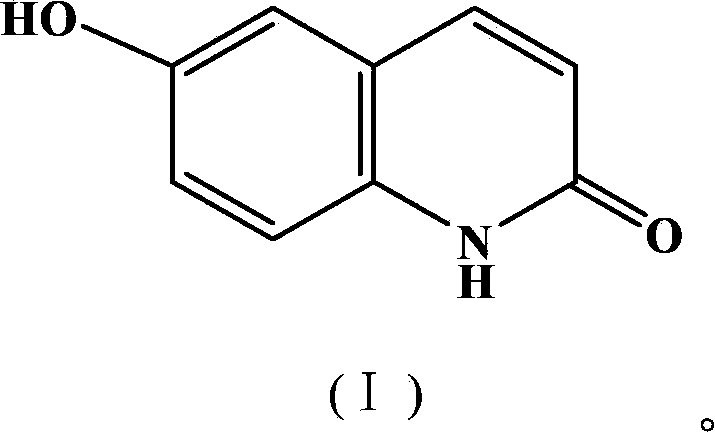
Synthetic method of 6-hydroxyl-2(1H)-quinolinone compound
A synthesis method and quinolinone technology are applied in directions such as organic chemistry to achieve the effects of simple synthesis method, environmental friendliness and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
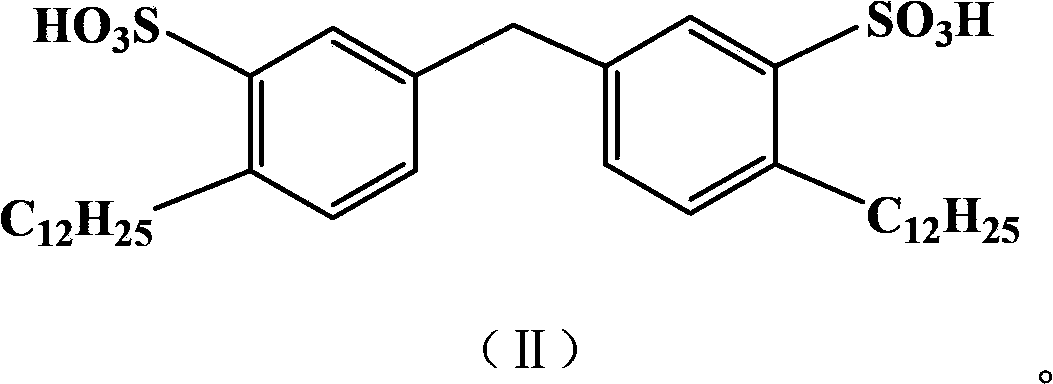
[0029] The preparation of embodiment 1 gemini surface active agent
[0030] 1) Alkylation Add 16.8g (0.1mol) of diphenylmethane, anhydrous AlCl 3 13g, fully stirred to disperse the catalyst evenly, at 45-50°C, add 34g (0.2mol) of dodecene dropwise, the dropping time is controlled at 20-30min, then gradually raise the reaction temperature to 70°C, and keep No change, react for 8 hours, after the reaction, adjust the reaction solution to neutral, extract with toluene, dry over anhydrous magnesium sulfate, filter, concentrate to obtain the alkylated product for the next step of sulfonation;
[0031] 2) Sulfonation In a four-necked reaction flask equipped with an electric stirrer, a thermometer, a reflux condenser, and a dropping funnel, add the alkylated product obtained above, and add 24 g (0.2 mol) of chlorosulfonic acid dropwise under vigorous stirring. ), the dropping time is 1h, the reaction temperature is controlled at 20-25°C, and the reaction is carried out for 2 hours. ...
Embodiment 2
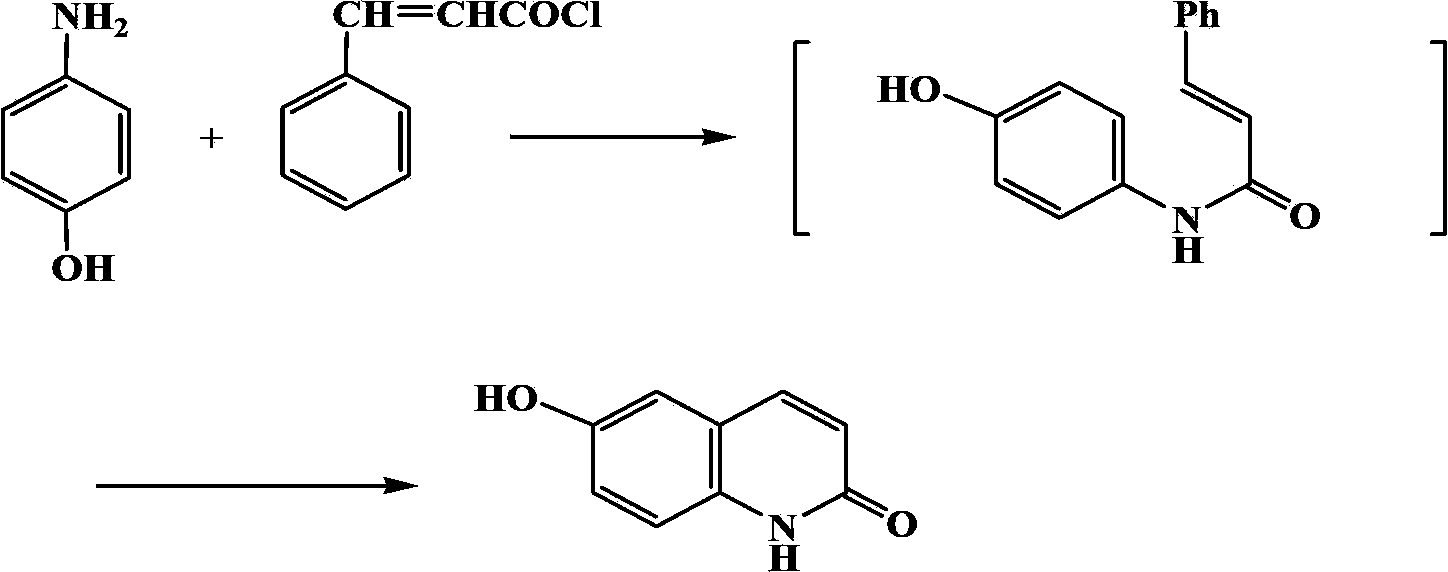
[0033] Example 2: Preparation of 6-hydroxyl-2(1H)-quinolinone compound
[0034] In a 150ml three-necked flask, add 3.27g (0.3mol) of 4-hydroxyaniline, 5.3g (0.32mol) of cinnamoyl chloride, and 9.8g of the gemini surfactant prepared by the method in Example 1, and react at 100°C for 10 hours while stirring. After the completion, the reaction solution was cooled to room temperature (25°C) and extracted three times with 30 mL of toluene. The toluene layers were combined and concentrated under reduced pressure until no toluene flowed out. The concentrate was recrystallized with industrial methanol to obtain 1.2 g of a white solid, namely 6-hydroxy- 2(1H)-Quinolinone compound, yield 61%, m.p.>300℃. IR(KBr):3108,2852,1658,1629,1429,1298cm -1 . 1H NMR (DMSO-d 6 )δ: 6.48 (1H, d, J=9.52, H-3), 7.16~7.25 (3H, m, ArH), 7.81 (1H, d, J=9.52, H-4), 9.46 (1H, s, OH), 11.64 (1H, s, H-1).
Embodiment 3
[0035] Example 3: Preparation of 6-hydroxyl-2(1H)-quinolinone compound
[0036] In a 150ml three-necked flask, add 3.27g (0.3mol) of 4-hydroxyaniline, 7.5g (0.45mol) of cinnamoyl chloride, and 33g of the gemini surfactant prepared by the method in Example 1, and react under stirring at 150°C for 10 hours, and the reaction ends The reaction solution was extracted three times with 30 mL of toluene, the toluene layers were combined, concentrated until no toluene flowed out, and the concentrate was recrystallized with industrial methanol to obtain 1.1 g of a white solid, namely 6-hydroxy-2(1H)-quinolinone compound. The rate is 59%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com