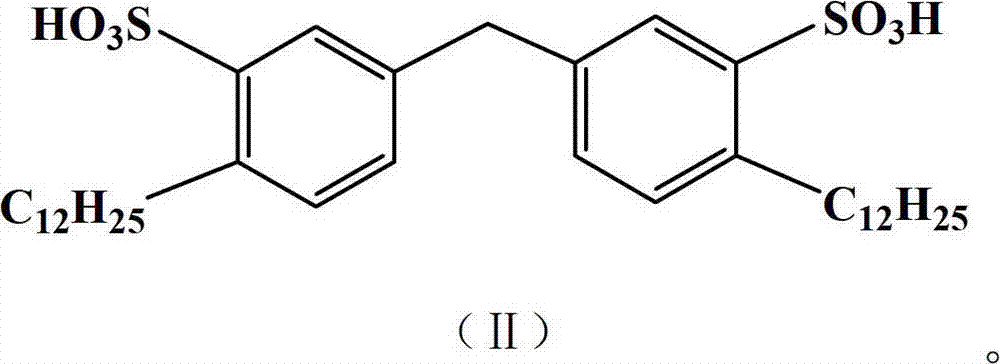
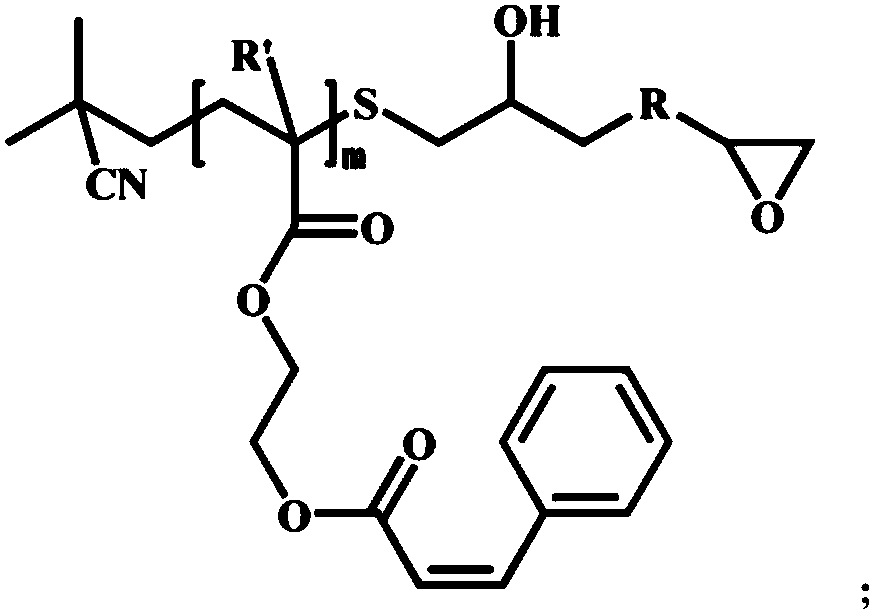
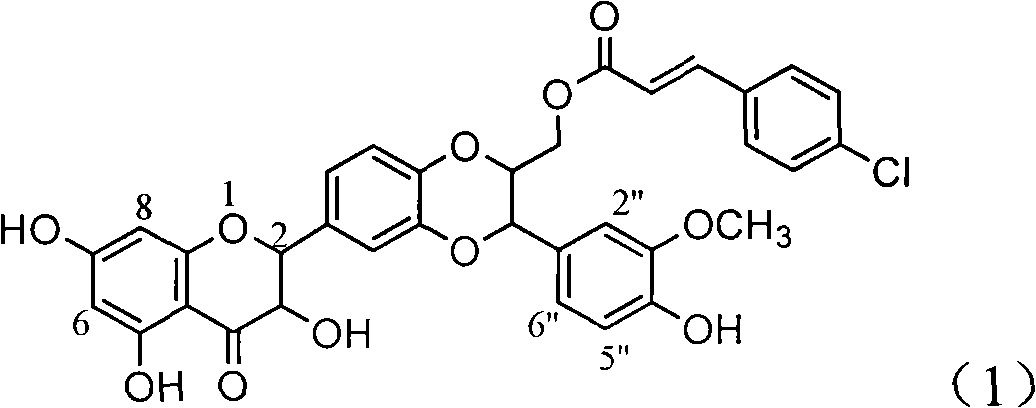
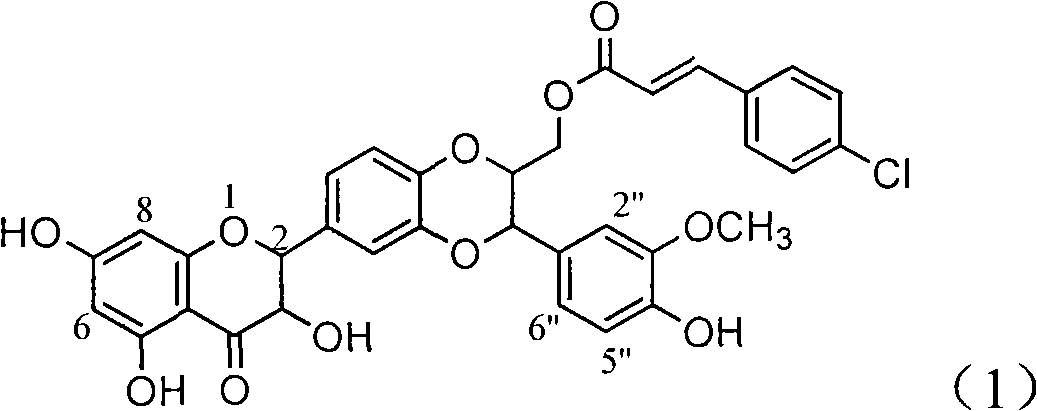
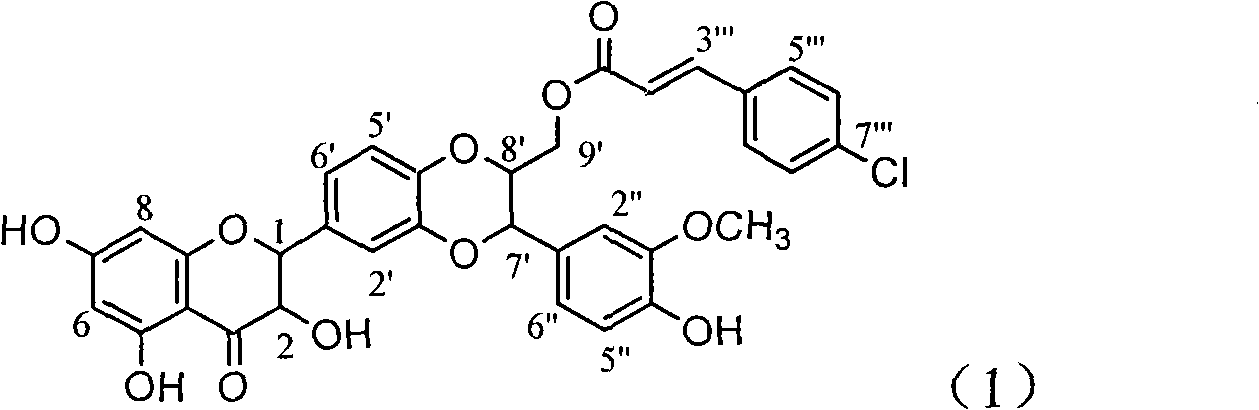
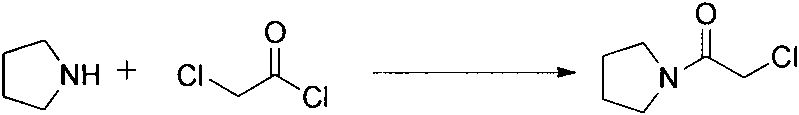Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
39 results about "Cinnamoyl chloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
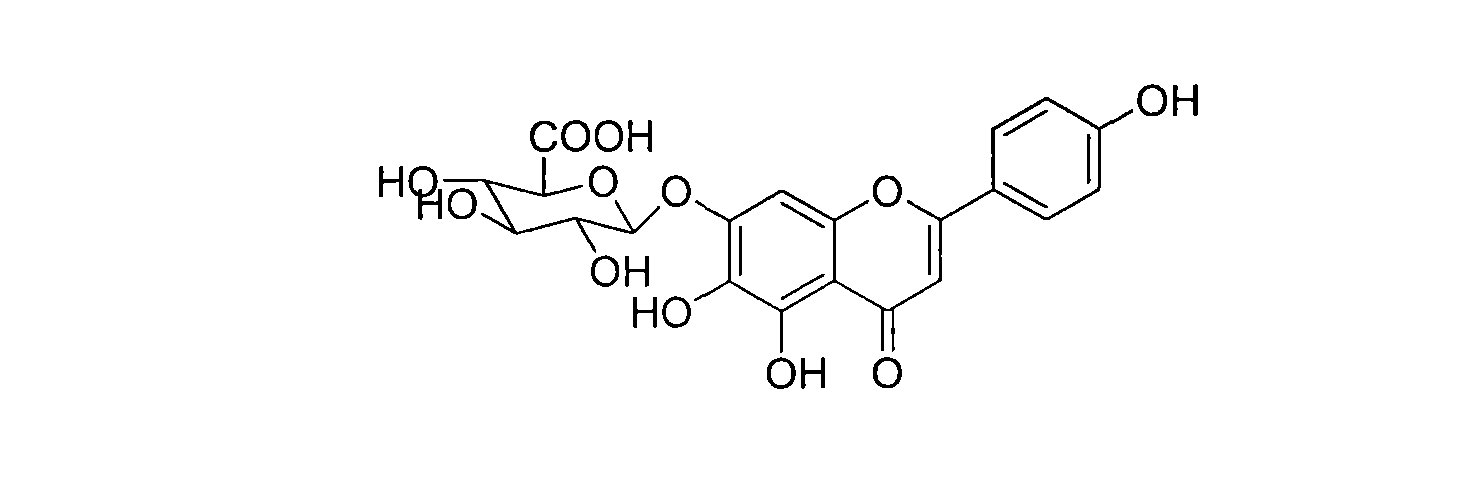
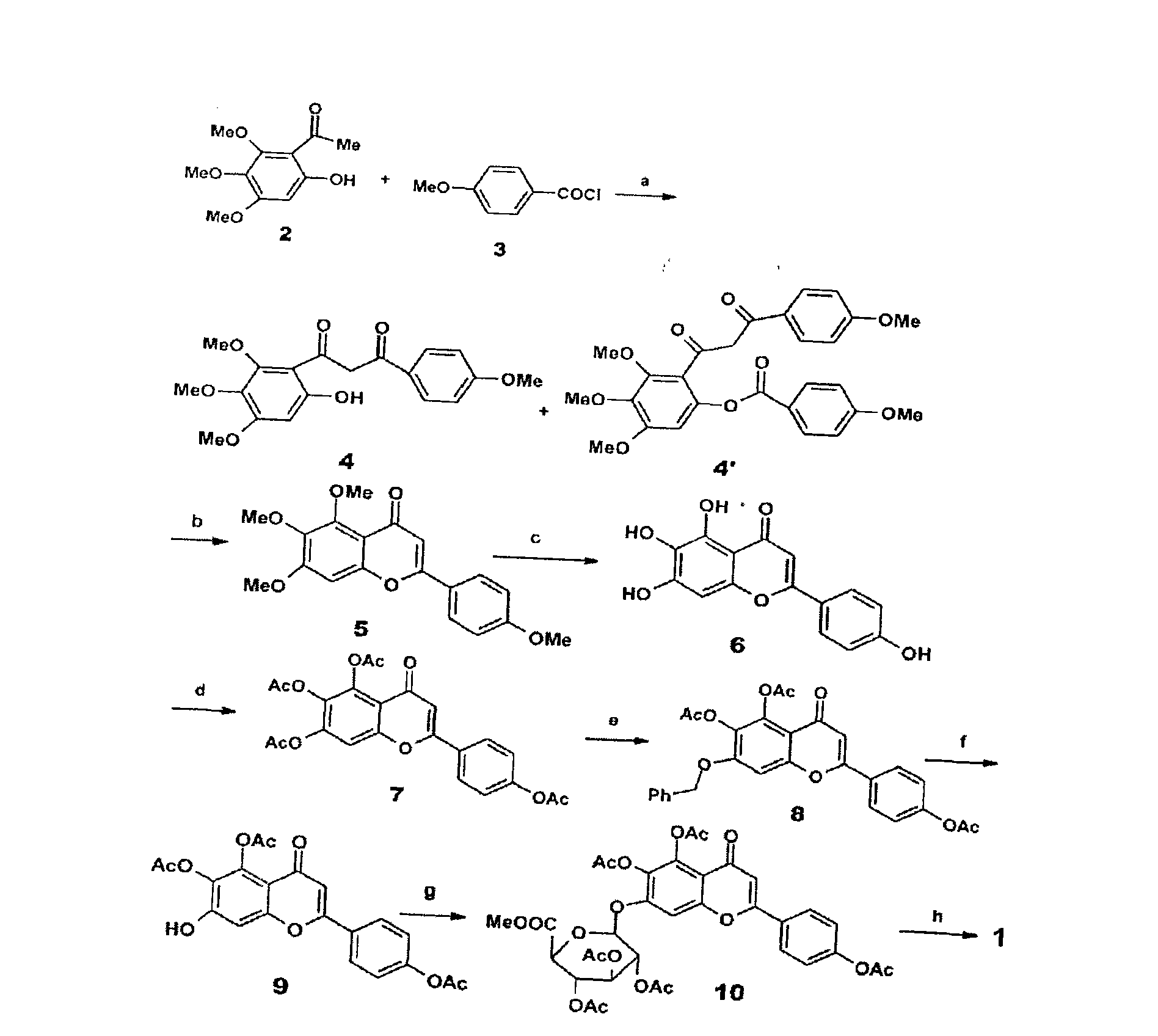
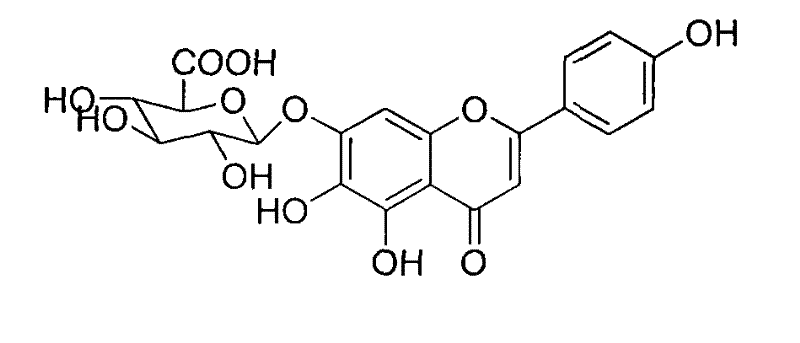
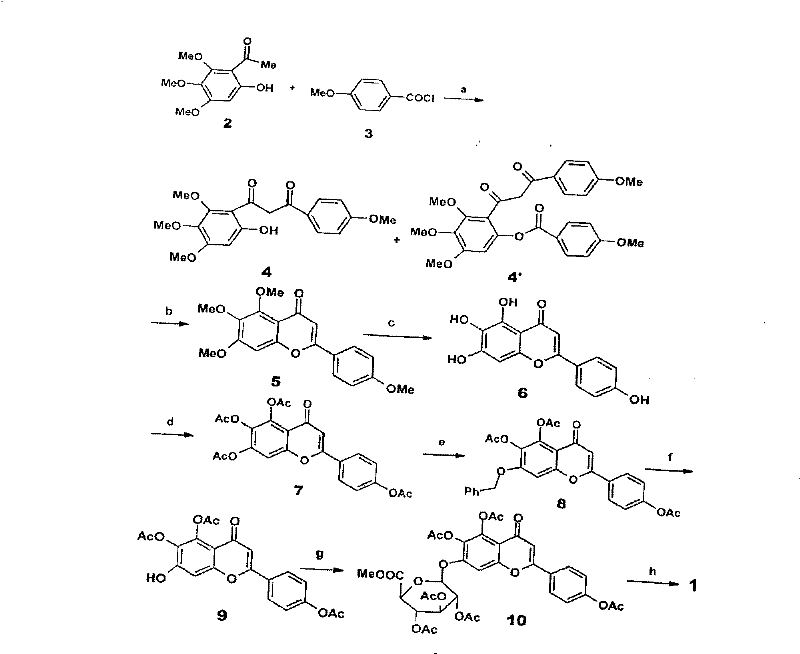
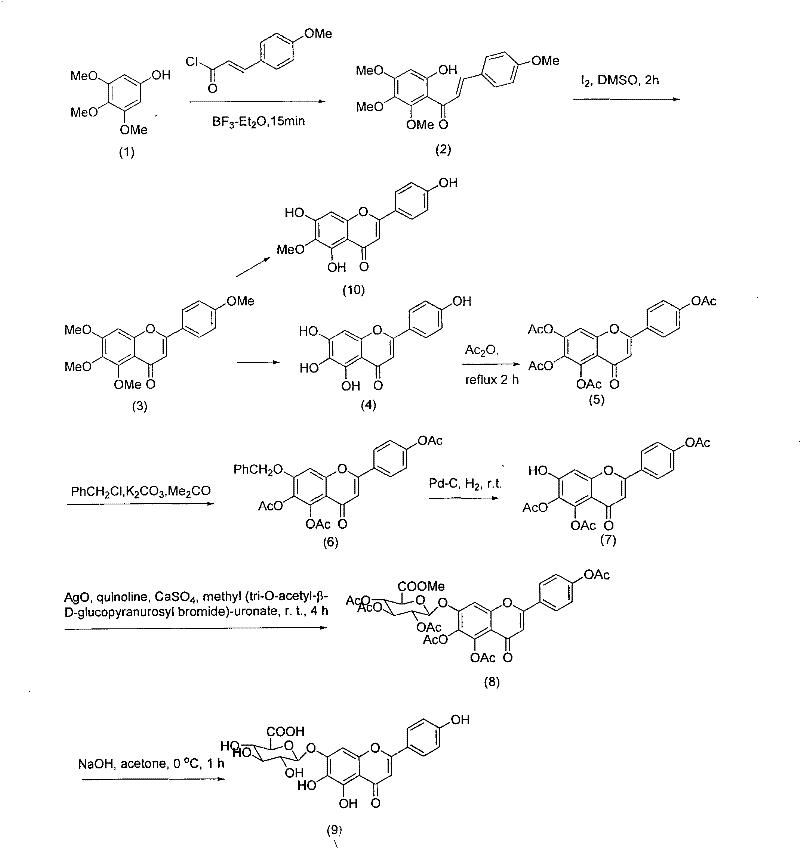
Method for synthesizing 5,6,4'-trihydroxyflavone-7-O-D-glucuronic acid
InactiveCN101538299AStrong space selectivityEasy to industrializeOrganic active ingredientsSugar derivativesChemical reactionCinnamoyl chloride
The invention provides a chemical method for preparing 5,6,4'-trihydroxyflavone-7-O-D-glucuronic acid to solve problems of unavailable raw materials, long synthetic route and low synthesis yield in the existing synthetic method. The method comprises a plurality of chemical reaction steps: first, taking 3,4,5-trimethoxy phenol and p-methoxy cinnamoyl chloride as the raw materials to construct a chalcone structure by a Friedel-Crafts acylation reaction, and cycloetherifying to obtain a flavone parent substance; then protecting and selectively deprotecting phenolic hydroxyl of the flavone parent substance, and glycosylating 7-phenolic hydroxyl; and finally removing a protective group to obtain the target compound. The obtained product has a beta-steric configuration. The method can be used for preparing high-purity and high-quality 5,6,4'-trihydroxyflavone-7-O-D-glucuronic acid products.
Owner:四川抗菌素工业研究所有限公司
Photosensitive degradable comb-like copolymer film with controllable surface appearance and performance
ActiveCN103524687APhotosensitivityDegradableEnergy modified materialsPharmaceutical non-active ingredientsGlycidyl methacrylatePolymer science
The invention relates to a photosensitive degradable comb-like copolymer film with controllable surface appearance and performance and belongs to the field of functional polymer materials. Firstly a reaction is carried out on cinnamoyl chloride (CC) and epsilon-caprolactone modified methacrylate (FMn, n is equal to 1-3), so that a series of photosensitive macromolecule monomers (FMnC) are obtained; the FMnC and polyethylene glycol methacrylate (MAPEG) are taken as a first monomer and a second monomer, then free radial copolymerization is carried out on the first monomer, the second monomer and a third monomer such as styrene (St), glycidyl methacrylate (GMA) and tert-butyl methacrylate (BMA), so that a photosensitive degradable terpolymer with a comb-like structure is prepared; a solvent volatilization method is adopted to prepare the product photosensitive degradable terpolymer with the comb-like structure into the film, and study shows that the photosensitive degradable comb-like copolymer film has photosensitivity and degradability; surface appearance and wettability of the photosensitive degradable comb-like copolymer film can be controlled by changing polarity and addition amount of a selective solvent. The obtained photosensitive degradable copolymer film with controllable surface appearance and performance is expected to be taken as an intelligent degradable material and widely applied to the fields of tissue engineering materials, medical materials and the like.
Owner:JIANGNAN UNIV
Method for compounding baicalein derivative
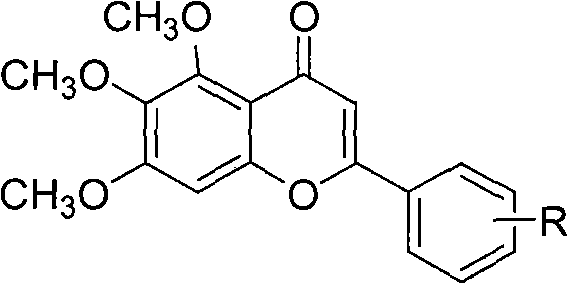
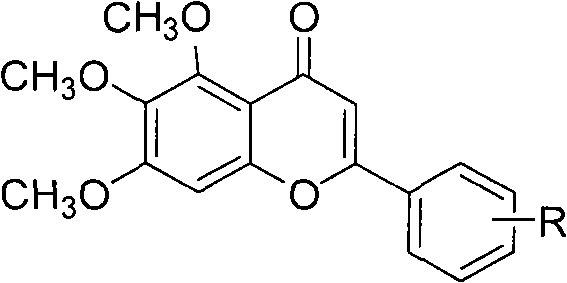
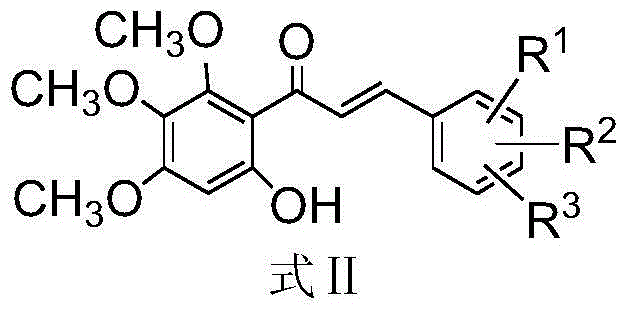
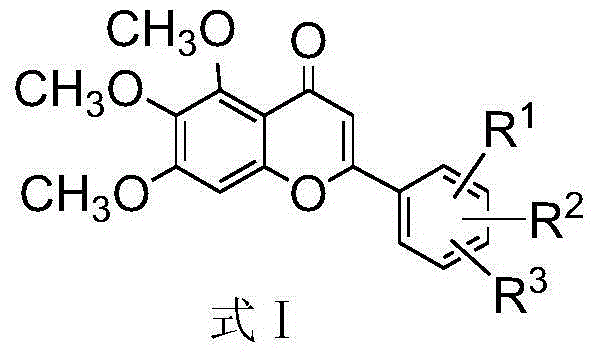
The invention discloses a method for compounding a novel baicalein derivative as shown in a formula I, wherein R can be H, hydroxyl, alkyl, alkoxy, nitro and the like. By modifying a baicalein B-ring skeleton, the baicalein derivative which has greatly-improved dissolubility and anticancer activity and the like compared with baicalein is compounded. In the method, the B-ring substituted baicalein derivative is prepared by using benzaldehyde or substituted benzaldehyde and 3,4,5-trimethoxy-phenol as basic raw materials through four steps. The method comprises the detailed steps: the benzaldehyde or the substituted benzaldehyde is subjected to a condensation reaction with anhydride under the action of a basic catalyst to obtain substituted cinnamylate; the substituted cinnamylate is subjected to a halogenating reaction under the action of a catalyst to obtain substituted cinnamoyl chloride; the substituted cinnamoyl chloride is subjected to an acylation reaction with the 3,4,5-trimethoxy-phenol under the action of a catalyst to obtain a chalcone compound; and the chalcone compound is subjected to a ring-closure reaction under the action of a catalyst to obtain the baicalein derivative. The method has the advantages of simple process, rich raw materials, high yield, good product purity and low cost, and has broad application prospect. The formula I is shown in the specification.
Owner:JIANGNAN UNIV
Synthetic method for polysubstituted baicalein derivatives
The invention discloses a synthetic method for a kind of polysubstituted baicalein derivatives shown as a formula I, and in the formula I, R1, R2 and R3 are H, hydroxyl, alkyl, alkoxy and the like. By modifying baicalein B-ring skeleton, the baicalein derivatives are synthesized, which are substantially improved in dissolvability, anticancer activity and other aspects compared with baicalein. The method employs substituted benzaldehyde and 3,4,5-trimethoxyphenol as basic raw materials, and the B-ring polysubstituted baicalein derivatives are prepared through 3 steps. The method concretely comprises performing condensation reaction on the substituted benzaldehyde and an acid anhydride under the effect of an alkali catalyst, so as to obtain substituted cinnamic acid, and further synthesizing substituted cinnamoyl chloride, performing acylation reaction on the substituted cinnamoyl chloride and 3,4,5-trimethoxyphenolunder the effect of a catalyst, so as to obtain a chalcone compound, and performing cyclization reaction on the chalcone compound under the effect of a catalyst, so as to obtain the corresponding polysubstituted baicalein derivative. The method is simple in technology, abundant in raw materials, high in yield, good in product purity and low in cost, and has wide application prospect.
Owner:JIANGNAN UNIV
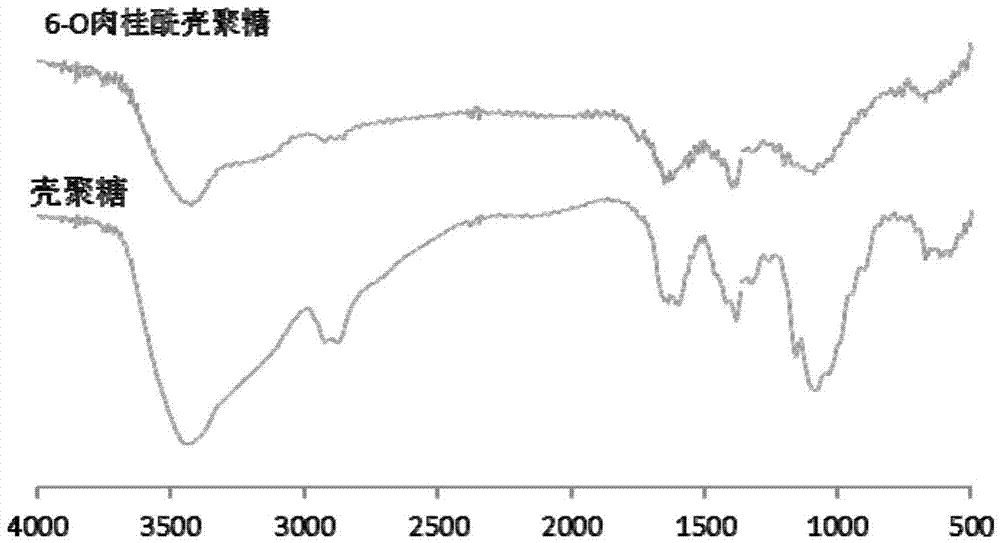
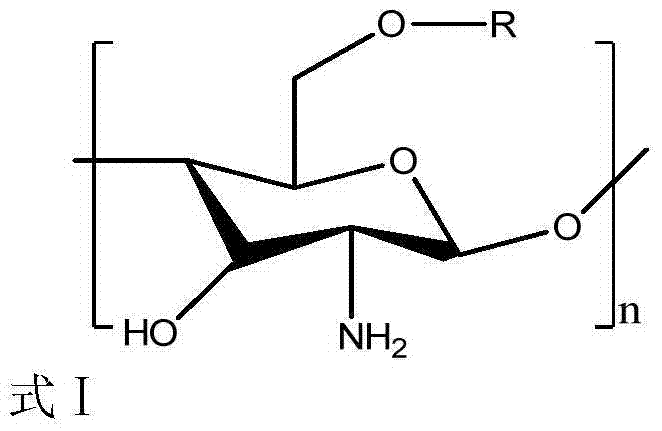
Novel chitosan grafted cinnamoyl product as well as preparation method and application thereof
The invention discloses a chitosan grafted cinnamic acid derivative as well as a preparation method and application thereof. The chitosan grafted cinnamic acid derivative is 6-O-chitosan cinnamate in a formula I in the specification, wherein in the formula I, R is cinnamoyl; n is the degree of polymerization and is not less than 2. The derivative is obtained by grafting a cinnamic acid active structure in the 6-O bit of a chitosan molecule. The preparation method is characterized by reacting cinnamoyl chloride with an amino protector of chitosan, thus preparing the derivative. The chitosan grafted cinnamoyl derivative is applied to preparation of water-based bactericides.
Owner:HENAN INST OF SCI & TECH
Preparation method of shape memory polymer with two-stage ultraviolet-light reversible curable lock
InactiveCN108276537AUnique UV ResponsivenessExcellent shape memory performancePolymer scienceCinnamoyl chloride
The invention discloses a preparation method of a shape memory polymer with a two-stage ultraviolet-light reversible curable lock. The method comprises the following steps: (1) dissolving diethyl iminodiacetate and cinnamoyl chloride into dichloromethane; adding triethylamine, washing and drying to obtain a monomer compound containing a cinnamon structure unit; (2) adding dihydric alcohol and stannous octoate into the monomer compound in step (1) and reacting in a vacuum environment to obtain a shape memory polymer pre-polymer containing hydroxyls at two ends; (3) adding a small molecular monomer containing double bonds into the pre-polymer in step (2) to prepare a shape memory polymer pre-polymer with a double-bond functional group; (4) dissolving the pre-polymer in step (3) into chloroform and adding an initiator; pouring into a polytetrafluoroethylene mold and reacting; de-molding to obtain the shape memory polymer. By adopting the shape memory polymer prepared by the preparation method, the contradiction problem of mechanic and heat-resisting performance and shape memory performance of materials can be effectively solved.
Owner:HARBIN INST OF TECH
Photocureable double-layer polysiloxane supra-molecular elastomer dressing for chronic skin wounds and preparation method of dressing
ActiveCN106039381APromote healingStrong water absorptionPharmaceutical delivery mechanismAbsorbent padsElastomerIrritation
The invention discloses a photocureable double-layer polysiloxane supra-molecular elastomer dressing for chronic skin wounds and a preparation method of the dressing. The method comprises the following steps: making carboxyl-terminated polysiloxane sequentially react with mono-functionality and dual-functionality primary amino compounds, so that a siloxane oligomer containing a secondary amine group is obtained; reacting the siloxane oligomer with hexyl diisocyanate and cinnamoyl chloride, so that a polysiloxane supra-molecular elastomer and a photocureable prepolymer are obtained; and coating the photocureable prepolymer on the polysiloxane supra-molecular elastomer and curing the prepolymer under ultraviolet irradiation to obtain a polysiloxane supra-molecular elastomer is obtained; therefore, the polysiloxane supra-molecular elastomer dressing is prepared. The dressing is good in air permeability and hydroscopicity; a substrate of the dressing is relatively good in elasticity and low in viscosity while a binding layer of the dressing is good in viscosity; the dressing, when used as a chronic wound dressing, is conducive to the growth and the regeneration of wound surface tissues and is capable of accelerating healing of wounds. The dressing, which is prepared from the polysiloxane, is free from cytotoxicity and skin irritation and is good in good biocompatibility.
Owner:SOUTH CHINA UNIV OF TECH
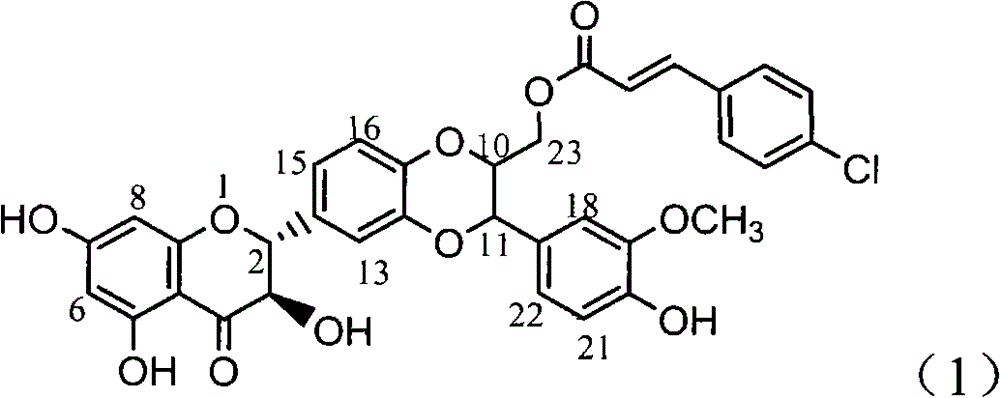
Application of 4-cinnamoyl chloride substituted silybin to preparing glycosidase inhibitors
InactiveCN102000057AConvenient sourceSimple manufacturing methodOrganic active ingredientsMetabolism disorderPositive controlCinnamoyl chloride
The invention relates to application of 4-cinnamoyl chloride substituted silybin to preparing glycosidase inhibitors and in particular discloses application of 4-cinnamoyl chloride substituted silybin ester flavonolignans or pharmaceutical salt thereof to preparing drugs for inhibiting alpha-glycosidase and preventing and treating type II diabetes. The flavonolignans has extremely obvious activity for inhibiting alpha-glycosidase and the intensity of activity of the 40mcg / ml flavonolignans for inhibiting alpha-glycosidase reaches 92.1%. The measured half-inhibitory concentration of the flavonolignans shows that the intensity of activity of the flavonolignans for inhibiting alpha-glycosidase is 30 times that of the positive control drug acarbose. The pharmacodynamics result shows that the flavonolignans or pharmaceutical salt thereof can be expected to be applied to preparing glycosidase inhibitors, especially the drugs for preventing and treating type II diabetes.
Owner:DALI UNIV
Method for preparing cinnamyl cinnamate
InactiveCN101239910AHigh yieldSimple methodOrganic compound preparationCarboxylic acid esters preparationOrganic solventCinnamoyl chloride
The invention discloses a method for prepaing cinnamates, comprising steps of: A. heating materials of cinnamic acid and thionyl chloride to prepare rough cinnamoyl chloride, recoverying non-reacted thionyl chloride under atmospheric pressure, and air extracting to collect cinnamoyl chloride products; B. the cinnamoyl chloride products of the step A and cinnamyl alcohol materials are performed with heat preservation reaction and synthesis in the presence of an organic solvent to obatin rough cinnamates, recoverying the residual wastes of solvent by reduction and re-crystallizing the residual wastes by using methanol or ethanol to obtain cinnamate products. The method of the invention is simple, convenient in operation, low in material cost and high in yield. The method also has no pollution, and is very suitable for industrial production. The yield of producing cinnamates can reach 81.2%.
Owner:湖北远成赛创科技有限公司
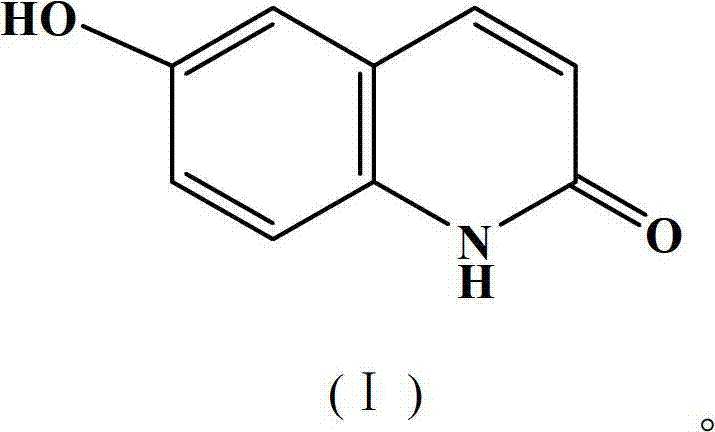
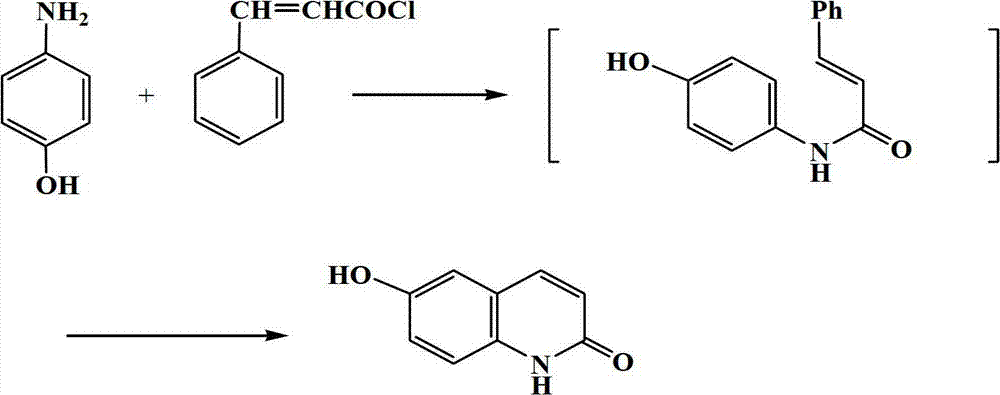
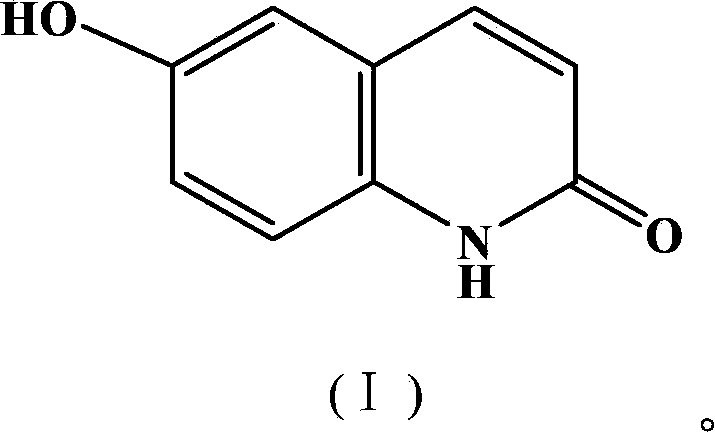
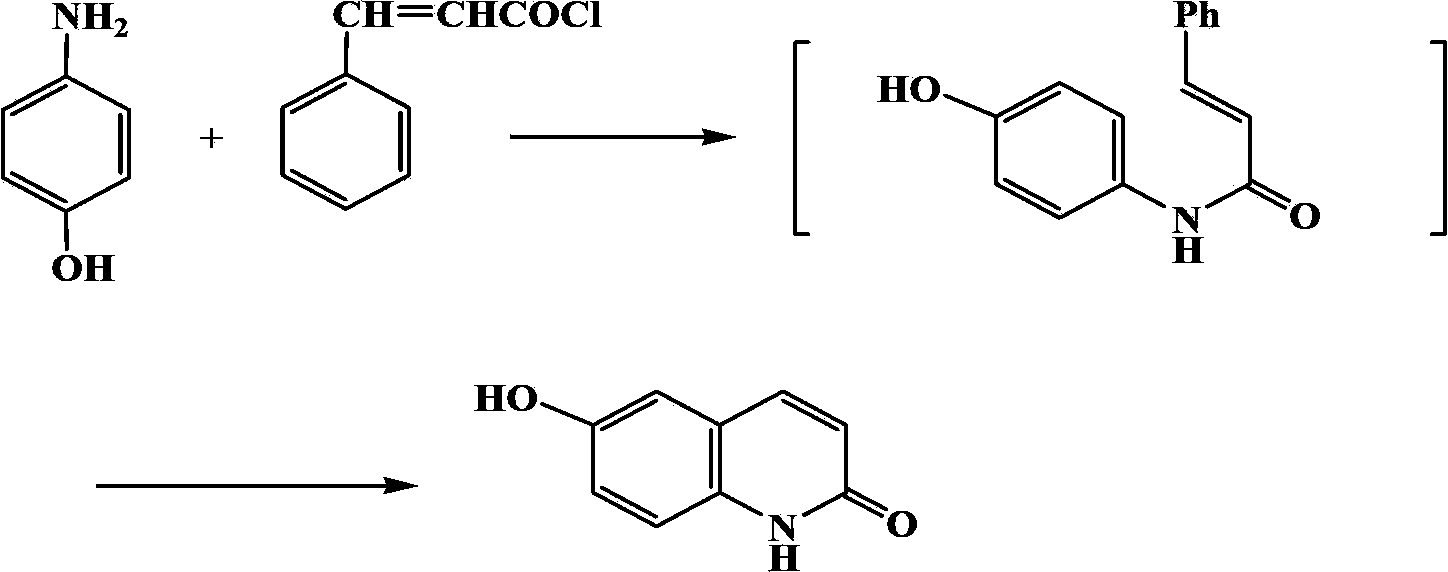
Synthetic method of 6-hydroxyl-2(1H)-quinolinone compound
ActiveCN102816116AThe synthesis method is simpleRaw materials are cheap and easy to getOrganic chemistryCinnamoyl chlorideAniline
The invention discloses a synthetic method of a 6-hydroxyl-2(1H)-quinolinone compound, comprising the following steps of: mixing 4-hydroxy aniline and cinnamoyl chloride, performing a reaction with stirring under the action of a gemini surfactant at the temperature of 50-150 DEG C for 1-20 h, and carrying out post-processing on a reacting liquid after the reaction, so as to obtain the 6-hydroxyl-2(1H)-quinolinone compound. The synthetic method provided by the invention is simple, has an advantage of cheap and easily available raw materials, is environmentally friendly, requires low cost, and is suitable for industrial operation.
Owner:苏州卫优知识产权运营有限公司
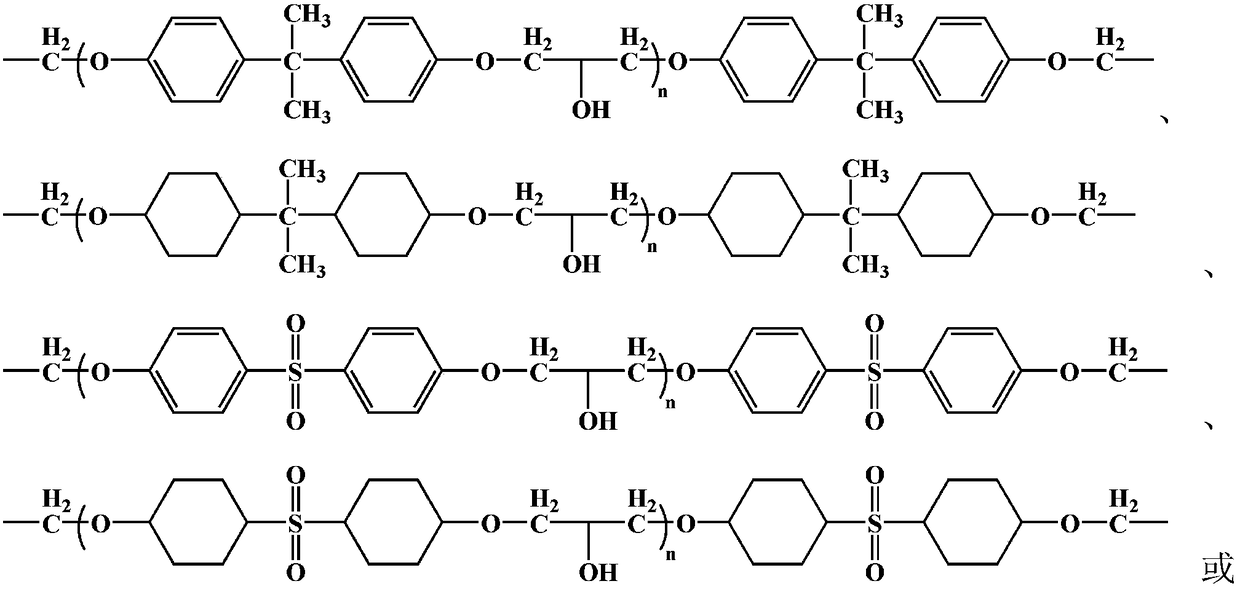
Modified epoxy resin and preparation and application thereof
InactiveCN109134825AImprove curing effectHigh bonding strengthNon-macromolecular adhesive additivesMacromolecular adhesive additivesEpoxyCross-link
The invention relates to a modified epoxy resin and preparation and application thereof. The structural general formula of the modified epoxy resin is shown in the description. The modified epoxy resin preparation method achieves the chemically modified epoxy resin, which is prepared from the suitable size epoxy resin modified by mercapto polymethacrylic acid cinnamoyl chloride ethyl ester or a mercapto polyacrylic acid cinnamoyl chloride ethyl ester with proper polymerization degree. When the curing adhensive is prepared from the modified epoxy resin, the cross-linking and curing under cross-linking adhensive system which is obtained does not need to depend on components such as photoinitiator and the like, but self-cured under room temperature and purple light; In addition, the time forcross-linking and curing can be well controlled through the adjustment to the size of mercaptopolymethacrylic acid cinnamoyl chloride ethyl ester or the mercapto polyacrylic acid cinnamoyl chloride ethyl ester, and truly realize the curing during a very short time. The modified epoxy resin can not only guarantee the curing effect but have good bonding strength as well.
Owner:GUANGZHOU BAIYUN CHEM IND
Method for preparing cinnamoyl chloride from silicon tetrachloride
InactiveCN105777531ALow costOrganic compound preparationCarboxylic compound preparationCinnamoyl chlorideAdditional values
The invention provides a method for preparing cinnamoyl chloride from silicon tetrachloride; the method includes the steps: with silicon tetrachloride as an acyl chlorination reagent, and carrying out a reaction with cinnamic acid for 2-4 h at the temperature of 60-70 DEG C, to obtain a cinnamoyl chloride crude product. A large amount of by-product silicon tetrachloride in polysilicon production and organosilicon production is effectively transformed to produce cinnamoyl chloride having high additional value; while the difficult-to-treat by-product silicon tetrachloride brought from the polysilicon production is transformed and treated, the cost of preparation of cinnamoyl chloride is significantly reduced.
Owner:SICHUAN UNIVERSITY OF SCIENCE AND ENGINEERING
Preparation method of cinnamoyl modified fabric
InactiveCN109023936AImproves UV protectionLong UV protection cycle timeLight resistant fibresCinnamoyl chlorideRetention time
The invention provides a preparation method of cinnamoyl modified fabric, comprising the following steps: treating fabric with alkali lye; preparing carboxyl modified fabric by using acrylic acid; preparing amino-modified fabric through PEG which is amino-modified at two ends; grafting cinnamoyl chloride to the surface of the amino-modified fabric to obtain cinnamoyl modified fabric; and putting the cinnamoyl modified fabric into an ethanol solution containing dihexadecyldimethylammonium bromide, polydimethylsiloxane diquaternary ammonium salt, amino silicon oil, methyl salicylate and polyhexamethylene biguanidine hydrochloride so as to prepare the fabric finished product. It shows through test results that the prepared fabric has good anti-ultraviolet capability and the retention time ofanti-ultraviolet period is long. Thus, the fabric has higher use value when used as anti-ultraviolet fabric.
Owner:SUZHOU TIANAO SPECIAL EMBROIDERY CO LTD
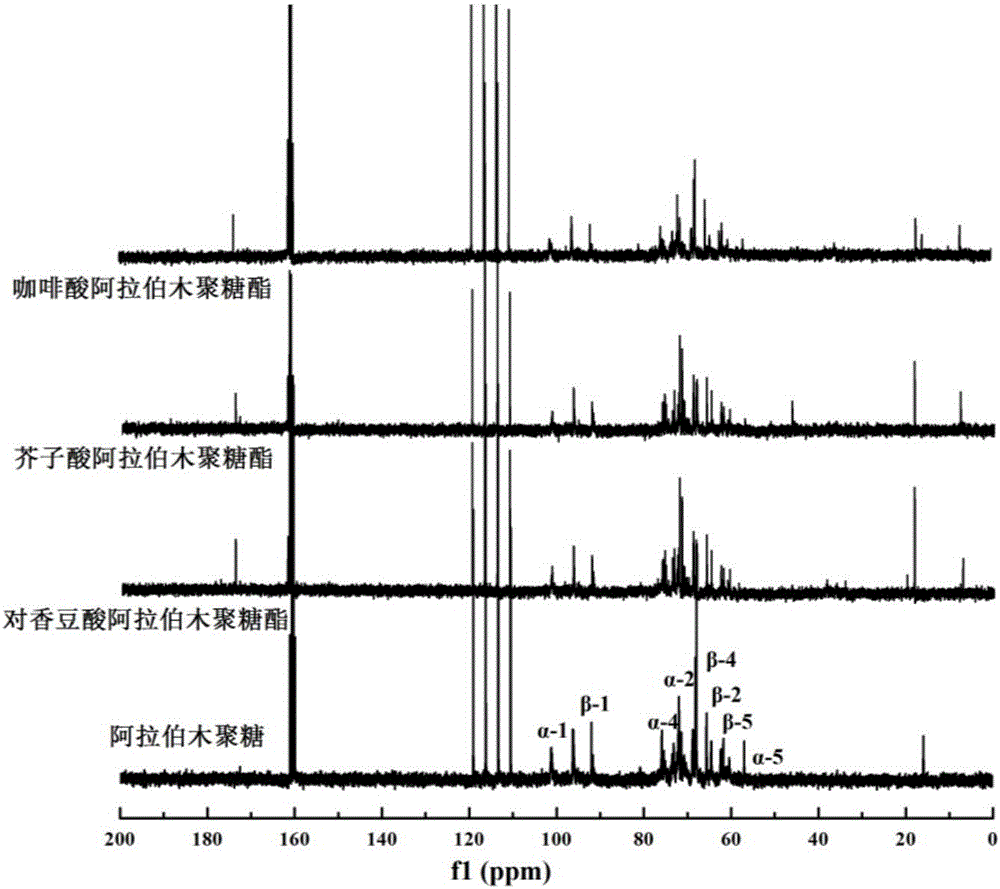
Multifunctional araboxylan and preparation method thereof
The invention discloses multifunctional araboxylan and a preparation method thereof, belongs to the field of natural high-molecular modification, and relates to a multifunctional polysaccharide antioxidant and a preparation method thereof. The preparation method comprises the following steps: grafting a small molecular antioxidant to araboxylan; making hydroxycinnamic acid react under the action of thionyl chloride to generate hydroxy cinnamoyl chloride; then, carrying out an esterification reaction on the araboxylan and the hydroxy cinnamoyl chloride under the action of triethylamine (acid-binding agent) to generate hydroxycinnamic acid araboxylan ester. The hydroxycinnamic acid araboxylan ester has high oxygen resistance and thickening performance, can be widely applied to the industries of foods, health care products and makeups.
Owner:JIANGNAN UNIV
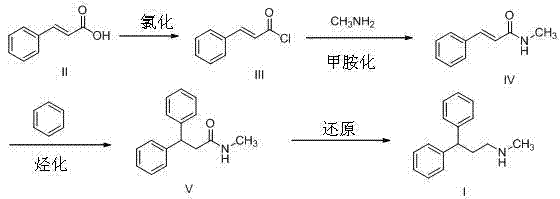
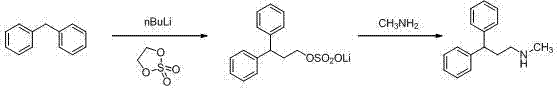
Synthesis method of lercanidipine intermediates
ActiveCN103360263AOrganic compound preparationAmino compound preparationSynthesis methodsCinnamoyl chloride
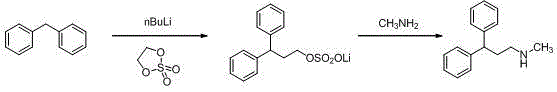
The invention discloses a synthesis method of lercanidipine intermediates. The method comprises the following steps of: taking cinnamic acid as an initial material, obtaining cinnamoyl chloride by chlorination, and then obtaining N-methyl cinnamon amide by amination; further carrying out friedel-crafts alkylation reaction together with benzene to obtain N-methyl-1,1-diphenylacetamide under the effect of a catalyst; and finally obtaining the N-methyl-3,3-diphenyl alanine by carbonyl reduction. The route is cheap and available in material, convenient to react and operate, high in yield, low in cost and suitable for industrial application.
Owner:CHONGQING SHENGHUAXI PHARMA CO LTD +1
Food additive and preparation method thereof
ActiveCN102613658BHigh antibacterial activityGood oil soluble antioxidantFruit and vegetables preservationOrganic chemistryFood additivePreservative
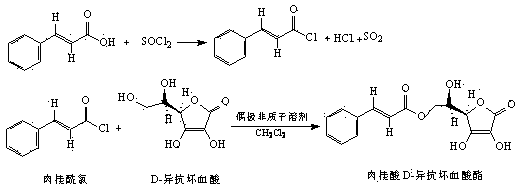
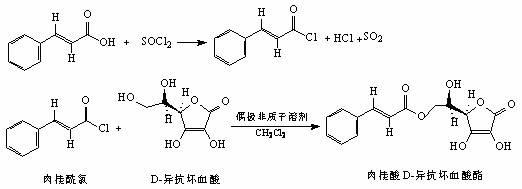
The invention relates to food additive cinnamic acid D-isoascorbic acid ester and a preparation method thereof. Cinnamic acid is reacted with thionyl chloride to generate cinnamoyl chloride, and the cinnamoyl chloride is then reacted with D-isoascorbic acid to generate cinnamic acid D-isoascorbic acid ester. Purity of a product is higher than 95%, spectral characterization data completely match with the structure of the product, and the food additive cinnamic acid D-isoascorbic acid ester has obvious oxidation resistance and antibacterial activity, and can be prepared into food antioxidant and food sterilizing preservative.
Owner:江西省德兴市百勤异VC钠有限公司 +1
Food additive and preparation method thereof
ActiveCN102613658AHigh antibacterial activityGood oil soluble antioxidantOrganic chemistryFruit and vegetables preservationBiotechnologyFood additive
The invention relates to food additive cinnamic acid D-isoascorbic acid ester and a preparation method thereof. Cinnamic acid is reacted with thionyl chloride to generate cinnamoyl chloride, and the cinnamoyl chloride is then reacted with D-isoascorbic acid to generate cinnamic acid D-isoascorbic acid ester. Purity of a product is higher than 95%, spectral characterization data completely match with the structure of the product, and the food additive cinnamic acid D-isoascorbic acid ester has obvious oxidation resistance and antibacterial activity, and can be prepared into food antioxidant and food sterilizing preservative.
Owner:江西省德兴市百勤异VC钠有限公司 +1
Application of 4-cinnamoyl chloride silybin in preparing medicaments for treating viral hepatitis B
InactiveCN101829099AInhibitory activityInhibition of replicationOrganic active ingredientsDigestive systemDiseaseHigh concentration
The invention relates to application of 4-cinnamoyl chloride silybin in preparing medicaments for treating viral hepatitis B, in particular to application of 4-cinnamoyl chloride substituted silybin ester type flavonolignan or a pharmaceutically acceptable salt thereof in preparing medicaments for suppressing HBV (Hepatitis B Virus) DNA replication and treating HBV infection diseases. The flavonolignan has definite activity on suppressing the HBV DNA, and the suppression activity on the replication of the HBV DNA under a high dose approaches that of alpha-interferon under a highest concentration of 10,000 units / milliliter, thus the flavonolignan belongs to effective non-nucleoside natural products for resisting the HBV. The pharmacodynamical results indicate that the flavonolignan or the pharmaceutically acceptable salt thereof can be expected to be used for preparing the medicaments for suppressing the HBV DNA replication and treating the HBV infection diseases.
Owner:DALI UNIV
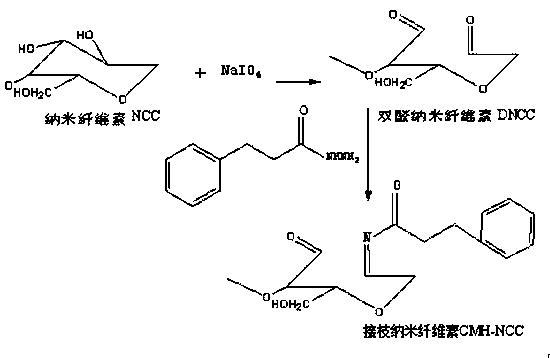
Preparation method of photoresponsive cinnamic acid derivative-grafted nanocellulose
InactiveCN109608556AGood photoresponse performanceEasy to produceCinnamoyl chlorideMechanical property
The invention belongs to the field of biomass functional polymer materials, and particularly relates to a preparation method of photoresponsive cinnamic acid derivative-grafted nanocellulose. According to the method, cellulose raw materials are added to a sulfuric acid solution, and nanocellulose is prepared under a certain temperature and ultrasonic condition; a nanocellulose solution is obtainedthrough dialysis and then reacts with sodium periodate to obtain dialdehyde nanocellulose; and cinnamoyl chloride and hydrazine hydrate react to prepare 3-phenylpropanehydrazide, and subsequently, the 3-phenylpropanehydrazide and an aldehyde group on the dialdehyde nanocellulose undergo a condensation reaction in a DMF solution, so that the cinnamic acid derivative-grafted nanocellulose is successfully prepared by connection of imine bonds. The nanocellulose of the invention can be used as a photoresponsive filler, is green, friendly to environment and high in safety, and has dual functions of improving mechanical properties and imparting light response performance.
Owner:FUJIAN AGRI & FORESTRY UNIV
Synthesis method of lercanidipine intermediates
ActiveCN103360263BOrganic compound preparationAmino compound preparationSynthesis methodsCinnamoyl chloride
The invention discloses a synthesis method of lercanidipine intermediates. The method comprises the following steps of: taking cinnamic acid as an initial material, obtaining cinnamoyl chloride by chlorination, and then obtaining N-methyl cinnamon amide by amination; further carrying out friedel-crafts alkylation reaction together with benzene to obtain N-methyl-1,1-diphenylacetamide under the effect of a catalyst; and finally obtaining the N-methyl-3,3-diphenyl alanine by carbonyl reduction. The route is cheap and available in material, convenient to react and operate, high in yield, low in cost and suitable for industrial application.
Owner:CHONGQING SHENGHUAXI PHARMA CO LTD +1
Novel method for preparing new antipsychotic drug brexpiprazole
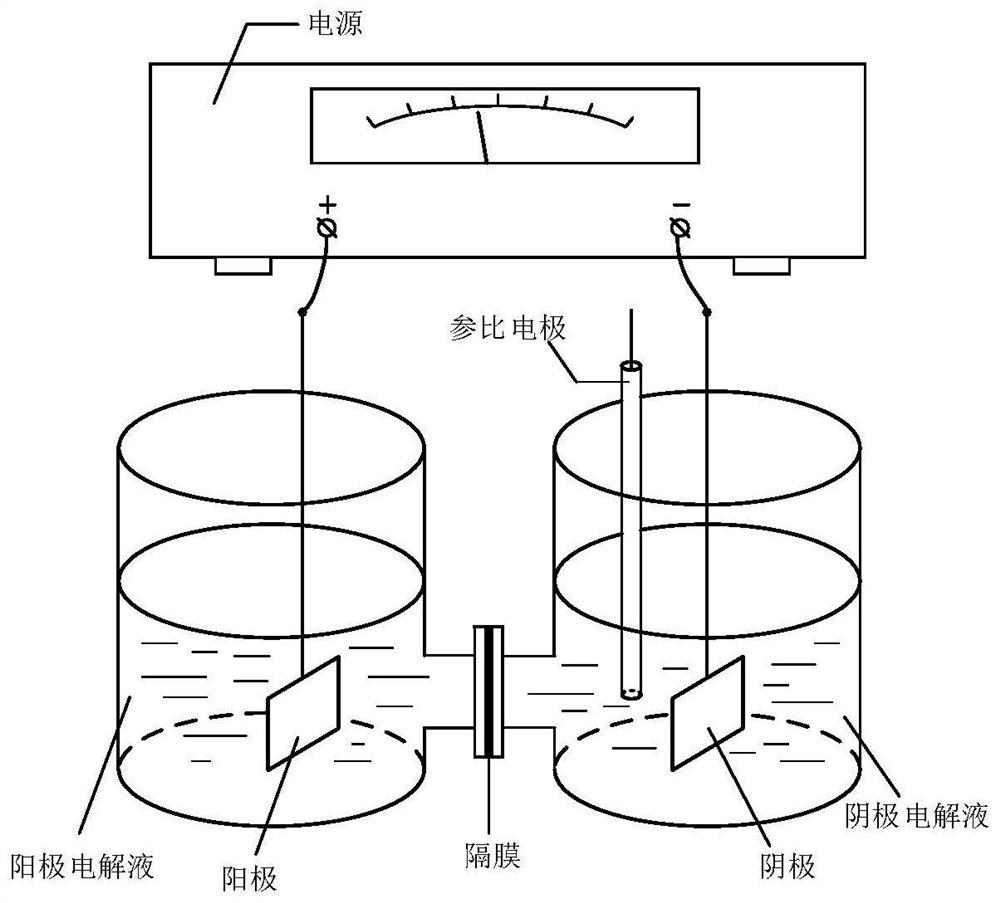
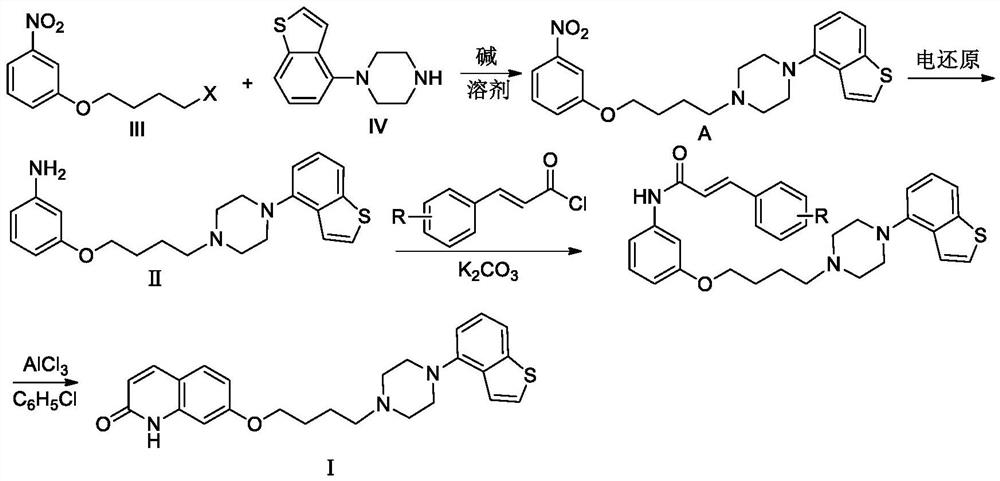
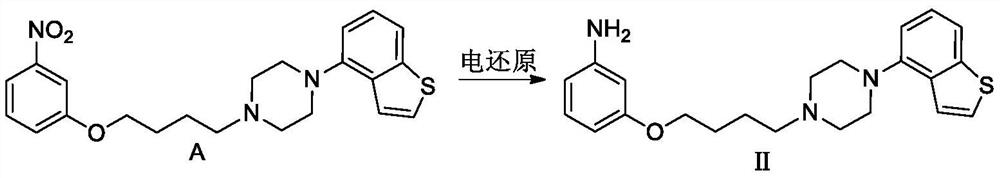
ActiveCN112125896AHigh yieldHigh purityOrganic chemistryElectrolysis componentsCinnamoyl chlorideNitrobenzene
The invention discloses a new electrochemical preparation method of a new antipsychotic drug brexpiprazole. The preparation method specifically comprises the following steps of: preparing a key intermediate, namely -1-(benzo [b] thiophene-4-yl)-4-(4-(-3nitrophenoxy) butyl) piperazine, and carrying out electric reduction on the key intermediate to prepare 3-[4-[4-(benzo [b] thiophene-4-yl) piperazine-1y-l] butoxy] aniline; and carrying out acylation reaction with cinnamyl chloride and intramolecular Friedel-Crafts acylation reaction to prepare brexpiprazole.
Owner:湖南省湘中制药有限公司
Application of 4-cinnamoyl chloride substituted silybin in preparing glycosidase inhibitors
InactiveCN102000057BInhibitory activityExact originalityOrganic active ingredientsMetabolism disorderPositive controlCinnamoyl chloride
The invention relates to application of 4-cinnamoyl chloride substituted silybin to preparing glycosidase inhibitors and in particular discloses application of 4-cinnamoyl chloride substituted silybin ester flavonolignans or pharmaceutical salt thereof to preparing drugs for inhibiting alpha-glycosidase and preventing and treating type II diabetes. The flavonolignans has extremely obvious activity for inhibiting alpha-glycosidase and the intensity of activity of the 40mcg / ml flavonolignans for inhibiting alpha-glycosidase reaches 92.1%. The measured half-inhibitory concentration of the flavonolignans shows that the intensity of activity of the flavonolignans for inhibiting alpha-glycosidase is 30 times that of the positive control drug acarbose. The pharmacodynamics result shows that the flavonolignans or pharmaceutical salt thereof can be expected to be applied to preparing glycosidase inhibitors, especially the drugs for preventing and treating type II diabetes.
Owner:DALI UNIV
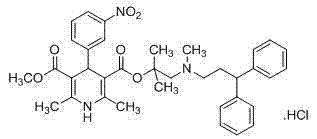
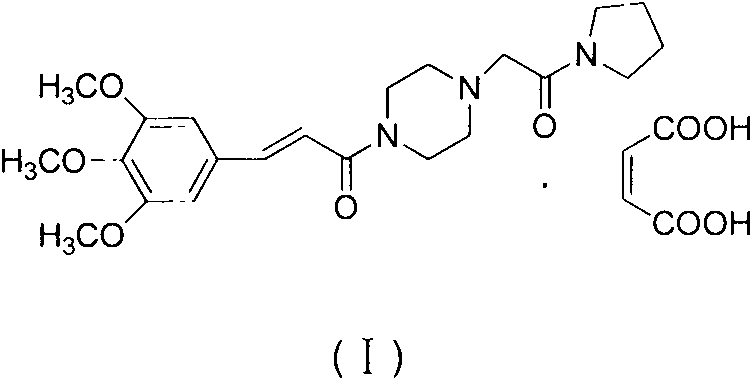
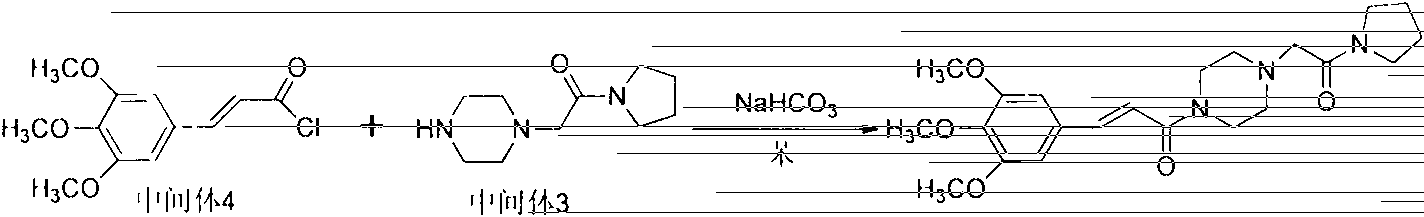
Improved synthetic method of cinepazide maleate
InactiveCN103664830ARaw materials are easy to getThe reaction equipment is simpleOrganic chemistryCinnamoyl chloridePyrrolidine
The invention relates to an improved synthetic method of cinepazide maleate. The synthetic method sequentially comprises the following steps: 1, preparation of chloracetyl pyrrolidine; 2, preparation of 1-piperazine acetyl pyrrolidine; 3, preparation of 3, 4, 5-trimethoxyl cinnamoyl chloride; 4, preparation of cinepazide free alkali; 5, preparation of cinepazide maleate. The synthetic method is characterized in that in the preparation processes of steps 1 and 4, a feeding manner of dropping an alkaline raw material is adopted; in the step 2, by taking triethylamine as an acid-binding agent, a post-treatment method for removing piperazine by layered extraction is adopted, or by taking potassium carbonate as an acid-binding agent, a method for removing piperazine by acetone crystallization is adopted. The invention provides the improved synthetic method of cinepazide maleate, which is simple to operate, economic and applicable and can be used for industrialized production easily by changing the feeding sequence and the post-treatment method.
Owner:凌沛学
Method for synthesizing 5,6,4'-trihydroxyflavone-7-O-D-glucuronic acid
InactiveCN101538298BRaw materials are easy to getThe synthetic route is simpleSugar derivativesSugar derivatives preparationChemical reactionCinnamoyl chloride
The invention discloses a chemical method for preparing 5,6,4'-trihydroxyflavone-7-O-D-glucuronic acid to solve problems of unavailable raw materials, long synthetic route and low synthesis yield in the existing synthetic method. The method comprises a plurality of chemical reaction steps: first, taking 3,4,5-trimethoxy phenol and p-methoxy cinnamoyl chloride as raw materials to construct a chalcone structure by a Friedel-Crafts acylation reaction, and cycloetherifying to obtain a flavone parent substance; then protecting and selectively deprotecting phenolic hydroxyl of the flavone parent substance, and glycosylating 7-phenolic hydroxyl; and finally removing a protective group to obtain the target compound. The obtained product has a beta-steric configuration. The method can be used for preparing high-purity and high-quality 5,6,4'-trihydroxyflavone-7-O-D-glucuronic acid products.
Owner:四川抗菌素工业研究所有限公司
Method used for measuring (E)3, 4, 5-triethoxy cinnamoyl chloride with LC-MS/MS
InactiveCN109115900ALow detection limitImprove stabilityComponent separationCinnamoyl chlorideColumn temperature
The invention belongs to the technical field of medicine impurity analysis, and more specifically discloses a method used for measuring (E)3, 4, 5-triethoxy cinnamoyl chloride with LC-MS / MS. Accordingto the method, the method is invented based on the principle that (E)3, 4, 5-triethoxy cinnamoyl chloride is capable of reacting with diethylamine to generate stable compound (E) N, N-dimethyl-3, 4,5-triethoxy cinnamamide. A derivatization product is subjected to liquid chromatography tandem mass spectrometry analysis, and the conditions are as following: chromatographic column Agilent Poroshell120 EC-C18(100*2.1mm, 2.7<mu>m); mobile phase methanol-0.1% formic acid (V / V, 50:50); flow speed 0.4ml / min; column temperature 35 DEG C; sample introduction volume 5<mu>l; ion source electrospray iontrap (ESI); detection method positive ion multi-reaction monitoring mode; the monitoring ion pairs of (E) N, N-dimethyl-3, 4, 5-triethoxy cinnamamide are m / s 294.0 / 221.0 and 294.0 / 190.0. The method is high in specificity, low in detection limit, high in stability, high in recovery rate, and can be used for quality control of impurity (E)3, 4, 5-triethoxy cinnamoyl chloride in cinepazide maleate production process.
Owner:中南粮油食品科学研究院有限公司 +1
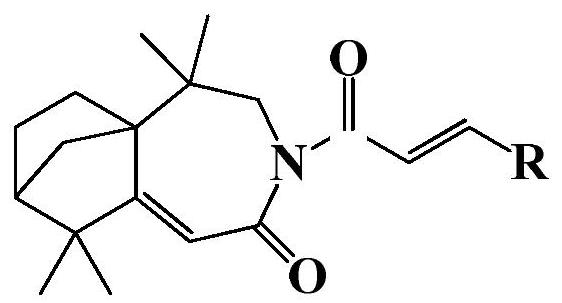
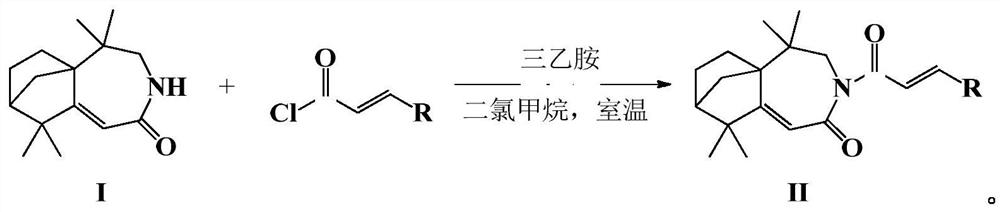
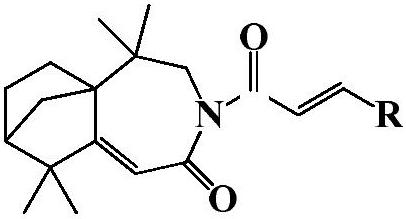
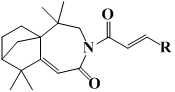
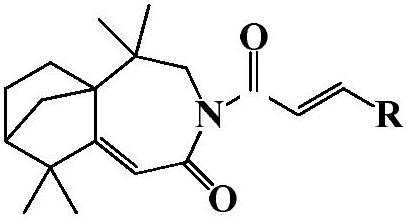
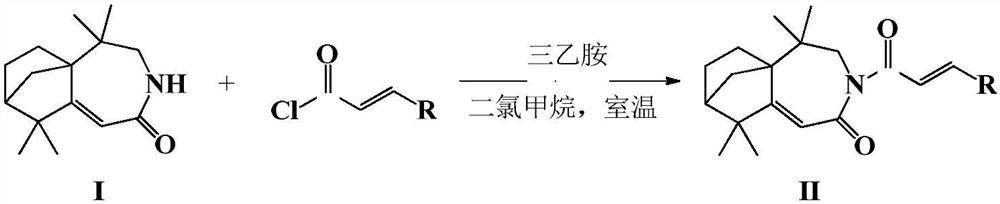
Isolongifolenone caprolactam derivative as well as preparation method and application thereof
ActiveCN111892539ARich sourcesEase of industrial productionOrganic chemistryAntineoplastic agentsIsolongifolenoneCinnamoyl chloride
The invention discloses an isolongifolenone caprolactam derivative as well as a preparation method and application thereof. The 3-cinnamoyl-5,5,9,9-tetramethyl-4,5,6,7,8,9-hexahydro-5a,8-bridged methylene benzo[d]azepine-2(3H)-one compound is prepared by taking an isolongifolenone caprolactam derivative as a raw material and taking a cinnamoyl chloride derivative as an acylation reagent through acylation reaction under the catalytic action of alkali. Experiments prove that the 3-cinnamoyl-5,5,9,9-tetramethyl-4,5,6,7,8,9-hexahydro-5a,8-bridged methylene benzo[d]azepine-2(3H)-one compound has good inhibitory activity on human breast cancer cells MCF-7, and has potential application value in preparation of antitumor drugs.
Owner:NANJING FORESTRY UNIV
Synthesis method of bagasse xylan cinnamic acid/m-chlorobenzoic acid diester with antiviral activity
InactiveCN110724213AImprove physical and chemical propertiesStrong antiviral activityAntiviralsM-chlorobenzoic acidBenzoic acid
The invention discloses a synthesis method of bagasse xylan cinnamic acid / m-chlorobenzoic acid diester with antiviral activity. The method comprises: carrying out an esterification reaction in a dichloromethane solvent by using bagasse xylan as a main raw material, using cinnamoyl chloride as an esterifying agent and using triethylamine as a catalyst to synthesize bagasse xylan cinnamate; and carrying out a secondary esterification reaction by using a sodium m-chlorobenzoate solution as an esterifying agent and using a 732 type strong-acid cation exchange resin and N,N'-diisopropylcarbodiimideas a composite catalyst to synthesize the bagasse xylan cinnamic acid / m-chlorobenzoate diester. According to the invention, the product bagasse xylan cinnamic acid / m-chlorobenzoic acid diester is synthesized through the two-step esterification composite catalytic reaction, wherein the two active groups of cinnamic acid and m-chlorobenzoic acid are introduced, so that the physicochemical properties of the original bagasse xylan are improved, and the antiviral activity of the bagasse xylan derivative is enhanced.
Owner:GUILIN UNIVERSITY OF TECHNOLOGY
Syntheses method of cinnamic acid/o-chlorobenzoic acid bagasse xylan diester with anti-HIV activity
InactiveCN110724212AEnhanced anti-HIV activityImprove physical and chemical propertiesAntiviralsBenzoic acidChlorobenzene
The invention discloses a synthesis method of cinnamic acid / o-chlorobenzoic acid bagasse xylan diester with anti-HIV activity. The method comprises: carrying out first-step esterification reaction ina dichloromethane solvent by using bagasse xylan as a main raw material, using cinnamyl chloride as an esterifying agent and using triethylamine as a catalyst to synthesize cinnamate bagasse xylan; and carrying out a second-step composite catalytic esterification reaction by using o-chlorobenzoyl chloride as an esterifying agent and using pyridine and N,N'-diisopropylcarbodiimide (DIC) as a composite catalyst to synthesize the cinnamic acid / o-chlorobenzoic acid bagasse xylan diester. According to the invention, through the two esterification reactions, the product has the properties of cinnamic acid bagasse xylan ester and o-chlorobenzoic acid bagasse xylan ester, has the enhanced biological activity, and widens the application range of the xylan derivative in the fields of biology, medicine and the like.
Owner:GUILIN UNIVERSITY OF TECHNOLOGY
Synthetic method of 6-hydroxyl-2(1H)-quinolinone compound
ActiveCN102816116BThe synthesis method is simpleRaw materials are cheap and easy to getOrganic chemistryCinnamoyl chlorideAniline
The invention discloses a synthetic method of a 6-hydroxyl-2(1H)-quinolinone compound, comprising the following steps of: mixing 4-hydroxy aniline and cinnamoyl chloride, performing a reaction with stirring under the action of a gemini surfactant at the temperature of 50-150 DEG C for 1-20 h, and carrying out post-processing on a reacting liquid after the reaction, so as to obtain the 6-hydroxyl-2(1H)-quinolinone compound. The synthetic method provided by the invention is simple, has an advantage of cheap and easily available raw materials, is environmentally friendly, requires low cost, and is suitable for industrial operation.
Owner:苏州卫优知识产权运营有限公司
Isophyllenone-caprolactam derivatives and their preparation methods and applications
ActiveCN111892539BRich sourcesEase of industrial productionOrganic chemistryAntineoplastic agentsCinnamoyl chlorideAcyl group
The invention discloses an isolongifolenone-caprolactam derivative, a preparation method and an application thereof. The present invention uses isolongifolyl caprolactam derivatives as raw materials and cinnamoyl chloride derivatives as acylating reagents to undergo an acylation reaction under the catalysis of a base to prepare 3-cinnamoyl-5,5,9,9 ‑Tetramethyl‑4, 5, 6, 7, 8, 9‑hexahydro‑5a, 8‑endomethylenebenzo[d]azepine‑2(3H)‑ketones. The application has been proved by experiments that 3-cinnamoyl-5,5,9,9-tetramethyl-4,5,6,7,8,9-hexahydro-5a, 8-endomethylene benzo[d The ]azepine-2(3H)-ketone compound has good inhibitory activity on human breast cancer cell MCF-7, and has potential application value in the preparation of anti-tumor drugs.
Owner:NANJING FORESTRY UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com