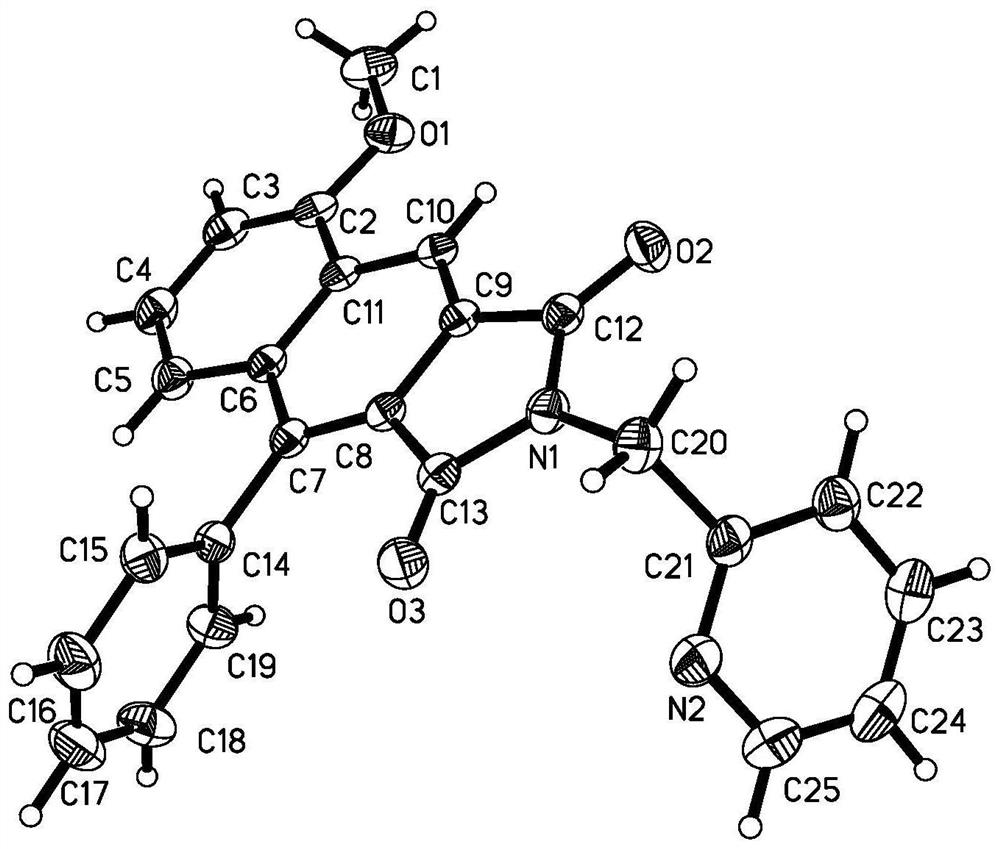
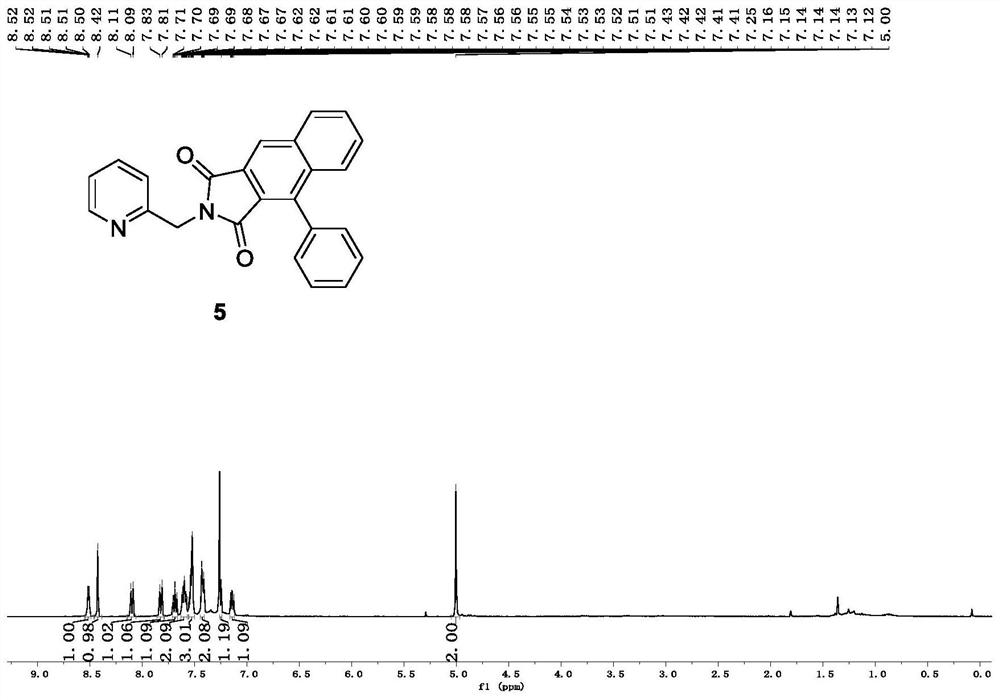
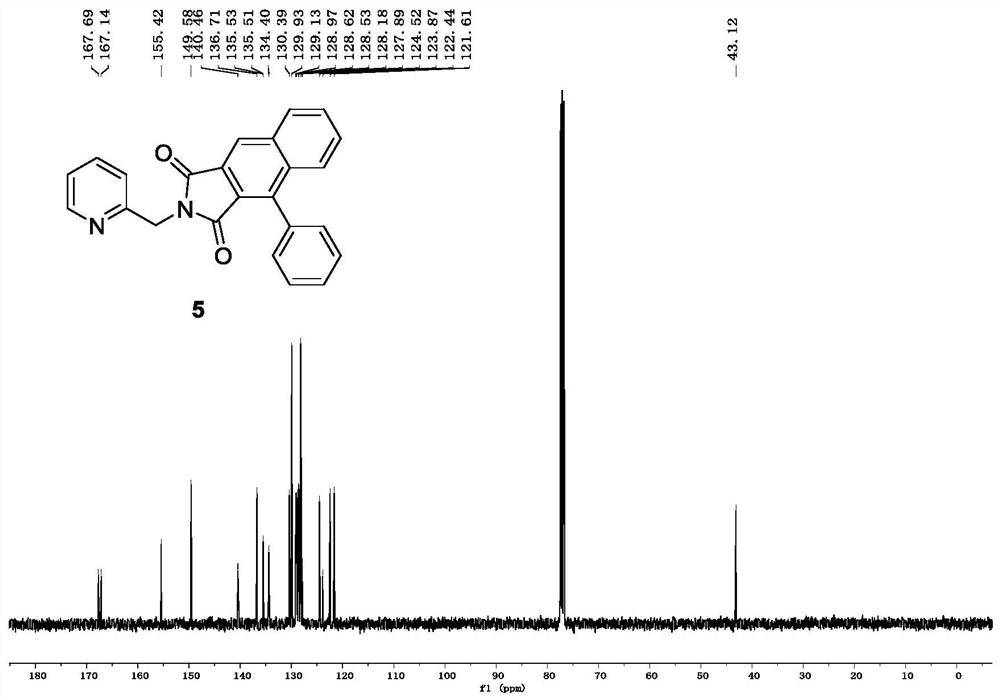
[0033] The compound alkyne amide derivative (Formula 3) is dehydrogenated under the action of a base, and then reacts with a cinnamoyl chloride derivative (Formula 4) to form an imide transition state, which is directly subjected to didehydrogenation DA reaction and oxidation reaction to obtain a related The ring product naphthalimide derivative (compound of formula 5, its color is yellow solid or white solid, brown solid).
[0034] Its synthetic route is as follows:
[0035]
[0036] (In this synthetic route, 0°C-r.t represents the first stage, such as starting 20-50 minutes, reacting at 0°C, that is, adding a base at this temperature, such as sodium hydride, and moving to room temperature after adding, for example, acid chloride Reaction, proceed to the second stage; since organic base or inorganic base can be used in this reaction, adjust the temperature acco...
Embodiment 1
[0043] Step (1) DidehydroDA reaction:
[0044] In air, add 3-phenyl-N-(pyridine-2-methylene) propynamide (0.10 g, 0.42 mmol) to a 25 ml eggplant-shaped flask, and add 2.5 ml of anhydrous tetrahydrofuran to the reaction flask , the reaction bottle was placed in a low temperature and constant temperature reaction bath for stirring at 0°C, sodium hydride (60% dispersed in mineral oil) (25.2 mg, 0.63 mmol) was slowly added to the reaction bottle in batches, and continued to stir at 0°C for 30 minutes , Cinnamoyl chloride (90.6 mg, 0.55 mmol) was added to the reaction flask, and the reaction flask was moved to room temperature and continued to stir for 3 hours; after the reaction was completed, the ammonium chloride solution was added to the reaction flask to quench the reaction, Ethyl acetate and water were used as two-phase extraction for 3 times, the ethyl acetate layer was collected, dried by adding anhydrous sodium sulfate, and finally separated and purified by chromatographic...
Embodiment 2
[0055] Step (1) DidehydroDA reaction:
[0056] 3-Phenyl-N-(pyridine-2-methylene)propynamide (0.10 g, 0.42 mmol) was added to a 25 ml Schlenk bottle with a rubber stopper in air, and 2.5 ml of anhydrous THF was added to the reaction In the bottle, the reaction bottle was placed in a low temperature and constant temperature reaction bath for stirring at 0°C, and sodium hydride (dispersed in mineral oil, and the mass fraction of sodium hydride in the mineral oil dispersion system was 60%) (25.2 mg, 0.63 mmol) Slowly add to the reaction flask in batches, continue to stir at 0°C for 30 minutes, add (2E,4E)-5-phenylpenta-2,4-dienoyl chloride (0.10 g, 0.55 mmol) to the reaction flask , the reaction bottle was moved to room temperature and continued to stir for 3 hours; after the reaction was completed, the ammonium chloride solution was added to the reaction bottle to quench the reaction, and ethyl acetate and water were used as two-phase extraction for 3 times, and the ethyl acetate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com