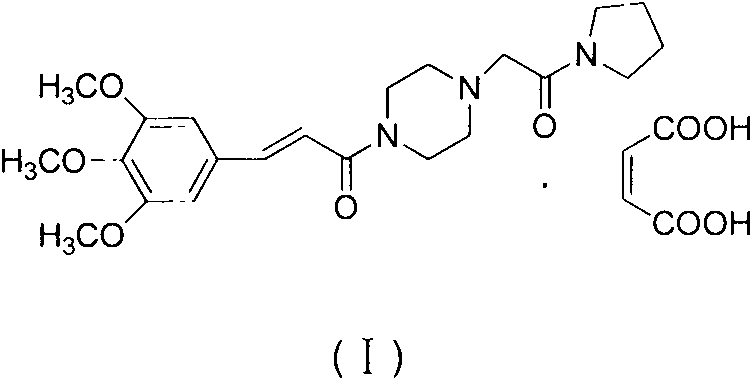
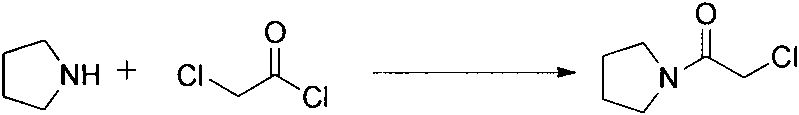Improved synthetic method of cinepazide maleate
A technology of cinepazide maleate and a synthesis method, which is applied in the synthesis field of -1{4-]-1-piperazine} acetylpyrrolidine maleate and can meet the requirements of production conditions and equipment High, unfavorable industrial production, easy to block pipelines and other problems, to achieve the effect of low cost, easy operation, simple reaction equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
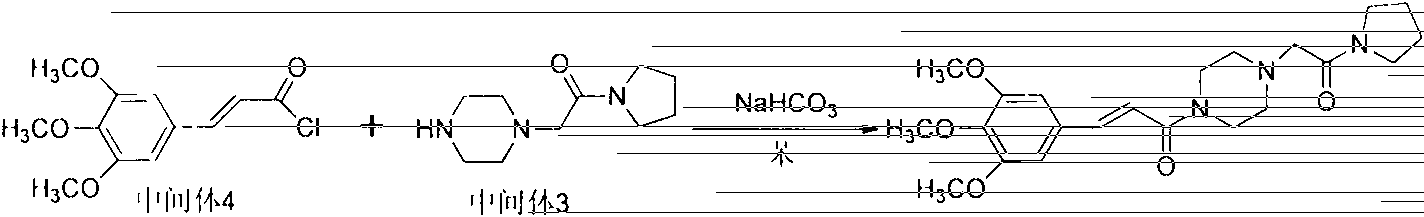
[0014] An improved preparation method of cinepazide maleate, comprising the following steps: 1: preparation of chloroacetylpyrrolidine; step 2: preparation of 1-piperazine acetylpyrrolidine; step 3: 3, 4, Preparation of 5-trimethoxycinnamoyl chloride; step 4: preparation of cinepazide free base; step 5: preparation of cinepazide maleate. In the preparation process of step 1 and step 4, the feeding method of dripping alkaline raw materials was adopted; in step 2, triethylamine was used as the acid-binding agent, and the post-treatment method of removing piperazine by layered extraction was adopted, also Or use potassium carbonate as an acid-binding agent and use acetone crystallization to remove piperazine.
[0015]
[0016] In the preparation method of step 2, different acid-binding agents adopt different methods to remove excess piperazine.
[0017] As an optimized solution, when organic bases such as organic amines or pyridines are used as acid-binding agents for the rea...
Embodiment 1
[0037] Embodiment 1: the synthesis of cinepazide maleate
[0038] Step 1: Preparation of Chloroacetylpyrrolidine
[0039] Put chloroacetyl chloride (3.7kg, 33mol) and dichloromethane (21.5kg) into a 50L reaction kettle in turn, stir and cool down to -10~0°C, slowly add tetrahydropyrrole (2.15kg, 30mol), triethyl Amine (3.12kg, 33mol) in dichloromethane (2.5kg) solution, the temperature is controlled not to exceed 0°C during the dropwise addition, after the drop is completed, react at 0°C for 0.5h, raise the temperature to reflux and continue stirring for 2h, cool down to room temperature, and react The solution was washed with water and saturated NaCl successively, dried over anhydrous sodium sulfate, the filtrate was concentrated, dichloromethane was recovered, and the residue solidified at room temperature to give 2 (3.7kg, yield 83.9%), Mp43-45°C.
[0040] Step 2: Preparation of 1-piperazine acetylpyrrolidine
[0041] Put anhydrous piperazine (8.2kg, 95.2mol) and absolute...
Embodiment 2
[0049] Embodiment 2: the synthesis of cinepazide maleate
[0050] Step 1: Preparation of Chloroacetylpyrrolidine
[0051] Put chloroacetyl chloride (3.7kg, 33mol) and dichloromethane (21.5kg) into a 50L reaction kettle in turn, stir and cool down to -10~0°C, slowly add tetrahydropyrrole (2.15kg, 30mol), triethyl Amine (3.12kg, 33mol) in dichloromethane (2.5kg) solution, the temperature is controlled not to exceed 0°C during the dropwise addition, after the drop is completed, react at 0°C for 0.5h, raise the temperature to reflux and continue stirring for 2h, cool down to room temperature, and react The solution was washed with water and saturated NaCl successively, dried over anhydrous sodium sulfate, the filtrate was concentrated, dichloromethane was recovered, and the residue solidified at room temperature to give 2 (3.7kg, yield 83.9%), Mp43-45°C.
[0052] Step 2: Preparation of 1-piperazine acetylpyrrolidine
[0053] Put anhydrous piperazine (8.2kg, 95.2mol), triethylami...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com